Enhancement of Immune Activities of Mixtures with Sasa quelpaertensis Nakai and Ficus erecta var. sieboldii
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Samples
2.2. Cell Culture
2.3. Cell Viability
2.4. Measurement of Nitric Oxide (NO) Production In Vitro
2.5. Enzyme-Linked Immunosorbent Assay (ELISA) on RAW 264.7 Cells
2.6. Animal Experiments
2.7. Measurement of NK Cell Cytotoxicity
2.8. ELISA on Spleen Cells
2.9. Measurement of NO Production In Vivo
2.10. Immune Cell Phenotype Analysis
2.11. Statistical Analysis
3. Results and Discussion
3.1. Effect of Mixtures on RAW264.7 Cells Viability
3.2. Effect of Mixtures on NO Production of RAW264.7 Cells
3.3. Effect of Mixtures on Cytokines Production of RAW264.7 Cells
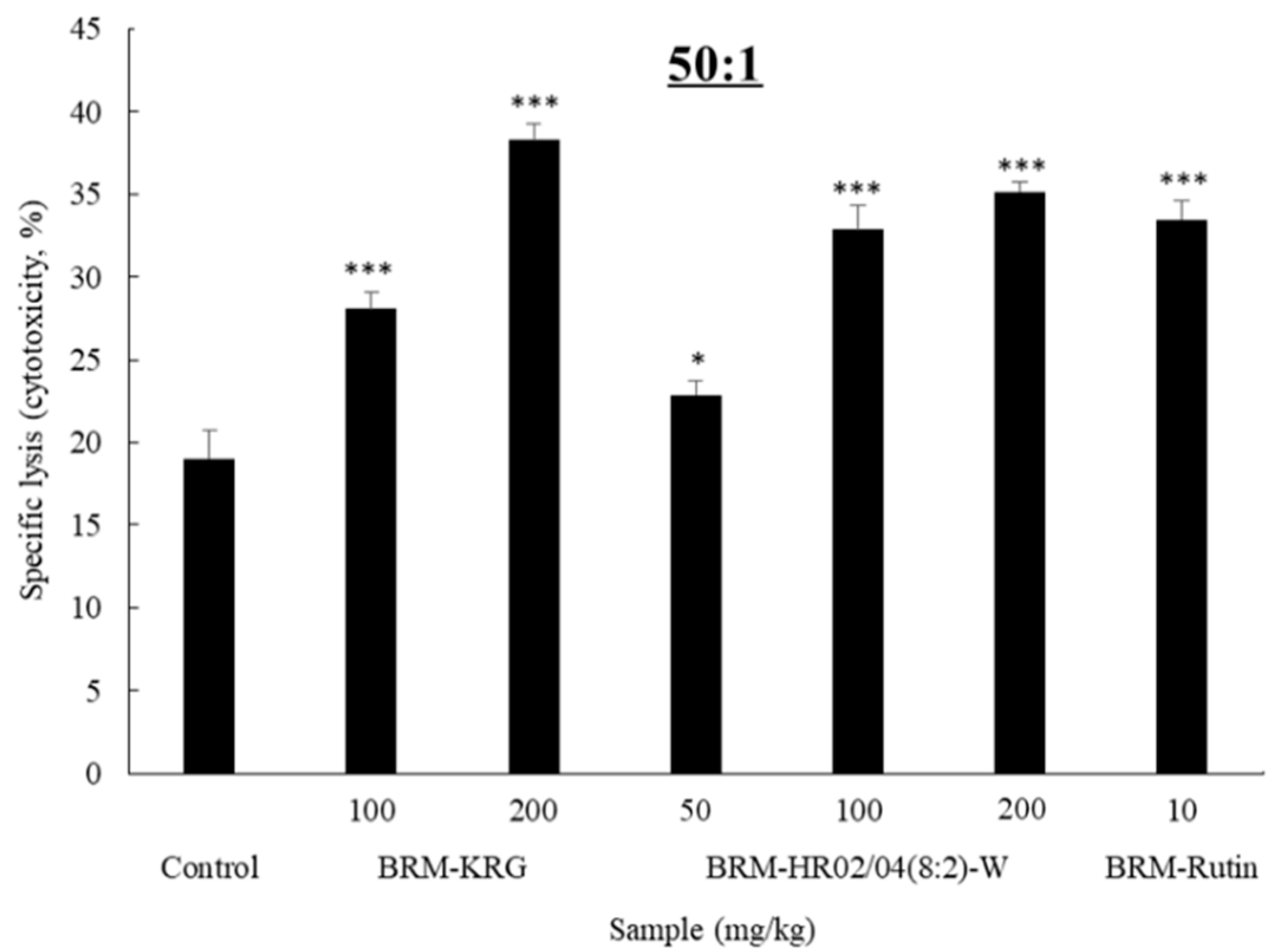
3.4. NK Cytotoxicity Activity
3.5. Effect of HR02/04(8:2)-W on the Expression of Cytokines in Splenocytes
3.6. Effect of HR02/04(8:2)-W on NO Production in Splenocytes
3.7. Effect of HR02/04(8:2)-W on Total Cell Number
3.8. Effect of HR02/04(8:2)-W on Albsolute Nunber of Immune Cell Subtypes
3.8.1. Immune Cell Activity and Number in the Spleen
3.8.2. Immune Cell Activity and Number in DLN
3.8.3. Immune Cell Activity and Number in PEC, Thymus, and Peyer’s Patches
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Azuma, I. Synthetic immunoadjuvants: Application to non-specific host stimulation and potentiation of vaccine immunogenicity. Vaccine 1992, 10, 1000–1006. [Google Scholar] [CrossRef]
- Sfondrini, L.; Balsari, A. Innate immunity in breast carcinoma. Endocr. Relat. Cancer 2003, 10, 301–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janis, K.; Thomas, J.K.; Richard, A.G.; Barbara, A.O. Immunology, 5th ed.; W.H. Freeman: New York, NY, USA, 2003; pp. 32–38, 288–297. [Google Scholar]
- Lee, H.J.; Seung, Y.C.; Lee, M.S. Immune regulation effects of Gentianae Radix extract in LPS-induced acute inflammatory mice. Korea J. Herbol. 2018, 33, 79–84. [Google Scholar]
- Lee, E.B.; Lee, S.H.; Park, Y.G.; Choi, J.H.; Lee, H.K.; Jang, H.H.; Hwang, G.A.; Choe, J.S.; Park, S.Y.; Choi, A.J.; et al. Platycodon grandiflorum extract ameliorates cyclophosphamide-induced immunosuppression in mice. J. East Asian Soc. Diet. Life 2019, 29, 303–309. [Google Scholar] [CrossRef]
- Hyun, S.H.; Kim, Y.S.; Lee, J.W.; Han, C.K.; Park, M.S.; So, S.H. Immunomodulatory effects of arginine-fructose-glucose enriched extracts of red ginseng. J. Korean Soc. Food Sci. Nutr. 2018, 17, 1–6. [Google Scholar] [CrossRef]
