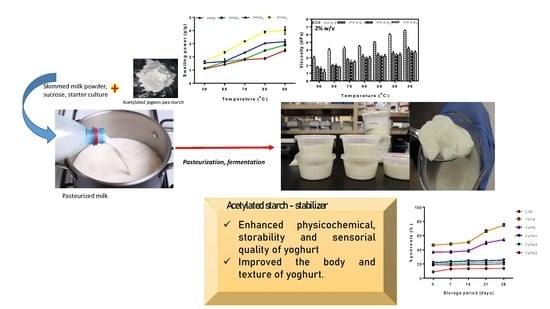
Technological Properties of Acetylated Pigeon Pea Starch and Its Stabilized Set-Type Yoghurt
Abstract
1. Introduction
2. Materials and Methods
2.1. Determination of Functional Properties of Starch
2.1.1. Determination of Solubility and Swelling Power
2.1.2. Determination of Paste Clarity
2.1.3. Determination of Pasting Properties of Acetylated Starches
2.2. Preparation of Set-Type Yoghurt
2.3. Chemical Attributes of Yoghurt
2.3.1. Acidity
2.3.2. Determination of Titratable Acidity (TA)
2.3.3. Determination of Total Solids (TS)
2.3.4. Determination of Syneresis
2.3.5. Determination of Water Holding Capacity
2.4. Sensory Evaluation of Yoghurt
2.5. Statistical Analysis
3. Results
3.1. Effect of Acetylation on Solubility and Swelling Power of Pigeon Pea Starch
3.2. Effect of Storage Period on Light Transmittance of Native and Acetylated Pigeon Pea Starches
3.3. Pasting Properties of Native and Acetylated Pigeon Pea Starch
3.4. Physicochemical Properties of Yoghurt Stabilized with Acetylated Pigeon Pea Starch
3.5. Effect of Acetylated Pigeon Pea Starch as Yoghurt Stabilizer during Refrigerated Storage
3.6. Sensory Attributes of Yoghurt Stabilized with Pigeon Pea Starch
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bierzuńska, P.; Cais-Sokolińska, D.; Yiğit, A. Storage stability of texture and sensory properties of yogurt with the addition of polymerized whey proteins. Foods 2019, 8, 548. [Google Scholar] [CrossRef] [PubMed]
- El Bouchikhi, S.; Pagès, P.; El Alaoui, Y.; Ibrahimi, A.; Bensouda, Y. Syneresis investigations of lacto-fermented sodium caseinate in a mixed model system. BMC Biotechnol. 2019, 19, 57. [Google Scholar] [CrossRef] [PubMed]
- Amatayakul, T.; Sherkat, F.; Shah, N.P. Physical characteristics of set yoghurt made with altered casein to whey protein ratios and EPS-producing starter cultures at 9 and 14% total solids. Food Hydrocoll. 2006, 20, 314–324. [Google Scholar] [CrossRef]
- El-Sayed, E.M.; El-Gawad, I.A.; Murad, H.A.; Salah, S.H. Utilization of laboratory-produced xanthan gum in the manufacture of yogurt and soy yogurt. Eur. Food Res. Technol. 2002, 215, 298–304. [Google Scholar]
- Temesgen, M. Effect of application of stabilizers on gelation and syneresis in yoghurt. Food Sci. Qual. Manag. 2015, 37, 90–102. [Google Scholar]
- Gyawali, R.; Ibrahim, S.A. Effects of hydrocolloids and processing conditions on acid whey production with reference to Greek yogurt. Trends Food Sci. Technol. 2016, 56, 61–76. [Google Scholar] [CrossRef]
- Macit, E.; Bakirci, I. Effect of different stabilizers on quality characteristics of the set-type yoghurt. Afr. J. Biotechnol. 2017, 16, 2142–2151. [Google Scholar]
- Cui, B.; Lu, Y.; Tan, C.; Wang, G.; Ki, G. Effect of cross-linked acetylated starch content on the structure and stability of set yoghurt. Food Hydrocoll. 2014, 35, 576–582. [Google Scholar] [CrossRef]
- Okoth, E.M.; Kinyanjui, P.K.; Kinyuru, J.N.; Juma, F.O. Effects of substituting skimmed milk powder with modified starch in yoghurt production. J. Agric. Sci. Technol. 2011, 13, 15–32. [Google Scholar]
- Schmidt, K.A.; Herald, T.J.; Khatib, K.A. Modified wheat starches used as stabilizers in set-type yogurt. J. Food Qual. 2001, 24, 421–434. [Google Scholar] [CrossRef]
