Chicken Egg Proteins and Derived Peptides with Antioxidant Properties
Abstract
:1. Introduction
2. Antioxidant Activity of Chicken Egg Proteins
2.1. Ovalbumin
2.2. Ovotransferrin
2.3. Lysozyme
2.4. Cystatin
2.5. Phosvitin
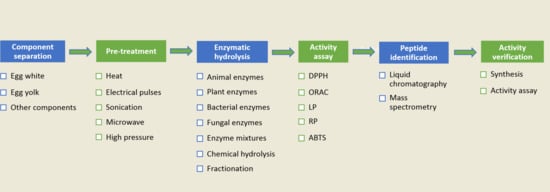
3. Production of Antioxidant Peptides from Chicken Egg Proteins
3.1. Hydrolysis of Egg White
3.2. Hydrolysis of Egg Yolk
3.3. Hydrolysis of Other Egg Components
4. Peptides from Individual Egg White Proteins
4.1. Ovotransferrin
4.2. Lysozyme
4.3. Ovalbumin
4.4. Other Egg Proteins
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Nimalaratne, C.; Bandara, N.; Wu, J. Purification and characterization of antioxidant peptides from enzymatically hydrolyzed chicken egg white. Food Chem. 2015, 188, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Miranda, J.M.; Anton, X.; Redondo-Valbuena, C.; Roca-Saavedra, P.; Rodriguez, J.A.; Lamas, A.; Franco, C.M.; Cepeda, A. Egg and Egg-Derived Foods: Effects on Human Health and Use as Functional Foods. Nutrients 2015, 7, 706–729. [Google Scholar] [CrossRef] [PubMed]
- Kovacs-Nolan, J.; Phillips, M.; Mine, Y. Advances in the Value of Eggs and Egg Components for Human Health. J. Agric. Food Chem. 2005, 53, 8421–8431. [Google Scholar] [CrossRef] [PubMed]
- Abeyrathne, E.D.N.S.; Lee, H.Y.; Ahn, D.U. Egg white proteins and their potential use in food processing or as nutraceutical and pharmaceutical agents—A review. Poult. Sci. 2013, 92, 3292–3299. [Google Scholar] [CrossRef] [PubMed]
- Mann, K. Proteomic analysis of the chicken egg vitelline membrane. Proteomics 2008, 8, 2322–2332. [Google Scholar] [CrossRef]
- Martinez-Villaluenga, C.; Penas, E.; Frias, J. Bioactive peptides in fermented foods: Production and evidence for health effects. In Fermented Foods in Health and Disease Prevention; Frias, J., MartinezVillaluenga, C., Penas, E., Eds.; Academic Press Ltd-Elsevier Science Ltd: London, UK, 2017; pp. 23–27. [Google Scholar] [CrossRef]
- Udenigwe, C.C.; E Aluko, R. Chemometric Analysis of the Amino Acid Requirements of Antioxidant Food Protein Hydrolysates. Int. J. Mol. Sci. 2011, 12, 3148–3161. [Google Scholar] [CrossRef] [Green Version]
- Agyei, D.; Danquah, M.K. Industrial-scale manufacturing of pharmaceutical-grade bioactive peptides. Biotechnol. Adv. 2011, 29, 272–277. [Google Scholar] [CrossRef]
- Zambrowicz, A.; Eckert, E.; Pokora, M.; Bobak, L.; Szołtysik, M.; Trziszka, T.; Chrzanowska, J.; Dąbrowska, A. Antioxidant and antidiabetic activities of peptides isolated from a hydrolysate of an egg-yolk protein by-product prepared with a proteinase from Asian pumpkin (Cucurbita ficifolia). RSC Adv. 2015, 5, 10460–10467. [Google Scholar] [CrossRef]
- Kim, J.; Moon, S.H.; Ahn, D.U.; Paik, H.-D.; Park, E. Antioxidant effects of ovotransferrin and its hydrolysates. Poult. Sci. 2012, 91, 2747–2754. [Google Scholar] [CrossRef]
- Shen, S.; Chahal, B.; Majumder, K.; You, S.-J.; Wu, J. Identification of Novel Antioxidative Peptides Derived from a Thermolytic Hydrolysate of Ovotransferrin by LC-MS/MS. J. Agric. Food Chem. 2010, 58, 7664–7672. [Google Scholar] [CrossRef]