- Kim, E.Y.; Gwak, J.Y.; Chung, M.J. Immunomodulatory activity of Platycodon grandiflorum, Codonopsis lanceolata, and Adenophora triphylla extracts in macrophage cells. J. Korean Soc. Food Sci. Nutr. 2018, 47, 1069–1075. [Google Scholar] [CrossRef]
- Ahn, D.K. Illustrated Book of Korean Medicinal Herbs, 1st ed.; Kyohaksa Publishing Co.: Seoul, Korea, 1998; p. 855. [Google Scholar]
- Park, S.H.; Kim, J.E.; Yeum, H.S.; Lee, N.M. Isolation and evaluation of anti-oxidative constituents from the extracts of Ficus erecta var. sieboldii king leaves. J. Soc. Cosmet. Sci. Korea 2016, 42, 321–328. [Google Scholar]
- Ham, Y.M.; Yoon, W.J.; Sohn, E.H.; Park, D.W.; Jeon, H.L.; Jung, Y.H.; Lee, S.R.; Kang, S.C. The CH2Cl2 extract fraction from Ficus erecta var. sieboldii (Miq.) king suppresses lipopolysaccharide-mediated inflammatory responses in raw 264.7 cells. J. Food Nutr. Res. 2018, 6, 356–364. [Google Scholar] [CrossRef] [Green Version]
- Okabe, S.; Takeuchi, K.; Takagi, K.; Shibata, M. Stimulatory effect of the water extract of bamboo grass (Folin solution) on gastric acid secretion in pylorus-ligated rats. J. Pharmacol. 1975, 25, 608–609. [Google Scholar] [CrossRef] [Green Version]
- Bae, K. The Medicinal plants of Korea, 1st ed.; KyoHak Publishing Co.: Seoul, Korea, 2000; pp. 565–567. [Google Scholar]
- Park, Y.O.; Lim, H.S. Antioxidant activities of bamboo (Sasa Borealis) leaf extract according to extraction solvent. J. Korean Soc. Food Sci. Nutr. 2009, 38, 1640–1648. [Google Scholar] [CrossRef]
- Jang, M.R.; Lee, D.U.; Kim, G.H. Antibacterial activity of ethanol extract and fraction of Sasa borealis. Korean J. Food Cook. Sci. 2010, 26, 848–852. [Google Scholar]
- Song, W.Y.; Byeon, S.J.; Choi, J.H. Anti-oxidative and anti-inflammatory activities of Sasa borealis extracts. J. Agric. Life Sci. 2015, 49, 145–154. [Google Scholar] [CrossRef]
- Yang, J.H.; Lim, H.S.; Heo, Y.R. Sasa borealis leaves extract improves insulin resistance by modulating inflammatory cytokine secretion in high fat diet-induced obese C57/BL6J mice. Nutr. Res. Pract. 2010, 4, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.O. Sasa quelpartensis Nakai Extracts Anti-Obesity propeRties in Korean Obese Adults. Ph.D. Thesis, Ewah Womans University, Seoul, Korea, 2015. [Google Scholar]
- Lee, J.Y.; Song, H.; Ko, H.C.; Jang, M.G.; Kim, S.J. Anti-oxidant and anti-inflammatory potentials of Sasa quelpaertensis leaf residue extracts. J. Life Sci. 2018, 28, 738–744. [Google Scholar]
- Kim, J.H.; Kim, K.M. Antiproliferative and apoptotic effects of Sasa quelpaertensis Nakai in human cancer cells. J. Life Sci. 2014, 24, 903–909. [Google Scholar] [CrossRef]
