Influence of the Microalga Chlorella vulgaris on the Growth and Metabolic Activity of Lactobacillus spp. Bacteria
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains
2.2. The Research Material
2.3. Statistical Analysis
2.4. Growth of Lactobacillus brevis Cultured in the Presence of Chlorella vulgaris
2.5. Acidifying Activity of Lactobacillus spp.
2.6. Proportion of Lactic Acid Isomers
2.7. Measurement of the Enzymatic Profile
3. Results
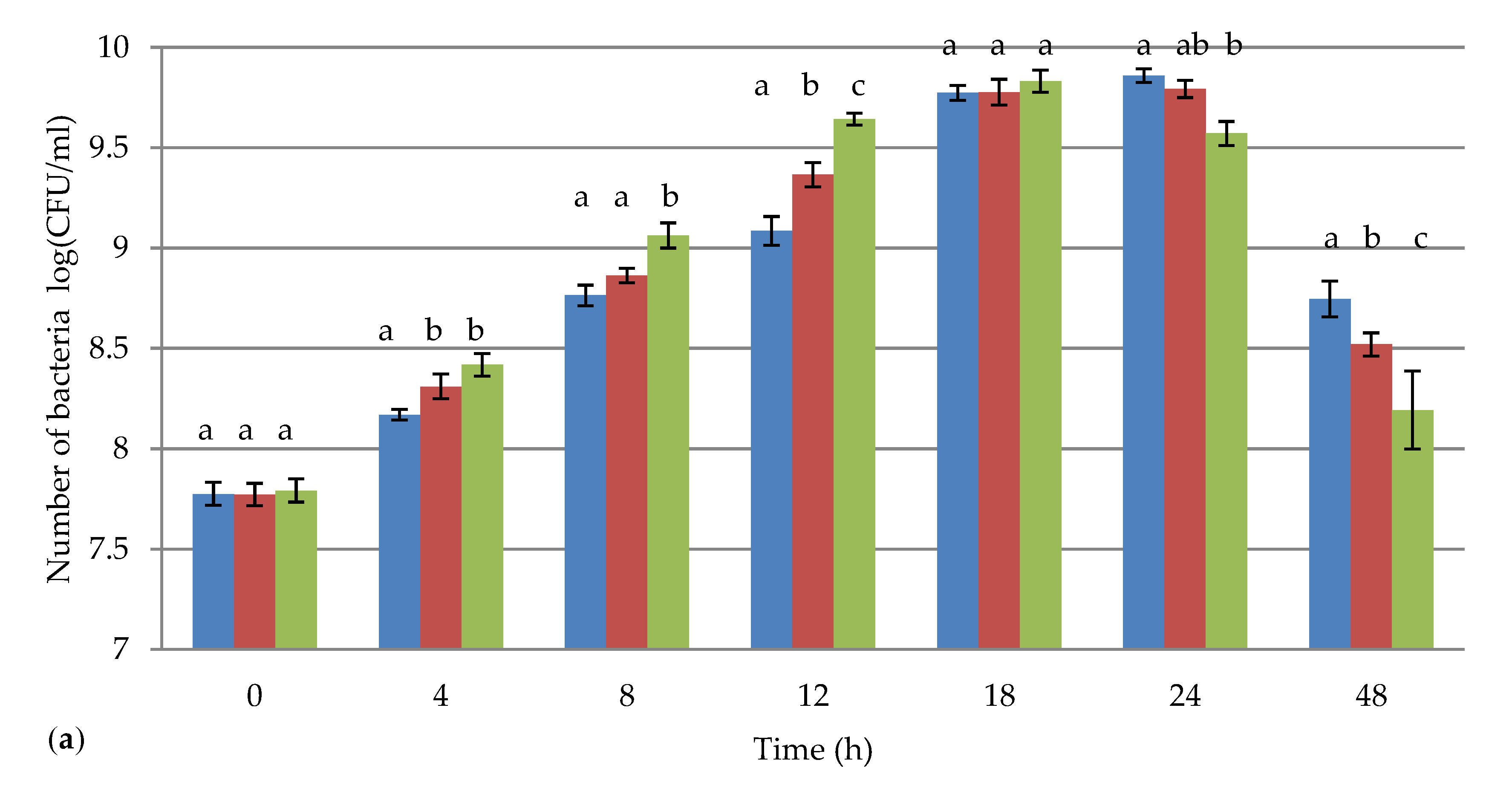
3.1. The Impact of Chlorella vulgaris on the Growth of Lactobacillus spp.
3.2. The Effect of Chlorella vulgaris on the Total Titratable Acidity of Lactobacillus spp.
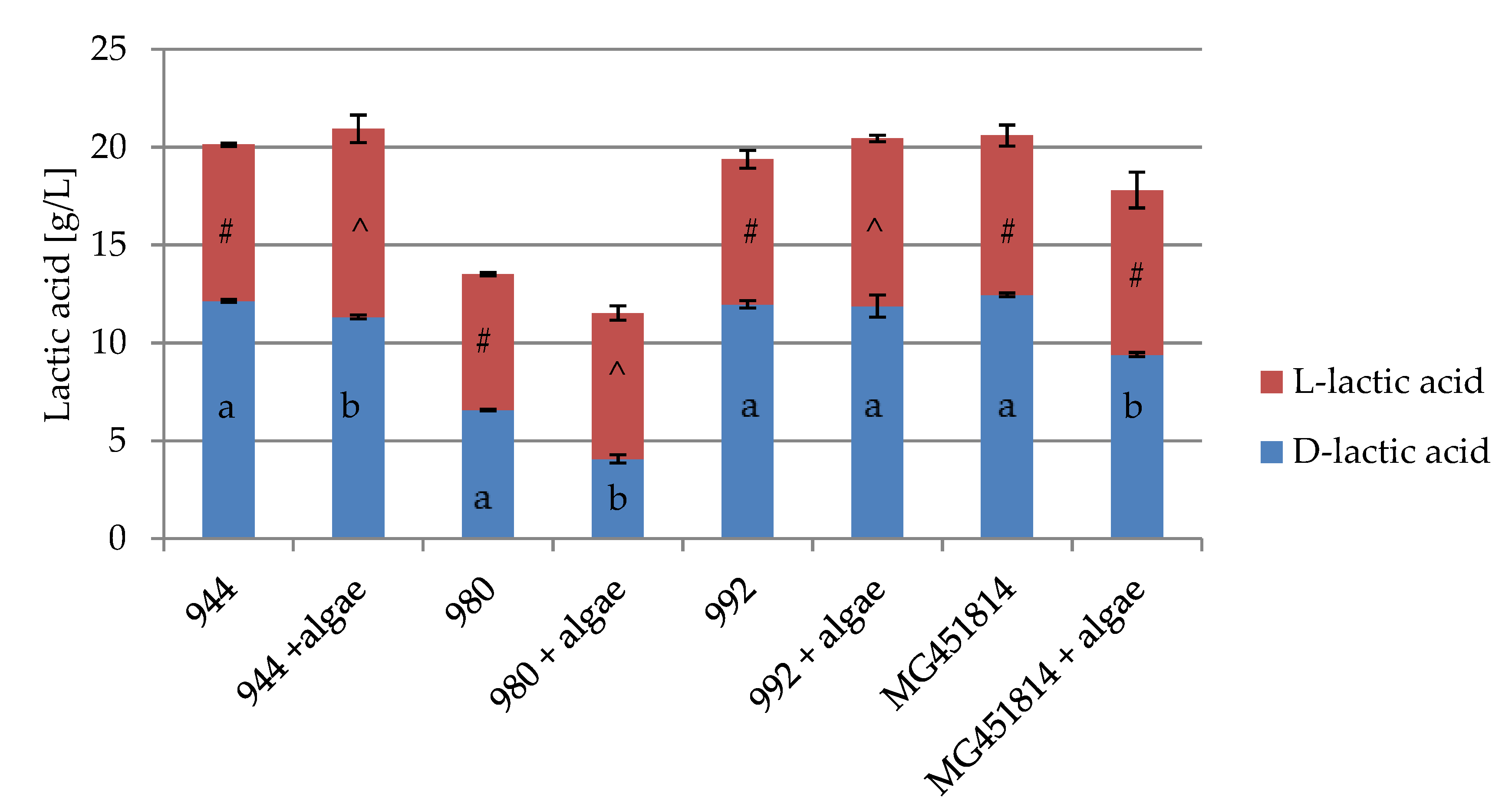
3.3. Influence of Chlorella vulgaris on d- and l-Lactic Acid Production by Lactobacillus spp.
3.4. The Impact of Chlorella vulgaris on the Enzymatic Activity of Lactobacillus spp.
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lafarga, T. Effect of microalgal biomass incorporation into foods: Nutritional and sensorial attributes of the end products. Algal Res. 2019, 41, 101566. [Google Scholar] [CrossRef]
- Caporgno, M.P.; Mathys, A. Trends in microalgae incorporation into innovative food products with potential health benefits. Front. Nutr. 2018, 5, 58. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; He, Y.; Luo, M.; Chen, F. Utilization of enzymatic cell disruption hydrolysate of Chlorella pyrenoidosa as potential carbon source in algae mixotrophic cultivation. Algal Res. 2020, 45, 101730. [Google Scholar] [CrossRef]
- Graça, C.; Fradinho, P.; Sousa, I.; Raymundo, A. Impact of Chlorella vulgaris on the rheology of wheat flour dough and bread texture. LWT Food Sci. Technol. 2018, 89, 466–474. [Google Scholar] [CrossRef]
- Uribe-Wandurraga, Z.N.; Igual, M.; García-Segovia, P.; Martínez-Monzó, J. Effect of microalgae addition on mineral content, colour and mechanical properties of breadsticks. Food Funct. 2019, 10, 4685–4692. [Google Scholar] [CrossRef]