- Alim, M.A.; Wadehra, A.; Singh, A.K. Effect of various plant starches on the quality characteristics of starch-based sweetened cow milk yoghurt. J. Bangladesh Agric. Univ. 2016, 14, 119–126. [Google Scholar] [CrossRef]
- Mwizerwa, H.; Abong, G.O.; Okoth, M.W.; Ongol, M.P.; Onyango, C.; Thavarajah, P. Effect of resistant cassava starch on quality parameters and sensory attributes of yoghurt. Curr. Res. Nutr. Food Sci. 2017, 5, 353–367. [Google Scholar] [CrossRef]
- Altemimi, A.B. Extraction and optimization of potato starch and its application as a stabilizer in yogurt manufacturing. Foods 2018, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Wani, I.A.; Sogi, D.S.; Hamdani, A.M.; Gani, A.; Bhat, N.A.; Shah, A. Isolation, composition and physicochemical properties of starch from legumes: A review. Starch 2016, 68, 834–845. [Google Scholar] [CrossRef]
- Talari, A.; Shakappa, D. Role of pigeon pea (Cajanus cajan L.) in human nutrition and health: A review. Asian J. Dairy Food Res. 2018, 37, 212–220. [Google Scholar] [CrossRef]
- Sharma, S.; Agarwal, N.; Verma, P. Pigeon pea (Cajanus cajan L.): A hidden treasure of regime nutrition. J. Funct. Environ. Bot. 2011, 1, 91–101. [Google Scholar] [CrossRef]
- Adenekan, M.K.; Fadimu, G.; Odunmbaku, L.A.; Oke, E.K. Effect of isolation technique on the characteristics of pigeon pea (Cajanus cajan) protein isolates. Food Sci. Nutr. 2018, 6, 146–152. [Google Scholar] [CrossRef]
- Olagunju, A.I.; Omoba, O.S.; Enujiugha, V.N.; Alashi, A.M.; Aluko, R.E. Pigeon pea enzymatic protein hydrolysates and ultrafiltered peptide fractions as potential sources of antioxidant peptides: An in vitro study. Food Sci. Technol. 2018, 97, 269–278. [Google Scholar]
- Olagunju, A.I.; Omoba, O.S.; Enujiugha, V.N.; Alashi, A.M.; Aluko, R.E. Antioxidant properties, ACE/renin inhibitory activities of pigeon pea hydrolysates and effects on systolic blood pressure of spontaneously hypertensive rats. Food Sci. Nutr. 2018, 6, 1879–1889. [Google Scholar] [CrossRef] [PubMed]
- Olagunju, A.I.; Omoba, O.S.; Enujiugha, V.N.; Wiens, R.A.; Gough, K.M.; Aluko, R.E. Influence of acetylation on physicochemical and morphological characteristics of pigeon pea starch. Food Hydrocoll. 2020, 100, 105424. [Google Scholar] [CrossRef]
- Gani, A.; Haq, S.S.; Masoodi, F.A.; Broadway, A.A.; Gani, A. Physico-chemical, morphological and pasting properties of starches extracted from water chestnuts (Trapa natans) from three lakes of Kashmir, India. Braz. Arch. Biol. Technol. 2010, 53, 731–740. [Google Scholar] [CrossRef]
- Craig, S.A.S.; Maningat, C.C.; Seib, P.A.; Hoseney, R.C. Starch paste clarity. Cereal Chem. 1989, 66, 173–182. [Google Scholar]
- AACC International. Approved Methods of the American Association of Cereal Chemists, 10th ed.; The Association: St. Paul, MN, USA, 2000. [Google Scholar]
- Guzman-Gonzalez, M.; Morais, F.; Amigo, L. Influence of skimmed milk concentrate replacement by dry dairy products in a low-fat set-type yoghurt model system. Use of caseinates, co-precipitate and blended dairy powders. J. Sci. Food Agric. 2000, 80, 433–438. [Google Scholar] [CrossRef]
- Guzmán-González, M.; Morais, F.; Ramos, M.; Amigo, L. Influence of skimmed milk concentrate replacement by dry dairy products in a low fat set-type yoghurt model system. I: Use of whey protein concentrates, milk protein concentrates and skimmed milk powder. J. Sci. Food Agric. 1999, 79, 1117–1122. [Google Scholar]