- Jacobs, D.R.; Gross, M.D.; Tapsell, L.C. Food synergy: An operational concept for understanding nutrition. Am. J. Clin. Nutr. 2009, 89, 1543S–1548S. [Google Scholar] [CrossRef]
- Gertsch, J. Botanical drugs, synergy, and network pharmacology: Forth and back to intelligent mixtures. Planta Med. 2011, 77, 1086–1098. [Google Scholar] [CrossRef] [Green Version]
- Pepe, G.; Salviati, E.; Rapa, S.F.; Ostacolo, C.; Cascioferro, S.; Manfra, M.; Campiglia, P. Citrus sinensis and Vitis vinifera Protect Cardiomyocytes from Doxorubicin-Induced Oxidative Stress: Evaluation of Onconutraceutical Potential of Vegetable Smoothies. Antioxidants 2020, 9, 378. [Google Scholar] [CrossRef]
- Bahmani, M.; Taherikalani, M.; Khaksarian, M.; Rafieian-Kopaei, M.; Ashrafi, B.; Nazer, M.; Rashidipour, M. The synergistic effect of hydroalcoholic extracts of Origanum vulgare, Hypericum perforatum and their active components carvacrol and hypericin against Staphylococcus aureus. Future Sci. OA 2019, 5, FSO371. [Google Scholar] [CrossRef] [Green Version]
- Mensah, J.K.; Ibrahim, A.; Jibira, Y. Co-extract mixture from Strophanthus hispidus (roots) and Aframomum meleguta (seeds) show phytochemical synergy in its anti-inflammatory activity. J. Pharm. Pharm. Sci. 2019, 3, 89–100. [Google Scholar]
- Won, J.K.; Yang, H.W.; Shin, S.Y.; Lee, J.H.; Heo, W.D.; Cho, K.H. The crossregulation between ERK and PI3K signaling pathways determines the tumoricidal efficacy of MEK inhibitor. J. Mol. Cell Biol. 2012, 4, 156–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Cheng, Y.X.; Liu, A.L.; Wang, H.D.; Wang, Y.L.; Du, G.H. Antioxidant, anti-inflammatory and anti-influenza properties of components from Chaenomeles speciosa. Molecules 2010, 15, 8507–8517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hehlgans, T.; Pfeffer, K. The intriguing biology of the tumour necrosis factor/tumour necrosis factor receptor superfamily: Players, rules and the games. Immunology 2005, 115, 1–20. [Google Scholar] [CrossRef]
- Monmai, C.; You, S.; Park, W.J. Immune-enhancing effects of anionic macromolecules extracted from Codium fragile on cyclophosphamide-treated mice. PLoS ONE 2019, 14, e0211570. [Google Scholar] [CrossRef] [PubMed]
- Ji, K.Y.; Jang, J.H.; Lee, E.H.; Kim, S.M.; Song, H.W.; Yang, W.K.; Kim, H.Y.; Kim, K.H.; Lee, Y.S.; Kim, D.S.; et al. Canavalia gladiata and Arctium lappa extracts ameliorate dextran sulphate sodium-induced inflammatory bowel disease by enhancing immune responses. J. Funct. Foods 2018, 45, 24–33. [Google Scholar] [CrossRef]