- Lin, S.; Jin, Y.; Liu, M.; Yang, Y.; Zhang, M.; Guo, Y.; Jones, G.; Liu, J.; Yin, Y. Research on the preparation of antioxidant peptides derived from egg white with assisting of high-intensity pulsed electric field. Food Chem. 2013, 139, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Bamdad, F.; Khey, K.; Sunwoo, H.H. Antioxidant and anti-inflammatory properties of chicken egg vitelline membrane hydrolysates. Poult. Sci. 2017, 96, 3510–3516. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Ojalvo, D.; Molina, E.; López-Fandiño, R. Regulation of Exacerbated Immune Responses in Human Peripheral Blood Cells by Hydrolysed Egg White Proteins. PLoS ONE 2016, 11, e0151813. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D. Methods for determination of antioxidant capacity: A review. Int. J. Pharm. Sci. Res. 2015, 6, 546–566. [Google Scholar] [CrossRef]
- Dontha, S. A review on antioxidant methods. Asian J. Pharm. Clin. Res. 2016, 9, 14–32. [Google Scholar]
- Sheng, Z.W.; Ma, W.H.; Gao, J.H.; Bi, Y.; Zhang, W.M.; Dou, H.T.; Jin, Z.Q. Antioxidant properties of banana flower of two cultivars in China using 2,2-diphenyl-1-picrylhydrazyl (DPPH,) reducing power, 2,2 ’-azinobis-(3-ethylbenzthiazoline-6-sulphonate (ABTS) and inhibition of lipid peroxidation assays. Afr. J. Biotechnol. 2011, 10, 4470–4477. [Google Scholar]
- Rodríguez-Bonilla, P.; Gandía-Herrero, F.; Matencio, A.; García-Carmona, F.; López-Nicolás, J.M. Comparative Study of the Antioxidant Capacity of Four Stilbenes Using ORAC, ABTS+, and FRAP Techniques. Food Anal. Methods 2017, 164, 191–3000. [Google Scholar] [CrossRef]
- Ilyasov, I.; Beloborodov, V.; Selivanova, I.; Terekhov, R.P. ABTS/PP Decolorization Assay of Antioxidant Capacity Reaction Pathways. Int. J. Mol. Sci. 2020, 21, 1131. [Google Scholar] [CrossRef] [Green Version]
- Wu, J. Eggs as Functional Foods and Nutraceuticals for Human Health. Food Chem. Funct. Anal. 2019, 14, 1–406. [Google Scholar]
- Huang, X.; Tu, Z.; Xiao, H.; Wang, H.; Zhang, L.; Hu, Y.; Zhang, Q.; Niu, P. Characteristics and antioxidant activities of ovalbumin glycated with different saccharides under heat moisture treatment. Food Res. Int. 2012, 48, 866–872. [Google Scholar] [CrossRef]
- Tu, Z.; Hu, Y.-M.; Wang, H.; Huang, X.-Q.; Xia, S.-Q.; Niu, P.-P. Microwave heating enhances antioxidant and emulsifying activities of ovalbumin glycated with glucose in solid-state. J. Food Sci. Technol. 2013, 52, 1453–1461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, W.; Tu, Z.; Wang, H.; Zhang, L.; Song, Q. Glycation of ovalbumin after high-intensity ultrasound pretreatment: Effects on conformation, immunoglobulin (Ig)G/IgE binding ability and antioxidant activity. J. Sci. Food Agric. 2018, 98, 3767–3773. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Kato, A.; Kobayashi, K. Enhanced antioxidative effect of ovalbumin due to covalent binding of polysaccharides. J. Agric. Food Chem. 1992, 40, 2033–2037. [Google Scholar] [CrossRef]
- Awatsuhara, R.; Nagao, K.; Harada, K.; Maeda, T.; Nomura, T. Antioxidative activity of the buckwheat polyphenol rutin in combination with ovalbumin. Mol. Med. Rep. 2009, 3, 121–125. [Google Scholar] [CrossRef] [Green Version]
- Li, C.-P.; He, Z.; Wang, X.; Yang, L.; Yin, C.; Zhang, N.; Lin, J.; Zhao, H. Selenization of ovalbumin by dry-heating in the presence of selenite: Effect on protein structure and antioxidant activity. Food Chem. 2014, 148, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Acero-Lopez, A. Ovotransferrin: Structure, bioactivities, and preparation. Food Res. Int. 2012, 46, 480–487. [Google Scholar] [CrossRef]
- Ibrahim, H.R.; Hoq, I.; Aoki, T. Ovotransferrin possesses SOD-like superoxide anion scavenging activity that is promoted by copper and manganese binding. Int. J. Boil. Macromol. 2007, 41, 631–640. [Google Scholar] [CrossRef]