- Batista, A.P.; Niccolai, A.; Fradinho, P.; Fragoso, S.; Bursic, I.; Rodolfi, L.; Biondi, N.; Tredici, M.R.; Sousa, I.; Raymundo, A. Microalgae biomass as an alternative ingredient in cookies: Sensory, physical and chemical properties, antioxidant activity and in vitro digestibility. Algal Res. 2017, 26, 161–171. [Google Scholar] [CrossRef]
- Beheshtipour, H.; Mortazavian, A.M.; Mohammadi, R.; Sohrabvandi, S.; Khosravi-Darani, K. Supplementation of Spirulina platensis and Chlorella vulgaris algae into probiotic fermented milks. Compr. Rev. Food Sci. Food Saf. 2013, 12, 144–154. [Google Scholar] [CrossRef]
- Heo, J.-Y.; Shin, H.-J.; Oh, D.-H.; Cho, S.-K.; Yang, C.-J.; Kong, I.-K.; Lee, S.-S.; Choi, K.-S.; Choi, S.-H.; Kim, S.-C.; et al. Quality properties of Appenzeller cheese added with Chlorella. Korean J. Food Sci. Anim. Resour. 2006, 26, 525–531. [Google Scholar]
- Tohamy, M.M.; Ali, M.A.; Shaaban, H.A.G.; Mohamad, A.G.; Hasanain, A.M. Production of functional spreadable processed cheese using Chlorella vulgaris. Acta Sci. Pol. Technol. Aliment. 2018, 17, 347–358. [Google Scholar] [CrossRef]
- Carrizo, S.L.; de LeBlanc, A.D.M.; LeBlanc, J.G.; Rollán, G.C. Quinoa pasta fermented with lactic acid bacteria prevents nutritional deficiencies in mice. Food Res. Int. 2020, 127, 108735. [Google Scholar] [CrossRef]
- Vieco-Saiz, N.; Belguesmia, Y.; Raspoet, R.; Auclair, E.; Gancel, F.; Kempf, I.; Drider, D. Benefits and inputs from lactic acid bacteria and their bacteriocins as alternatives to antibiotic growth promoters during food-animal production. Front. Microb. 2019, 10, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- EFSA Panel on Biological Hazards (BIOHAZ); Koutsoumanis, K.; Allende, A.; Álvarez-Ordóñez, A.; Bolton, D.; Bover-Cid, S.; Chemaly, M.; Davies, R.; Hilbert, F.; Lindqvist, R.; et al. Update of the list of QPS-recommended biological agents intentionally added to food or feed as notified to EFSA 9: Suitability of taxonomic units notified to EFSA until September 2018. Efsa J. 2019, 17, e05555. [Google Scholar] [CrossRef]