- Wang, Y.; Vodovotz, Y.; Kim, P.K.; Zamora, R.; Billiar, T.R. Mechanisms of hepatoprotection by nitric oxide. Ann. N. Y. Acad. Sci. 2002, 962, 415–422. [Google Scholar] [CrossRef]
- Dinarello, C.A. Interleukin-1. Rev. Infect. Dis. 1984, 6, 51–95. [Google Scholar] [CrossRef]
- Van Snick, J. Interleukin-6: An overview. Annu. Rev. Immunol. 1990, 8, 253–278. [Google Scholar] [CrossRef]
- Feuerstein, G.Z.; Liu, T.; Barone, F.C. Cytokines, inflammation, and brain injury: Role of tumor necrosis factor-alpha. Cerebrovasc. Brain Metab. Rev. 1994, 6, 341–360. [Google Scholar]
- Farag, S.S.; Fehniger, T.A.; Ruggeri, L.; Velardi, A.; Caligiuri, M.A. Natural killer cell receptors: New biology and insights into the graft-versus-leukemia effect. Blood 2002, 100, 1935–1947. [Google Scholar] [CrossRef]
- Strowig, T.; Brilot, F.; Münz, C. Noncytotoxic functions of NK cells: Direct pathogen restriction and assistance to adaptive immunity. J. Immunol. 2008, 180, 7785–7791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kishimoto, T.; Taga, T.; Akira, S. Cytokine signal transduction. Cell 1994, 76, 253–262. [Google Scholar] [CrossRef]
- Smith, K.A. Interleukin 2. Annu. Rev. Immunol. 1984, 2, 319–333. [Google Scholar] [CrossRef] [PubMed]
- Paul, W.E. Interleukin-4: A prototypic immunoregulatory lymphokine. Blood 1991, 77, 1859–1870. [Google Scholar] [CrossRef] [Green Version]
- Moore, K.W.; O’garra, A.; Malefyt, R.D.; Vieira, P.; Mosmann, T.R. Interleukin-10. Annu. Rev. Immunol. 1993, 11, 165–190. [Google Scholar] [CrossRef]
- MacNeil, I.A.; Suda, T.; Moore, K.W.; Mosmann, T.R.; Zlotnik, A. IL-10, a novel growth cofactor for mature and immature T cells. J. Immunol. 1990, 145, 4167–4173. [Google Scholar]
- Scott, P. IL-12: Initiation cytokine for cell-mediated immunity. Science 1993, 260, 496–498. [Google Scholar] [CrossRef]
- Catalona, W.J.; Ratliff, T.L.; McCool, R.E. γ Interferon induced by S. aureus protein A augments natural killing and ADCC. Nature 1981, 291, 77–79. [Google Scholar] [CrossRef]
- Arenzana-Seisdedos, F.; Virelizier, J.L.; Fiers, W. Interferons as macrophage-activating factors. III. Preferential effects of interferon-gamma on the interleukin 1 secretory potential of fresh or aged human monocytes. J. Immunol. 1985, 134, 2444–2448. [Google Scholar]
- Kang, B.K.; Kim, K.B.W.R.; Ahn, N.K.; Choi, Y.U.; Kim, M.J.; Bark, S.W.; Park, W.M.; Kim, B.R.; Park, J.H.; Bae, N.Y.; et al. Immuno-stimulating activities of skipjack tuna Katsuwonus pelamis cooking juice concentrates on mouse macrophages and spleen cells. Korean J. Fish Aquat. Sci. 2014, 47, 776–784. [Google Scholar]