- You, J.; Luo, Y.; Wu, J. Conjugation of Ovotransferrin with Catechin Shows Improved Antioxidant Activity. J. Agric. Food Chem. 2014, 62, 2581–2587. [Google Scholar] [CrossRef]
- Moon, S.H.; Lee, J.H.; Ahn, D.U.; Paik, H.-D. In vitro antioxidant and mineral-chelating properties of natural and autocleaved ovotransferrin. J. Sci. Food Agric. 2014, 95, 2065–2070. [Google Scholar] [CrossRef]
- Liu, T.; Navarro, S.; Lopata, A.L. Current advances of murine models for food allergy. Mol. Immunol. 2016, 70, 104–117. [Google Scholar] [CrossRef]
- Sheng, L.; Su, P.; Han, K.; Chen, J.; Cao, A.; Zhang, Z.; Jin, Y.; Ma, M. Synthesis and structural characterization of lysozyme–pullulan conjugates obtained by the Maillard reaction. Food Hydrocoll. 2017, 71, 1–7. [Google Scholar] [CrossRef]
- Hamdani, A.M.; Wani, I.A.; Bhat, N.A.; Siddiqi, R.A. Effect of guar gum conjugation on functional, antioxidant and antimicrobial activity of egg white lysozyme. Food Chem. 2017, 240, 1201–1209. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, M.M.; Aminlari, M.; Moosavinasab, M. Preparation of and studies on the functional properties and bactericidal activity of the lysozyme–xanthan gum conjugate. LWT 2014, 57, 594–602. [Google Scholar] [CrossRef]
- Verdot, L.; Lalmanach, G.; Vercruysse, V.; Hartmann, S.; Lucius, R.; Hoebeke, J.; Gauthier, F.; Vray, B. Cystatins Up-regulate Nitric Oxide Release from Interferon-γ- activated Mouse Peritoneal Macrophages. J. Boil. Chem. 1996, 271, 28077–28081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lehtinen, M.K.; Tegelberg, S.; Schipper, H.; Su, H.; Zukor, H.; Manninen, O.; Kopra, O.; Joensuu, T.; Hakala, P.; Bonni, A.; et al. Cystatin B deficiency sensitizes neurons to oxidative stress in progressive myoclonus epilepsy, EPM1. J. Neurosci. 2009, 29, 5910–5915. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Han, J.; Decker, E.A. Antioxidant Activity of Phosvitin in Phosphatidylcholine Liposomes and Meat Model Systems. J. Food Sci. 2002, 67, 37–41. [Google Scholar] [CrossRef]
- Castellani, O.; Guérin-Dubiard, C.; David-Briand, E.; Anton, M. Influence of physicochemical conditions and technological treatments on the iron binding capacity of egg yolk phosvitin. Food Chem. 2004, 85, 569–577. [Google Scholar] [CrossRef]
- Nimalaratne, C.; Lopes-Lutz, D.; Schieber, A.; Wu, J. Free aromatic amino acids in egg yolk show antioxidant properties. Food Chem. 2011, 129, 155–161. [Google Scholar] [CrossRef]
- Lee, J.; Paik, H.-D. Anticancer and immunomodulatory activity of egg proteins and peptides: A review. Poult. Sci. 2019, 98, 6505–6516. [Google Scholar] [CrossRef]
- Da Silva, A.C.; Queiroz, A.E.S.D.F.; Oliveira, J.T.C.; Medeiros, E.V.; De Souza-Motta, C.M.; Moreira, K.A. Antioxidant Activities of Chicken Egg White Hydrolysates Obtained by New Purified Protease of Aspergillus avenaceus URM 6706. Braz. Arch. Boil. Technol. 2019, 62, 14. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, X.W. Proteomics in Food Biotechnology. In Omics Technologies: Tools for Food Science; Benkeblia, N., Ed.; Crc Press-Taylor & Francis Group: Boca Raton, FL, USA, 2012; pp. 99–118. [Google Scholar]
- Yuan, J.; Zheng, Y.; Wu, Y.; Chen, H.; Tong, P.; Gao, J. Double enzyme hydrolysis for producing antioxidant peptide from egg white: Optimization, evaluation, and potential allergenicity. J. Food Biochem. 2019, 44, 12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wang, H.; Wang, Y.; Yu, Y.; Liu, J.; Liu, B.; Zhang, T. Identification of antioxidant peptides derived from egg-white protein and its protective effects on H 2 O 2 -induced cell damage. Int. J. Food Sci. Technol. 2019, 54, 2219–2227. [Google Scholar] [CrossRef]