- Ma, C.; Zhang, S.; Lu, J.; Zhang, C.; Pang, X.; Lv, J. Screening for cholesterol-lowering probiotics from lactic acid bacteria isolated from corn silage based on three hypothesized pathways. Int. J. Mol. Sci. 2019, 20, 2073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khare, A.; Gaur, S. Cholesterol-Lowering effects of Lactobacillus species. Curr. Microb. 2020, 77, 638–644. [Google Scholar] [CrossRef]
- Kim, J.A.; Kim, S.H.; Kim, I.S.; Yu, D.Y.; Kim, S.C.; Lee, S.H.; Lee, S.S.; Yun, C.-H.; Choi, I.S.; Cho, K.K. Anti-inflammatory effects of a mixture of lactic acid bacteria and sodium butyrate in atopic dermatitis murine model. J. Med. Food 2018, 21, 716–725. [Google Scholar] [CrossRef]
- Pinteus, S.; Silva, J.; Alves, C.; Horta, A.; Fino, N.; Rodrigues, A.I.; Mendes, S.; Pedrosa, R. Cytoprotective effect of seaweeds with high antioxidant activity from the Peniche coast (Portugal). Food Chem. 2017, 218, 591–599. [Google Scholar] [CrossRef]
- Tsai, C.C.; Lai, T.M.; Hsieh, Y.M. Evaluation of Lactobacilli for antagonistic activity against the growth, adhesion and invasion of Klebsiella pneumoniae and Gardnerella vaginalis. Indian J. Microb. 2019, 59, 81–89. [Google Scholar] [CrossRef]
- Iorizzo, M.; Lombardi, S.J.; Ganassi, S.; Testa, B.; Ianiro, M.; Letizia, F.; Succi, M.; Tremonte, P.; Vergalito, F.; Cozzolino, A.; et al. Antagonistic Activity against Ascosphaera apis and Functional Properties of Lactobacillus kunkeei Strains. Antibiotics 2020, 9, 262. [Google Scholar] [CrossRef]
- Mechai, A.; Debabza, M.; Zouari, S. Antagonistic activity of lactic acid bacteria isolated from Algerian traditional fermented milks against multi-drug resistant and β-lactamases-producing pathogenic bacteria. Res. J. Biotechnol. 2020, 15, 4. [Google Scholar]
- Harper, A.; Naghibi, M.M.; Garcha, D. The Role of Bacteria, Probiotics and Diet in Irritable Bowel Syndrome. Foods 2018, 7, 13. [Google Scholar] [CrossRef] [Green Version]
- Ellis, S.R.; Nguyen, M.; Vaughn, A.R.; Notay, M.; Burney, W.A.; Sandhu, S.; Sivamani, R.K. The Skin and Gut Microbiome and Its Role in Common Dermatologic Conditions. Microorganisms 2019, 7, 550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, R.; Ning, H.; Shen, M.; Li, J.; Zhang, J.; Chen, X. Probiotics for the Treatment of Atopic Dermatitis in Children: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front. Cell. Infect. Microbiol. 