- Hong, R. The thymus. Finally getting some respect. Chest Surg. Clin. N. Am. 2001, 11, 295–310. [Google Scholar]
- Mowat, A.M. Anatomical basis of tolerance and immunity to intestinal antigens. Nat. Rev. Immunol. 2003, 3, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Kuka, M.; Iannacone, M. The role of lymph node sinus macrophages in host defense. Ann. N. Y. Acad. Sci. 2014, 1319, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Levings, M.K.; Bacchetta, R.; Schulz, U.; Roncarolo, M.G. The role of IL-10 and TGF-β in the differentiation and effector function of T regulatory cells. Int. Arch. Allergy Immunol. 2002, 129, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Hida, M. Histamine releasing mechanism from rat peritoneal mast cells by pancreatic kallikrein. Arerugi 1982, 31, 1093. [Google Scholar]
- Mehlhop, P.D.; Van De Rijn, M.; Goldberg, A.B.; Brewer, J.P.; Kurup, V.P.; Martin, T.R.; Oettgen, H.C. Allergen-induced bronchial hyperreactivity and eosinophilic inflammation occur in the absence of IgE in a mouse model of asthma. Proc. Natl. Acad. Sci. USA 1997, 94, 1344–1349. [Google Scholar] [CrossRef] [Green Version]
- Gabrilovich, D.I.; Nagaraj, S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 2009, 9, 162–174. [Google Scholar] [CrossRef]
- Peng, H.; Sun, R.; Tang, L.; Wei, H.; Tian, Z. CD62L is critical for maturation and accumulation of murine hepatic NK cells in response to viral infection. J. Immunol. 2013, 190, 4255–4262. [Google Scholar] [CrossRef] [Green Version]
- Aktas, E.; Kucuksezer, U.C.; Bilgic, S.; Erten, G.; Deniz, G. Relationship between CD107a expression and cytotoxic activity. Cell. Immunol. 2009, 254, 149–154. [Google Scholar] [CrossRef]
- Xia, Y.H.; Wang, Z.M.; Chen, R.X.; Ye, S.L.; Sun, R.X.; Xue, Q.; Huang, Y. T-cell apoptosis induced by intratumoral activated hepatic stellate cells is associated with lung metastasis in hepatocellular carcinoma. Oncol. Rep. 2013, 30, 1175–1184. [Google Scholar] [CrossRef]
- Cibrián, D.; Sánchez-Madrid, F. CD69: From activation marker to metabolic gatekeeper. Eur. J. Immunol. 2017, 47, 946–953. [Google Scholar] [CrossRef] [PubMed]
- Cifarelli, V.; Libman, I.M.; DeLuca, A.; Becker, D.; Trucco, M.; Luppi, P. Increased expression of monocyte CD11b (Mac-1) in overweight recent-onset type 1 diabetic children. Rev. Diabet. Stud. 2007, 4, 113. [Google Scholar] [CrossRef] [PubMed]








| Name | Mixture Ratio FES (1):SQN (2) | Extraction Solvent | Extraction Yield (%) |
|---|---|---|---|
| HR1902-W | 10:0 | Water | 27.0 |
| HR1902-70E | 70% Ethanol | 22.1 | |
| HR02/04(2:8)-W | 2:8 | Water | 24.3 |