- Neto, Y.A.A.H.; Rosa, J.C.; Cabral, H. Peptides with antioxidant properties identified from casein, whey, and egg albumin hydrolysates generated by two novel fungal proteases. Prep. Biochem. Biotechnol. 2019, 49, 639–648. [Google Scholar] [CrossRef] [PubMed]
- Jing, H.; Yap, M.; Wong, P.Y.Y.; Kitts, D.D. Comparison of Physicochemical and Antioxidant Properties of Egg-White Proteins and Fructose and Inulin Maillard Reaction Products. Food Bioprocess Technol. 2009, 4, 1489–1496. [Google Scholar] [CrossRef]
- Van Der Plancken, I.; Van Loey, A.; Hendrickx, M. Combined effect of high pressure and temperature on selected properties of egg white proteins. Innov. Food Sci. Emerg. Technol. 2005, 6, 11–20. [Google Scholar] [CrossRef]
- Karadag, A.; Özçelik, B.; Saner, S. Review of Methods to Determine Antioxidant Capacities. Food Anal. Methods 2009, 2, 41–60. [Google Scholar] [CrossRef]
- Davalos, A.; Miguel, M.; Bartolomé, B.; López-Fandiño, R. Antioxidant Activity of Peptides Derived from Egg White Proteins by Enzymatic Hydrolysis. J. Food Prot. 2004, 67, 1939–1944. [Google Scholar] [CrossRef]
- Ou, B.; Huang, D.; Hampsch-Woodill, M.; Flanagan, J.A.; Deemer, E.K. Analysis of Antioxidant Activities of Common Vegetables Employing Oxygen Radical Absorbance Capacity (ORAC) and Ferric Reducing Antioxidant Power (FRAP) Assays: A Comparative Study. J. Agric. Food Chem. 2002, 50, 3122–3128. [Google Scholar] [CrossRef]
- Yu, Z.; Liu, B.; Zhao, W.; Yin, Y.; Liu, J.; Chen, F. Primary and secondary structure of novel ACE-inhibitory peptides from egg white protein. Food Chem. 2012, 133, 315–322. [Google Scholar] [CrossRef]
- Huang, W.-Y.; Majumder, K.; Wu, J. Oxygen radical absorbance capacity of peptides from egg white protein ovotransferrin and their interaction with phytochemicals. Food Chem. 2010, 123, 635–641. [Google Scholar] [CrossRef]
- Memarpoor-Yazdi, M.; Asoodeh, A.; Chamani, J. A novel antioxidant and antimicrobial peptide from hen egg white lysozyme hydrolysates. J. Funct. Foods 2012, 4, 278–286. [Google Scholar] [CrossRef]
- Rao, S.; Sun, J.; Liu, Y.; Zeng, H.; Su, Y.; Yang, Y. ACE inhibitory peptides and antioxidant peptides derived from in vitro digestion hydrolysate of hen egg white lysozyme. Food Chem. 2012, 135, 1245–1252. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, W.; Gómez-Ruiz, J.A.; Miralles, B.; Ramos, M.; Barrio, D.; Recio, I. Identification of antioxidant peptides of hen egg-white lysozyme and evaluation of inhibition of lipid peroxidation and cytotoxicity in the Zebrafish model. Eur. Food Res. Technol. 2016, 242, 1777–1785. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Chen, Z.; He, J.; Zhang, Y.; Zhang, T.; Jiang, Y. Anti-oxidative and anti-apoptosis effects of egg white peptide, Trp-Asn-Trp-Ala-Asp, against H2O2-induced oxidative stress in human embryonic kidney 293 cells. Food Funct. 2014, 5, 3179–3188. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Jin, Y.; Lin, S.; Jones, G.S.; Chen, F. Purification and identification of novel antioxidant peptides from egg white protein and their antioxidant activities. Food Chem. 2015, 175, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chi, Y.-J.; Zhao, M.-Y.; Lv, L. Purification and identification of antioxidant peptides from egg white protein hydrolysate. Amino Acids 2011, 43, 457–466. [Google Scholar] [CrossRef]