2017, 7, 392. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, K.; Kim, W. Cream cheese-derived Lactococcus chungangensis CAU 28 modulates the gut microbiota and alleviates atopic dermatitis in BALB/c mice. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uchida, M.; Murata, M.; Ishikawa, F. Lactic acid bacteria effective for regulating the growth of contaminant bacteria during the fermentation of Undaria pinnatifida (Phaeophyta). Fish Sci. 2007, 73, 694–704. [Google Scholar] [CrossRef]
- Niccolai, A.; Shannon, E.; Abu-Ghannam, N.; Biondi, N.; Rodolfi, L.; Tredici, M.R. Lactic acid fermentation of Arthrospira platensis (spirulina) biomass for probiotic-based products. J. Appl. Phycol. 2019, 31, 1077–1083. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.; Abu-Ghannam, N.; Scannell, A.G. Growth and kinetics of Lactobacillus plantarum in the fermentation of edible Irish brown seaweeds. Food Bioprod. Process. 2011, 89, 346–355. [Google Scholar] [CrossRef] [Green Version]
- Oh, S.T.; Zheng, L.; Kwon, H.J.; Choo, Y.K.; Lee, K.W.; Kang, C.W.; An, B.K. Effects of dietary fermented Chlorella vulgaris (CBT®) on growth performance, relative organ weights, cecal microflora, tibia bone characteristics, and meat qualities in Pekin ducks. Asian Australas. J Anim. Sci. 2015, 28, 95. [Google Scholar] [CrossRef]
- Loveday, S.M. Food proteins: Technological, nutritional, and sustainability attributes of traditional and emerging proteins. Annu. Rev. Food Sci. Technol. 2019, 10, 311–339. [Google Scholar] [CrossRef]
- Saadat, Y.R.; Khosroushahi, A.Y.; Gargari, B.P. A comprehensive review of anticancer, immunomodulatory and health beneficial effects of the lactic acid bacteria exopolysaccharides. Carbohydr. Polym. 2019, 217, 79–89. [Google Scholar] [CrossRef]
- Noda, M.; Danshiitsoodol, N.; Inoue, Y.; Okamoto, T.; Sultana, N.; Sugiyama, M. Antibiotic susceptibility of plant-derived lactic acid bacteria conferring health benefits to human. J. Antibiot. 2019, 72, 834–842. [Google Scholar] [CrossRef]