| HR02/04(2:8)-70E | 70% Ethanol | 17.3 | |
| HR02/04(5:5)-W | 5:5 | Water | 16.9 |
| HR02/04(5:5)-70E | 70% Ethanol | 21.8 | |
| HR02/04(8:2)-W | 8:2 | Water | 16.2 |
| HR02/04(8:2)-70E | 70% Ethanol | 19.3 | |
| HR1904-W | 0:10 | Water | 23.1 |
| HR1904-70E | 70% Ethanol | 16.0 |
| Spleen (×105 Cells) | Immune Cell Number in BALB/c Mice (Absolute No.) | ||||||
|---|---|---|---|---|---|---|---|
| BALB/c | BRM-KRG | BRM-HR02/04(8:2)-W | BRM-Rutin | ||||
| Normal | 100 mg/kg | 200 mg/kg | 50 mg/kg | 100 mg/kg | 200 mg/kg | 10 mg/kg | |
| CD3+ | 25.5 ± 1.40 | 40.7 ± 1.56 ** | 41.9 ± 3.66 ** | 45.1 ± 1.40 ** | 57.2 ± 7.70 ** | 55.5 ± 8.72 ** | 49.0 ± 2.78 *** |
| CD49b+ | 1.7 ± 0.04 | 2.5 ± 0.11 *** | 2.4 ± 0.12 ** | 2.0 ± 0.14 | 2.8 ± 0.28 ** | 2.7 ± 0.49 | 3.1 ± 0.09 *** |
| CD4+ | 9.8 ± 1.42 | 16.4 ± 0.66 *** | 17.1 ± 2.07 ** | 18.2 ± 1.65 ** | 19.4 ± 1.25 *** | 17.7 ± 2.63 ** | 17.7 ± 1.19 *** |
| CD8+ | 6.4 ± 0.30 | 11.2 ± 0.83 *** | 13.0 ± 1.07 *** | 11.0 ± 0.64 *** | 9.8 ± 0.45 *** | 9.3 ± 1.80 | 9.1 ± 0.06 *** |
| CD4+/CD25+ | 1.2 ± 0.24 | 2.1 ± 0.18 ** | 2.2 ± 0.40 * | 2.6 ± 0.18 *** | 2.2 ± 0.17 ** | 2.4 ± 0.67 | 2.0 ± 0.33 |
| CD8+/CD25+ | 0.4 ± 0.02 | 0.4 ± 0.01 ** | 0.8 ± 0.03 *** | 0.5 ± 0.11 | 0.4 ± 0.07 | 0.5 ± 0.13 | 0.5 ± 0.10 |
| CD23+/B220+ | 12.5 ± 1.23 | 20.7 ± 1.25 *** | 19.4 ± 2.13 ** | 18.8 ± 0.44 *** | 25.2 ± 1.44 *** | 25.9 ± 4.17 ** | 21.7 ± 2.73 ** |
| Gr-1+/CD11b+ | 1.3 ± 0.42 | 3.8 ± 0.53 ** | 4.1 ± 0.13 *** | 2.9 ± 0.05 ** | 3.5 ± 0.07 *** | 3.6 ± 0.71 ** | 5.1 ± 0.21 *** |
| DLN (×105 Cells) | Immune Cell Number in BALB/c Mice (Absolute No.) | ||||||
|---|---|---|---|---|---|---|---|
| BALB/c | BRM-KRG | BRM-HR02/04(8:2)-W | BRM-Rutin | ||||
| Normal | 100 mg/kg | 200 mg/kg | 50 mg/kg | 100 mg/kg | 200 mg/kg | 10 mg/kg | |
| CD3+ | 9.3 ± 2.07 | 55.8 ± 19.97 * | 67.2 ± 27.19 * | 76.8 ± 9.67 *** | 70.0 ± 7.08 *** | 93.7 ± 30.27 ** | 95.9 ± 2.35 *** |
| CD49b+ | 0.3 ± 0.00 | 1.0 ± 0.38 | 0.6 ± 0.15 | 0.8 ± 0.05 *** | 0.9 ± 0.03 *** | 1.0 ± 0.25 ** | 1.1 ± 0.29 ** |
| CD4+ | 7.3 ± 0.70 | 38.6 ± 13.64 * | 44.5 ± 18.34 * | 53.4 ± 6.19 *** | 45.7 ± 9.00 *** | 63.1 ± 20.29 ** | 63.8 ± 1.60 *** |
| CD8+ | 3.6 ± 0.61 | 16.7 ± 5.94 * | 21.0 ± 8.67 * | 21.3 ± 2.76 *** | 19.6 ± 3.89 *** | 26.0 ± 8.73 ** | 27.3 ± 0.27 *** |
| CD4+/CD25+ | 1.1 ± 0.22 | 6.5 ± 2.22 ** | 8.3 ± 3.71 | 8.6 ± 1.07 *** | 8.9 ± 1.54 *** | 11.7 ± 4.15 ** | 10.4 ± 0.30 *** |
| CD23+/B220+ | 0.7 ± 0.13 | 4.3 ± 1.30 ** | 12.1 ± 5.62 * | 6.3 ± 0.61 *** | 6.9 ± 0.96 *** | 7.8 ± 1.93 * | 7.3 ± 1.35 *** |
| PEC, Thymus, Peyer’s Patches | Immune Cell Number in BALB/c Mice (Absolute No.) | |||||||