- Stadtman, E.R.; Moskovitz, J.; Berlett, B.S.; Levine, R.L. Cyclic oxidation and reduction of protein methionine residues is an important antioxidant mechanism. Mol. Cell. Biochem. 2002, 234, 3–9. [Google Scholar] [CrossRef]
- Baradaran, A.; Nasri, H.; Rafieian-Kopaei, M. Oxidative stress and hypertension: Possibility of hypertension therapy with antioxidants. J. Res. Med. Sci. 2014, 19, 358–367. [Google Scholar]
- Manso, M.A.; Miguel, M.; Even, J.; Hernández, R.; Aleixandre, A.; López-Fandiño, R. Effect of the long-term intake of an egg white hydrolysate on the oxidative status and blood lipid profile of spontaneously hypertensive rats. Food Chem. 2008, 109, 361–367. [Google Scholar] [CrossRef]
- Garcés-Rimón, M.; Gonzalez, C.; Uranga, J.; López-Miranda, V.; López-Fandiño, R.; Miguel, M. Pepsin Egg White Hydrolysate Ameliorates Obesity-Related Oxidative Stress, Inflammation and Steatosis in Zucker Fatty Rats. PLoS ONE 2016, 11, e0151193. [Google Scholar] [CrossRef] [Green Version]
- Sun, S.; Niu, H.; Yang, T.; Lin, Q.; Luo, F. Antioxidant and anti-fatigue activities of egg white peptides prepared by pepsin digestion. J. Sci. Food Agric. 2014, 94, 3195–3200. [Google Scholar] [CrossRef] [PubMed]
- Garcés-Rimón, M.; López-Expósito, I.; López-Fandiño, R.; Miguel, M. Egg white hydrolysates with in vitro biological multiactivities to control complications associated with the metabolic syndrome. Eur. Food Res. Technol. 2015, 242, 61–69. [Google Scholar] [CrossRef] [Green Version]
- Xiang, J.Z.; Hong, Y.W.; Cai, Y.Y.; Qing, S.M.; Ji, Z. Technology optimization, antioxidant activities and characteristics of peptide from egg white. J. Food Sci. Biotechnol. 2013, 32, 844–853. [Google Scholar]
- Lin, S.Y.; Guo, Y.; Liu, J.B.; You, Q.; Yin, Y.G.; Cheng, S. Optimized enzymatic hydrolysis and pulsed electric field treatment for production of antioxidant peptides from egg white protein. Afr. J. Biotechnol. 2011, 10, 11648–11657. [Google Scholar]
- Cho, D.-Y.; Jo, K.; Cho, S.Y.; Kim, J.M.; Lim, K.; Suh, H.J.; Oh, S. Antioxidant Effect and Functional Properties of Hydrolysates Derived from Egg-White Protein. Food Sci. Anim. Resour. 2014, 34, 362–371. [Google Scholar] [CrossRef]
- Noh, D.O.; Suh, H.J. Preparation of Egg White Liquid Hydrolysate (ELH) and Its Radical-Scavenging Activity. Prev. Nutr. Food Sci. 2015, 20, 183–189. [Google Scholar] [CrossRef] [Green Version]
- Tanasković, S.J.; Luković, N.; Grbavčić, S.; Stefanović, A.; Jovanovic, J.; Bugarski, B.; Jugovic, Z.K. Production of egg white protein hydrolysates with improved antioxidant capacity in a continuous enzymatic membrane reactor: Optimization of operating parameters by statistical design. J. Food Sci. Technol. 2017, 55, 128–137. [Google Scholar] [CrossRef]
- Hernández-Ledesma, B.; Miralles, B.; Amigo, L.; Ramos, M.; Recio, I. Identification of antioxidant and ACE-inhibitory peptides in fermented milk. J. Sci. Food Agric. 2005, 85, 1041–1048. [Google Scholar] [CrossRef]
- Singh, A.; Ramaswamy, H.S. Effect of high-pressure treatment on trypsin hydrolysis and antioxidant activity of egg white proteins. Int. J. Food Sci. Technol. 2013, 49, 269–279. [Google Scholar] [CrossRef]
- Stefanović, A.B.; Jovanovic, J.; Grbavčić, S.Ž.; Šekuljica, N.Ž.; Manojlović, V.B.; Bugarski, B.M.; Knežević-Jugović, Z.D. Impact of ultrasound on egg white proteins as a pretreatment for functional hydrolysates production. Eur. Food Res. Technol. 2014, 239, 979–993. [Google Scholar] [CrossRef]