- Soukoulis, C.; Panagiotidis, P.; Koureli, R.; Tzia, C. Industrial yoghurt manufacture: Monitoring of fermentation process and improvement of final product quality. J. Dairy Sci. 2007, 90, 2641–2654. [Google Scholar] [CrossRef]
- Wani, I.A.; Sogi, D.S.; Gill, B.S. Physico-chemical properties of acetylated starches from Indian black gram (Phaseolus mungo L.) cultivars. J. Food Sci. Technol. 2015, 52, 4078–4089. [Google Scholar] [CrossRef]
- Phattanakulkaewmorie, N.; Paseephol, T.; Moongngarm, A. Chemical composition and physicochemical properties of malted sorghum flour and characteristics of gluten free bread. World Acad. Sci. Eng. Technol. 2011, 57, 454–460. [Google Scholar]
- Srichuwong, S.; Suharti, T.C.; Mishima, T.; Isono, M.; Hisamatsu, M. Starches from different botanical sources: Contribution of starch structure to swelling and pasting properties. Carbohydr. Polym. 2005, 62, 25–34. [Google Scholar] [CrossRef]
- Ayucitra, A. Preparation and characterisation of acetylated corn starches. Int. J. Chem. Eng. Appl. 2012, 3, 156–159. [Google Scholar] [CrossRef]
- Yadav, D.K.; Patki, P.E. Effect of acetyl esterification on physicochemical properties of chickpea (Cicer arietinum L.) starch. J. Food Sci. Technol. 2015, 52, 4176–4185. [Google Scholar] [CrossRef]
- Jacobson, M.R.; Obanni, M.; BeMiller, J.N. Retrogradation of starches from different botanical sources. Cereal Chem. 1997, 74, 571–578. [Google Scholar] [CrossRef]
- Zhang, Z.; Tian, X.; Wang, P.; Jiang, H.; Li, W. Compositional, morphological, and physicochemical properties of starches from red adzuki bean, chickpea, faba bean, and baiyue bean grown in China. Food Sci. Nutr. 2019, 7, 2485–2494. [Google Scholar] [CrossRef] [PubMed]
- Betancur-Ancona, D.; Chel, G.L.; Canizares, H.E. Acetylation and characterization of Canavalia ensiformis starch. J. Agric. Food Chem. 1997, 45, 378–382. [Google Scholar] [CrossRef]
- Adebowale, K.O.; Afolabi, T.A.; Olu-Owolabi, B.I. Functional, physicochemical and retrogradation properties of sword bean (Canavalia gladiata) acetylated and oxidized starches. Carbohydr. Polym. 2005, 65, 93–101. [Google Scholar] [CrossRef]
- Wojeicchowski, J.P.; Siqueira, G.L.; Lacerda, L.G.; Schnitzler, E.; Demiate, I.M. Physicochemical, structural and thermal properties of oxidized, acetylated and dual-modified common bean (Phaseolus vulgaris L.) starch. Food Sci. Technol. Camp. 2018, 38, 318–327. [Google Scholar] [CrossRef]
- Wani, I.A.; Sogi, D.S.; Gill, B.S. Physicochemical properties of acetylated starches from some Indian kidney bean (Phaseolus vulgaris L.) cultivars. Int. J. Food Sci. Technol. 2012, 47, 1993–1999. [Google Scholar] [CrossRef]
- Liao, L.; Liu, H.; Gan, Z.; Wu, W. Structural properties of sweet potato starch and its vermicelli quality as affected by heat-moisture treatment. Int. J. Food Prop. 2019, 22, 1122–1133. [Google Scholar] [CrossRef]
- Aryee, F.N.A.; Oduro, I.; Ellis, W.O.; Afuakwa, J.J. The physicochemical properties of flour samples from the roots of 31 varieties of cassava. Food Control 2006, 17, 916–922. [Google Scholar] [CrossRef]
- Hughes, T.; Hoover, R.; Liu, Q.; Donner, E.; Chibbar, R.; Jaiswal, S. Composition, morphology, molecular structure, and physicochemical properties of starches from newly released chickpea (Cicer arietinum L.) cultivars grown in Canada. Food Res. Int. 2009, 42, 627–635. [Google Scholar] [CrossRef]