- Shannon, E.; Abu-Ghannam, N. Seaweeds as nutraceuticals for health and nutrition. Phycologia 2019, 58, 563–577. [Google Scholar] [CrossRef] [Green Version]
- Klewicka, E.; Libudzisz, Z.; Śliżewska, K.; Otlewska, A. A New Strain of Lactic Acid Bacteria Lacobacillus brevis. Patent No. PL 214504, B1, 30 August 2013. [Google Scholar]
- Matejčeková, Z.; Liptáková, D.; Spodniaková, S.; Valík, Ľ. Characterization of the growth of Lactobacillus plantarum in milk in dependence on temperature. Acta Chim. Slov. 2016, 9, 104–108. [Google Scholar] [CrossRef] [Green Version]
- Bergqvist, S.W.; Sandberg, A.S.; Carlsson, N.G.; Andlid, T. Improved iron solubility in carrot juice fermented by homo-and hetero-fermentative lactic acid bacteria. Food Microbiol. 2005, 22, 53–61. [Google Scholar] [CrossRef]
- Yoon, K.Y.; Woodams, E.E.; Hang, Y.D. Production of probiotic cabbage juice by lactic acid bacteria. Biores. Technol. 2006, 97, 1427–1430. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, G.F.; Mao, X.; Wang, J.Y.; Duan, C.Y.; Wang, Z.J.; Liu, L.B. Growth and acid production of Lactobacillus delbrueckii ssp. bulgaricus ATCC 11842 in the fermentation of algal carcass. J. Dairy Sci. 2016, 99, 4243–4250. [Google Scholar] [CrossRef] [Green Version]
- Abbasiliasi, S.; Marikkar, M.N.; Ariff, A.; Amid, M.; Lamasudin, D.U.; Manap, M.Y.A.; Mustafa, S. Defatted coconut residue crude polysaccharides as potential prebiotics: Study of their effects on proliferation and acidifying activity of probiotics in vitro. J. Food Sci. Technol. 2017, 54, 164–173. [Google Scholar] [CrossRef] [Green Version]
- Fadaei, V.; Eslami-Moshkenani, A.; Khosravi-Darani, K. Effects of powdered Spirulina platensis biomass on pH and titratable acidity of probiotic doogh containing powdered mint during cold storage. Int. J. Biol. Biotechnol. 2013, 10, 631–635. [Google Scholar]
- Gavrilova, E.; Anisimova, E.; Gabdelkhadieva, A.; Nikitina, E.; Vafina, A.; Yarullina, D.; Bogachev, M.; Kayumov, A. Newly isolated lactic acid bacteria from silage targeting biofilms of foodborne pathogens during milk fermentation. BMC Microb. 2019, 19, 248. [Google Scholar] [CrossRef]