- Remanan, M.K.; Wu, J. Antioxidant activity in cooked and simulated digested eggs. Food Funct. 2014, 5, 1464–1474. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liao, W.; Nimalaratne, C.; Chakrabarti, S.; Wu, J. Purification and characterization of antioxidant peptides from cooked eggs using a dynamic in vitro gastrointestinal model in vascular smooth muscle A7r5 cells. NPJ Sci. Food 2018, 2, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chakrabarti, S.; Guha, S.; Majumder, K. Food-Derived Bioactive Peptides in Human Health: Challenges and Opportunities. Nutrients 2018, 10, 1738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jahandideh, F.; Majumder, K.; Chakrabarti, S.; Morton, J.S.; Panahi, S.; Kaufman, S.; Davidge, S.T.; Wu, J. Beneficial Effects of Simulated Gastro-Intestinal Digests of Fried Egg and Its Fractions on Blood Pressure, Plasma Lipids and Oxidative Stress in Spontaneously Hypertensive Rats. PLoS ONE 2014, 9, e115006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakanaka, S.; Tachibana, Y.; Ishihara, N.; Juneja, L.R. Antioxidant activity of egg-yolk protein hydrolysates in a linoleic acid oxidation system. Food Chem. 2004, 86, 99–103. [Google Scholar] [CrossRef]
- Zambrowicz, A.; Pokora, M.; Eckert, E.; Szołtysik, M.; Dąbrowska, A.; Chrzanowska, J.; Trziszka, T. Antioxidant and antimicrobial activity of lecithin free egg yolk protein preparation hydrolysates obtained with digestive enzymes. Funct. Foods Health Dis. 2012, 2, 487. [Google Scholar] [CrossRef]
- Pokora, M.; Eckert, E.; Zambrowicz, A.; Bobak, L.; Szołtysik, M.; Dąbrowska, A.; Chrzanowska, J.; Polanowski, A.; Trziszka, T. Biological and functional properties of proteolytic enzyme-modified egg protein by-products. Food Sci. Nutr. 2013, 1, 184–195. [Google Scholar] [CrossRef]
- Ishikawa, S.-I.; Yano, Y.; Arihara, K.; Itoh, M. Egg Yolk Phosvitin Inhibits Hydroxyl Radical Formation from the Fenton Reaction. Biosci. Biotechnol. Biochem. 2004, 68, 1324–1331. [Google Scholar] [CrossRef]
- Yousr, M.N.; Howell, N.K. Antioxidant and ACE Inhibitory Bioactive Peptides Purified from Egg Yolk Proteins. Int. J. Mol. Sci. 2015, 16, 29161–29178. [Google Scholar] [CrossRef] [Green Version]
- Zambrowicz, A.; Pokora, M.; Setner, B.; Dąbrowska, A.; Szołtysik, M.; Babij, K.; Szewczuk, Z.; Trziszka, T.; Lubec, G.; Chrzanowska, J. Multifunctional peptides derived from an egg yolk protein hydrolysate: Isolation and characterization. Amino Acids 2014, 47, 369–380. [Google Scholar] [CrossRef] [Green Version]
- Yoo, H.; Bamdad, F.; Gujral, N.; Suh, J.-W.; Sunwoo, H. High Hydrostatic Pressure-Assisted Enzymatic Treatment Improves Antioxidant and Anti-inflammatory Properties of Phosvitin. Curr. Pharm. Biotechnol. 2017, 18, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Mine, Y. Preparation of novel functional oligophosphopeptides from hen egg yolk phosvitin. J. Agric. Food Chem. 2000, 48, 990–994. [Google Scholar] [CrossRef] [PubMed]
- Feng, F.; Mine, Y. Phosvitin phosphopeptides increase iron uptake in a Caco-2 cell monolayer model. Int. J. Food Sci. Technol. 2006, 41, 455–458. [Google Scholar] [CrossRef]
- Katayama, S.; Xu, X.; Fan, M.Z.; Mine, Y. Antioxidative Stress Activity of Oligophosphopeptides Derived from Hen Egg Yolk Phosvitin in Caco-2 Cells. J. Agric. Food Chem. 2006, 54, 773–778. [Google Scholar] [CrossRef]
- Xu, X.; Katayama, S.; Mine, Y. Antioxidant activity of tryptic digests of hen egg yolk phosvitin. J. Sci. Food Agric. 2007, 87, 2604–2608. [Google Scholar] [CrossRef]