- Ashogbon, O.A. Comparative study of the physicochemical properties of starch blends-pigeon pea and rice starches versus Bambara groundnut and cassava starches. J. Curr. Chem. Pharm. Sci. 2014, 4, 142–151. [Google Scholar]
- Adebowale, K.O.; Lawal, O.S. Microstructure, physicochemical properties and retrogradation behaviour of Mucuna bean (Mucuna puriens) starch on heat moisture treatments. Food Hydrocoll. 2003, 17, 265–272. [Google Scholar] [CrossRef]
- Sandhu, K.S.; Kaur, M.; Singh, N.; Lim, S. A comparison of native and oxidized normal and waxy corn starches: Physicochemical, thermal, morphological and pasting properties. Food Sci. Technol. 2008, 41, 1000–1010. [Google Scholar] [CrossRef]
- Ott, A.A.; Hugi, B.M.; Chaintreau, A. Sensory investigation of yogurt flavor perception: Mutual influences of volatiles and acidity. J. Agric. Food Chem. 2000, 48, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Routray, W.; Mishra, H.N. Scientific and technical aspects of yogurt aroma and taste: A review. Compr. Rev. Food Sci. Food Saf. 2011, 10, 208–220. [Google Scholar] [CrossRef]
- Hashim, I.B.; Khalil, A.H.; Habib, H. Quality and acceptability of a set-type yoghurt made from camel milk. J. Dairy Sci. 2009, 92, 857–862. [Google Scholar] [CrossRef] [PubMed]
- Smit, G. Dairy Processing; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Lucey, J.A. Cultured dairy products: An overview of their gelation and texture properties. Int. J. Dairy Technol. 2019, 57, 77–84. [Google Scholar] [CrossRef]
- Ghasempour, Z.; Alizadeh, M.; Bari, M.R. Optimisation of probiotic yoghurt production containing Zedo gum. Int. J. Dairy Technol. 2012, 65, 118–125. [Google Scholar] [CrossRef]
- Yoon, W.B.; McCarthy, K.L. Rheology of yoghurt during pipe flow as characterized by magnetic resonance imaging. J. Texture Stud. 2002, 33, 431–444. [Google Scholar] [CrossRef]
- Alakali, J.S.; Okonkwo, T.M.; Iordye, E.M. Effect of stabilizers on the physico-chemical and sensory attributes of thermized yoghurt. Afr. J. Biotechnol. 2008, 7, 158–163. [Google Scholar]
- Soomro, A.H.; Dars, A.G.; Sheikg, S.A.; Khaskheli, S.G.; Magsi, A.S.; Talpur, A. Effect of milk source and stabilizers on the compositional and sensorial quality of yoghurt. Pure Appl. Biol. 2016, 5, 1316–1322. [Google Scholar] [CrossRef]


| Attribute | Description |
|---|---|
| Aroma | Detect any aroma defects (e.g., unclean, masked, unnatural, cooked, lacks freshness) by smelling and oral perception of samples |
| Colour | Assess the colour (white, whitish, yellowish, yellow) of yoghurt |
| Palatability | Evaluate the taste of samples considering several attributes associated with taste (e.g., Unclean, unnatural, whey and refreshing perception) and aftertaste (e.g., sourness, astringency, sweetness, bitterness, and saltiness) |
| Firmness | Estimate the hardness, brittleness, gumminess, and gelatin-like texture of the coagulum |
| Consistency | Evaluate the consistency when stirring the product with the spoon, determine the rheological behaviour of yoghurt in the mouth |
| Flavour | Evaluate the flavour (pleasant, palatable or unpleasant) |
| Syneresis | Visual observation of the yoghurt surface; examine whey drainage after inserting the spoon into the curd |
| Overall acceptability | Rate the overall score of the sample considering the appearance, taste, and flavour profiles. |
| Properties | PPNS | PPAS1 | PPAS2 | PPAS3 |
|---|---|---|---|---|