|---|---|---|---|---|---|---|---|---|
| BALB/c | BRM-KRG | BRM-HR02/04(8:2)-W | BRM-Rutin | |||||
| Normal | 100 mg/kg | 200 mg/kg | 50 mg/kg | 100 mg/kg | 200 mg/kg | 10 mg/kg | ||
| CD3+/CD4+ | PEC (×105 cells) | 7.9 ± 0.20 | 28.9 ± 10.66 | 35.3 ± 0.63 *** | 12.0 ± 1.17 ** | 11.8 ± 2.68 | 29.7 ± 1.24 *** | 32.8 ± 6.94 ** |
| CD3+/CD8+ | 5.3 ± 0.10 | 10.5 ± 1.99 ** | 16.0 ± 0.41 *** | 5.9 ± 0.07 *** | 5.2 ± 0.80 | 12.6 ± 0.58 *** | 11.8 ± 1.73 ** | |
| CD4+/CD25+ | 3.1 ± 0.04 | 5.1 ± 0.98 * | 3.6 ± 0.86 | 2.4 ± 0.43 | 2.3 ± 0.22 ** | 4.2 ± 0.74 | 7.3 ± 1.12 ** | |
| CD8+/CD25+ | 0.5 ± 0.01 | 1.0 ± 0.12 *** | 2.1 ± 0.05 *** | 0.7 ± 0.16 | 0.8 ± 0.22 | 1.3 ± 0.08 *** | 1.3 ± 0.31 ** | |
| B220+/CD23+ | 7.3 ± 0.82 | 16.1 ± 1.68 *** | 33.2 ± 3.54 *** | 13.6 ± 1.78 ** | 18.2 ± 3.09 ** | 28.5 ± 0.42 *** | 27.2 ± 3.73 *** | |
| B220+/CD69+ | 7.9 ± 0.14 | 14.9 ± 1.69 *** | 25.2 ± 0.76 *** | 12.2 ± 0.49 *** | 12.3 ± 0.46 *** | 21.6 ± 0.57 *** | 41.6 ± 6.55 *** | |
| CD11b+/CD69+ | 7.9 ± 0.32 | 10.2 ± 2.76 | 20.3 ± 4.20 ** | 8.9 ± 0.82 | 6.9 ± 1.12 | 11.8 ± 0.04 *** | 21.5 ± 4.46 ** | |
| CD4+ | Thymus (×106 cells) | 9.8 ± 1.00 | 12.8 ± 0.38 ** | 16.1 ± 2.96 ** | 11.2 ± 3.00 | 9.3 ± 0.59 | 18.6 ± 2.40 ** | 12.6 ± 1.82 |
| CD3+ | Peyer’s patch (×105 cells) | 7.4 ± 1.28 | 10.0 ± 5.91 | 11.4 ± 3.74 | 10.4 ± 0.81 * | 11.7 ± 1.38 * | 37.3 ± 3.76 *** | 26.8 ± 5.02 ** |
| CD3+/CD4+ | 2.2 ± 0.67 | 2.8 ± 1.40 | 2.8 ± 0.94 | 3.0 ± 0.35 | 3.7 ± 0.62 | 7.1 ± 1.01 ** | 4.1 ± 0.64 * | |
| CD3+/CD8+ | 2.5 ± 0.32 | 2.8 ± 1.18 | 4.1 ± 1.05 | 3.8 ± 0.82 | 4.9 ± 1.96 | 9.8 ± 2.04 ** | 11.0 ± 3.25 ** | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, H.-Y.; Choi, S.-I.; Han, X.; Men, X.; Jang, G.-W.; Choi, Y.-E.; Kim, S.-H.; Kang, J.-C.; Cho, J.-H.; Lee, O.-H. Enhancement of Immune Activities of Mixtures with Sasa quelpaertensis Nakai and Ficus erecta var. sieboldii. Foods 2020, 9, 868. https://doi.org/10.3390/foods9070868
Kwon H-Y, Choi S-I, Han X, Men X, Jang G-W, Choi Y-E, Kim S-H, Kang J-C, Cho J-H, Lee O-H. Enhancement of Immune Activities of Mixtures with Sasa quelpaertensis Nakai and Ficus erecta var. sieboldii. Foods. 2020; 9(7):868. https://doi.org/10.3390/foods9070868
Chicago/Turabian StyleKwon, Hee-Yeon, Sun-Il Choi, Xionggao Han, Xiao Men, Gill-Woong Jang, Ye-Eun Choi, Seung-Hyung Kim, Jun-Chul Kang, Ju-Hyun Cho, and Ok-Hwan Lee. 2020. "Enhancement of Immune Activities of Mixtures with Sasa quelpaertensis Nakai and Ficus erecta var. sieboldii" Foods 9, no. 7: 868. https://doi.org/10.3390/foods9070868