- Young, D.; Nau, F.; Pasco, M.; Mine, Y. Identification of Hen Egg Yolk-Derived Phosvitin Phosphopeptides and Their Effects on Gene Expression Profiling against Oxidative Stress-Induced Caco-2 Cells. J. Agric. Food Chem. 2011, 59, 9207–9218. [Google Scholar] [CrossRef]
- Young, D.; Fan, M.Z.; Mine, Y. Egg Yolk Peptides Up-regulate Glutathione Synthesis and Antioxidant Enzyme Activities in a Porcine Model of Intestinal Oxidative Stress. J. Agric. Food Chem. 2010, 58, 7624–7633. [Google Scholar] [CrossRef]
- Park, P.-J.; Jung, W.-K.; Nam, K.-S.; Shahidi, F.; Kim, S.-K. Purification and characterization of antioxidative peptides from protein hydrolysate of lecithin-free egg yolk. J. Am. Oil Chem. Soc. 2001, 78, 651–656. [Google Scholar] [CrossRef]
- Zambrowicz, A.; Eckert, E.; Pokora, M.; Dabrowska, A.; Szoltysik, M.; Bobak, L.; Trziszka, T.; Chrzanowska, J. Biological activity of egg-yolk protein by-product hydrolysates obtained with the use of non-commercial plant protease. Ital. J. Food Sci. 2015, 27, 450–458. [Google Scholar]
- Eckert, E.; Zambrowicz, A.; Bobak, L.; Zabłocka, A.; Chrzanowska, J.; Trziszka, T. Production and Identification of Biologically Active Peptides Derived from By-product of Hen Egg-Yolk Phospholipid Extraction. Int. J. Pept. Res. Ther. 2018, 25, 669–680. [Google Scholar] [CrossRef] [Green Version]
- Sakanaka, S.; Tachibana, Y. Active oxygen scavenging activity of egg-yolk protein hydrolysates and their effects on lipid oxidation in beef and tuna homogenates. Food Chem. 2006, 95, 243–249. [Google Scholar] [CrossRef]
- Eckert, E.; Zambrowicz, A.; Pokora, M.; Dabrowska, A.; Szoltysik, M.; Chrzanowska, J.; Trziszka, T. application of microbial proteases to obtain egg yolk protein hydrolysates with antioxidant and antimicrobial activity. ZYWN Nauk Technol. Jakosc 2013, 20, 105–118. [Google Scholar] [CrossRef]
- Jung, S.; Jo, C.; Kang, M.-G.; Ahn, D.U.; Nam, K.-C. Elucidation of Antioxidant Activity of Phosvitin Extracted from Egg Yolk using Ground Meat. Food Sci. Anim. Resour. 2012, 32, 162–167. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Kovacs-Nolan, J.; Jiang, B.; Tsao, R.; Mine, Y. Antioxidant activity of enzymatic hydrolysates from eggshell membrane proteins and its protective capacity in human intestinal epithelial Caco-2 cells. J. Funct. Foods 2014, 10, 35–45. [Google Scholar] [CrossRef]
- Shi, Y.; Kovacs-Nolan, J.; Jiang, B.; Tsao, R.; Mine, Y. Peptides derived from eggshell membrane improve antioxidant enzyme activity and glutathione synthesis against oxidative damage in Caco-2 cells. J. Funct. Foods 2014, 11, 571–580. [Google Scholar] [CrossRef]
- Nimalaratne, C.; Wu, J. Hen Egg as an Antioxidant Food Commodity: A Review. Nutrients 2015, 7, 8274–8293. [Google Scholar] [CrossRef] [Green Version]
- Jahandideh, F.; Chakrabarti, S.; Davidge, S.T.; Wu, J. Antioxidant Peptides Identified from Ovotransferrin by the ORAC Method Did Not Show Anti-Inflammatory and Antioxidant Activities in Endothelial Cells. J. Agric. Food Chem. 2015, 64, 113–119. [Google Scholar] [CrossRef]
- You, S.-J.; Udenigwe, C.C.; E Aluko, R.; Wu, J. Multifunctional peptides from egg white lysozyme. Food Res. Int. 2010, 43, 848–855. [Google Scholar] [CrossRef]
- Xu, M.; Shangguan, X.; Wang, W.; Chen, J. Antioxidative activity of hen egg ovalbumin hydrolysates. Asia Pac. J. Clin. Nutr. 2007, 16, 178–182. [Google Scholar]
- Abeyrathne, E.D.N.S.; Lee, H.Y.; Jo, C.; Nam, K.C.; Ahn, D.U. Enzymatic hydrolysis of ovalbumin and the functional properties of the hydrolysates. Poult. Sci. 2014, 93, 2678–2686. [Google Scholar] [CrossRef]