| Pasting temp (°C) | 89.95 ± 0.71 a | 87.81 ± 0.51 b | 87.40 ± 0.23 c | 85.60 ± 0.17 d |
| Pasting time (min) | 5.07 ± 0.11 a | 5.05 ± 0.10 a | 5.00 ± 0.10 a | 4.93 ± 0.20 a |
| Peak Viscosity (RVU) | 588.70 ± 0.83 a | 408.50 ± 0.51 b | 346.88 ± 0.99 c | 314.70 ± 1.27 d |
| Trough Viscosity (RVU) | 434.80 ± 1.12 a | 293.90 ± 0.83 b | 282.44 ± 1.07 c | 263.50 ± 0.92 d |
| Final Viscosity (RVU) | 794.40 ± 2.55 a | 576.70 ± 1.60 b | 503.10 ± 3.42 c | 465.90 ± 1.91 d |
| Break Down (RVU) | 153.90 ± 0.91 a | 116.72 ± 0.83 b | 114.80 ± 0.86 b | 78.20 ± 1.11 c |
| Set Back (RVU) | 359.60 ± 1.61 a | 222.80 ± 0.91 b | 223.10 ± 0.82 b | 202.40 ± 0.63 c |
| Sample | pH | Acidity (% Lactic Acid) | Total Solids (%) | Syneresis (%) |
|---|---|---|---|---|
| CTR | 4.63 ± 0.11 a | 0.90 ± 0.01 c | 18.53 ± 0.34 b | 10.80 ± 1.26 g |
| LCTR | 4.41 ± 0.05 b | 1.03 ± 0.01 b | 18.20 ± 0.20 b | 15.69 ± 0.55 f |
| YoCN | 4.31 ± 0.00 c | 1.21 ± 0.01 a | 14.86 ± 1.21 c | 46.54 ± 0.23 a |
| YoPN | 4.30 ± 0.01 c | 1.17 ± 0.06 a | 20.20 ± 0.62 a | 36.80 ± 0.09 b |
| YoPA1 | 4.32 ± 0.00 c | 1.10 ± 0.01 ab | 20.41 ± 0.50 a | 22.00 ± 0.11 c |
| YoPA2 | 4.31 ± 0.00 c | 1.21 ± 0.08 a | 20.33 ± 0.76 a | 20.18 ± 0.20 d |
| YoPA3 | 4.31 ± 0.00 c | 1.21 ± 0.01 a | 20.47 ± 0.23 a | 18.62 ± 1.63 e |
| Samples | |||||||
|---|---|---|---|---|---|---|---|
| Attributes | CTR | LCTR | YoCN | YoPN | YoPA1 | YoPA2 | YoPA3 |
| Aroma | 7.80 ± 1.61 a | 6.65 ± 0.85 b | 5.90 ± 0.99 b | 6.20 ± 1.03 b | 6.20 ± 1.03 b | 6.70 ± 1.34 b | 6.10 ± 0.87 b |
| Colour | 7.50 ± 1.58 a | 7.50 ± 1.20 a | 7.10 ± 0.84 a | 7.40 ± 0.84 a | 7.50 ± 0.70 a | 7.60 ± 0.70 a | 7.50 ± 0.97 a |
| Palatability | 7.90 ± 1.28 a | 7.30 ± 0.90 a | 5.30 ± 1.03 b | 5.80 ± 0.94 b | 5.70 ± 1.63 b | 5.90 ± 2.02 b | 6.00 ± 1.25 b |
| Firmness | 6.90 ± 1.81 ab | 7.00 ± 1.50 ab | 5.80 ± 1.03 b | 6.10 ± 0.99 ab | 6.20 ± 1.47 ab | 6.20 ± 0.37 ab | 7.20 ± 1.13 a |
| Consistency | 8.00 ± 1.24 a | 7.30 ± 1.50 b | 5.80 ± 1.23 c | 6.00 ± 0.94 c | 6.50 ± 1.34 c | 6.30 ± 1.56 c | 6.30 ± 1.33 c |
| Flavour | 7.70 ± 1.56 a | 6.50 ± 0.82 b | 4.20 ± 1.54 c | 6.10 ± 0.87 b | 6.00 ± 1.24 b | 6.10 ± 1.66 b | 6.20 ± 1.03 b |
| Syneresis | 7.90 ± 1.20 a | 7.75 ± 1.33 a | 4.40 ± 1.42 c | 5.40 ± 1.03 bc | 5.40 ± 1.07 bc | 5.70 ± 1.82 b | 5.80 ± 1.35 b |
| Overall acceptability | 7.90 ± 1.79 a | 6.80 ± 0.57 b | 5.30 ± 0.95 d | 5.70 ± 0.69 cd | 6.20 ± 0.82 bc | 6.40 ± 1.57 c | 6.50 ± 1.24 bc |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olagunju, A.; Omoba, O.; Enujiugha, V.; Alashi, A.; Aluko, R. Technological Properties of Acetylated Pigeon Pea Starch and Its Stabilized Set-Type Yoghurt. Foods 2020, 9, 957. https://doi.org/10.3390/foods9070957
Olagunju A, Omoba O, Enujiugha V, Alashi A, Aluko R. Technological Properties of Acetylated Pigeon Pea Starch and Its Stabilized Set-Type Yoghurt. Foods. 2020; 9(7):957. https://doi.org/10.3390/foods9070957
Chicago/Turabian StyleOlagunju, Aderonke, Olufunmilayo Omoba, Victor Enujiugha, Adeola Alashi, and Rotimi Aluko. 2020. "Technological Properties of Acetylated Pigeon Pea Starch and Its Stabilized Set-Type Yoghurt" Foods 9, no. 7: 957. https://doi.org/10.3390/foods9070957
APA StyleOlagunju, A., Omoba, O., Enujiugha, V., Alashi, A., & Aluko, R. (2020). Technological Properties of Acetylated Pigeon Pea Starch and Its Stabilized Set-Type Yoghurt. Foods, 9(7), 957. https://doi.org/10.3390/foods9070957