- Abeyrathne, E.D.N.S.; Lee, H.Y.; Jo, C.; Suh, J.W.; Ahn, D.U. Enzymatic hydrolysis of ovomucoid and the functional properties of its hydrolysates. Poult. Sci. 2015, 94, 2280–2287. [Google Scholar] [CrossRef] [PubMed]
- Abeyrathne, E.; Lee, H.; Jo, C.; Suh, J.; Ahn, D.U. Enzymatic hydrolysis of ovomucin and the functional and structural characteristics of peptides in the hydrolysates. Food Chem. 2016, 192, 107–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, O.K.; Ha, G.E.; Han, G.-S.; Seol, K.-H.; Kim, H.W.; Jeong, S.-G.; Oh, M.H.; Park, B.-Y.; Ham, J.-S. Novel Antioxidant Peptide Derived from the Ultrafiltrate of Ovomucin Hydrolysate. J. Agric. Food Chem. 2013, 61, 7294–7300. [Google Scholar] [CrossRef] [PubMed]
| Peptide Sequence | Protein of Origin | Starting Material | Enzymes | Antioxidant Assay | References |
|---|---|---|---|---|---|
| RVPSLM | OVT | EW | Alcalase | DPPH | [51] |
| TPSPR | |||||
| DLQGK | |||||
| AGLAPY | |||||
| RVPSL | |||||
| IRW | OVT | OVT | Thermolysin Pepsin | ORAC | [52] |
| LKP | |||||
| WNIP | OVT | OVT | Thermolysin | ORAC | [11] |
| GWNI | |||||
| YAEERYPIL | OVA | EW | Pepsin | DPPH, LP | [49] |
| SALAM | |||||
| YQIGL | |||||
| YRGGLEPINF | |||||
| DHPFLF | OVA | EW | Alcalase | DPPH | [51] |
| HAEIN | |||||
| QIGLF | |||||
| AEERYP | OVA | EW | Protease P | ORAC | [1] |
| AEERYP | |||||
| DEDTQAMP | |||||
| NTDGSTDYGILQINSR | LZ | LZ | Papain Trypsin | DPPH | [53] |
| RGY | LZ | LZ | Pepsin Trypsin α-chymotrypsin | RP, LP | [54] |
| WIR | |||||
| VAW | |||||
| VAWRNRCKGTD | LZ | LZ | Pepsin | ORAC, TBARS in Zebrafish larvae | [55] |
| IRGCRL | |||||
| WIRGCRL | |||||
| AWIRGCRL | |||||
| WRNRCKGTD | |||||
| WNWAD | OM | EW | Pepsin | ORAC, HEK-293 cells | [56] |
| PVDENDEG | CY | EW | Protease P | ORAC | [1] |
| HANENIF | EW | EW | Alcalase | DPPH | [51] |
| VKELY | |||||
| TNGIIR | |||||
| HTKE | EW | EW | Alcalase | RP, DPPH, ABTS, ORAC | [57] |
| FFGFN | |||||
| MPDAH | |||||
| DHTKE | |||||
| VYLPR | EW | EW | Alcalase | ORAC, ABTS, H2O2-induced oxidative damage on HEK-293 | [44] |
| EVYLPR | |||||
| VEVYLPR | |||||
| VVEVYLPR | |||||
| YLGAK | EW | EW | Papain | [58] | |
| GGLEPINFN |
| Starting Material | Pre-Treatment | Pepsin Activity | Enzyme to Substrate Ratio | T (°C) | Time | Stop Conditions | References |
|---|---|---|---|---|---|---|---|
| Dissolved EW (100 mg/mL) | - | 10,000 U/mg | 1/100 (w/w) | 37 | 3 h | pH 7 | [49,61] |
| Pasteurized EW | - | 3000 U/mg | 2:100 (w/w) | 38 | 8 h | pH 7 | [62] |
| Freeze-dried EW | 90 °C, 10 min | 9000 U/g | 30 g/L | 37 | 5 h | 85 °C, 30 min | [63] |
| EW powder 5.56% | 90 °C, 10 min | NS | 1.58% (w/w) | 37 | 1 h | 90 °C, 10 min | [12] |
| Liquid EW in water (1/4) | 95 °C, 10 min | NS | 0.4% (w/v) | 37 | 1 h | Heat (NS) | [43] |
| Pasteurized EW | - | 3000 U/mg | 2:100 (w/w) | 38 | 48 h | 95 °C, 15 min | [64] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benedé, S.; Molina, E. Chicken Egg Proteins and Derived Peptides with Antioxidant Properties. Foods 2020, 9, 735. https://doi.org/10.3390/foods9060735
Benedé S, Molina E. Chicken Egg Proteins and Derived Peptides with Antioxidant Properties. Foods. 2020; 9(6):735. https://doi.org/10.3390/foods9060735
Chicago/Turabian StyleBenedé, Sara, and Elena Molina. 2020. "Chicken Egg Proteins and Derived Peptides with Antioxidant Properties" Foods 9, no. 6: 735. https://doi.org/10.3390/foods9060735