- Komesu, A.; de Oliveira, J.A.R.; da Silva Martins, L.H.; Maciel, M.R.W.; Maciel Filho, R. Lactic acid production to purification: A review. BioResources 2017, 12, 4364–4383. [Google Scholar] [CrossRef] [Green Version]
- Mudryk, Z.J.; Podgorska, B. Enzymatic Activity of Bacterial Strains Isolated from Marine Beach Sediments. Pol. J. Environ. Stud. 2006, 15, 441–448. [Google Scholar]
- Abouloifa, H.; Rokni, Y.; Bellaouchi, R.; Ghabbour, N.; Karboune, S.; Brasca, M.; Salah, R.B.; Chihib, N.E.; Saalaoui, E.; Asehraou, A. Characterization of probiotic properties of antifungal Lactobacillus strains isolated from traditional fermenting green olives. Probiotics Antimicro. Prot. 2020, 12, 683–696. [Google Scholar] [CrossRef] [PubMed]
- Ghabbour, N.; Lamzira, Z.; Thonart, P.; Cidalia, P.; Markaoui, M.; Asehraou, A. Selection of oleuropein-degrading lactic acid bacteria strains isolated from fermenting Moroccan green olives. Grasas y Aceites 2011, 62, 84–89. [Google Scholar] [CrossRef] [Green Version]
- Nandan, A.; Nampoothiri, K.M. Therapeutic and biotechnological applications of substrate specific microbial aminopeptidases. Appl. Microbiol. Biotechnol. 2020, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Domingos-Lopes, M.F.P.; Stanton, C.; Ross, P.R.; Dapkevicius, M.L.E.; Silva, C.C.G. Genetic diversity, safety and technological characterization of lactic acid bacteria isolated from artisanal Pico cheese. Food Microbiol. 2017, 63, 178–190. [Google Scholar] [CrossRef]

| Time (h) | LAB | LAB+ Algae 0.1% | LAB+ Algae 1.5% |
|---|---|---|---|
| Total Acidity (mL 0.1-M NaOH/1 mL) | |||
| 0 | 0.70 ± 0.05 a | 0.80 ± 0.05 b | 0.90 ± 0.05 c |
| 4 | 0.80 ± 0.05 a | 0.90 ± 0.05 b | 1.00 ± 0.05 c |
| 8 | 1.20 ± 0.10 a | 1.30 ± 0.10 b | 1.45 ± 0.10 c |
| 12 | 1.80 ± 0.10 a | 1.95 ± 0.15 b | 2.20 ± 0.10 c |
| 18 | 2.40 ± 0.05 a | 2.45 ± 0.05 a | 2.55 ± 0.05 b |
| 24 | 2.50 ± 0.05 a | 2.50 ± 0.05 a | 2.60 ± 0.00 b |
| 48 | 2.50 ± 0.05 a | 2.50 ± 0.00 a | 2.65 ± 0.05 b |
| Time (h) | LAB | LAB+ Algae 0.1% | LAB+ Algae 1.5% |
|---|---|---|---|
| Total Acidity (mL 0.1-M NaOH/1 mL) | |||
| 0 | 0.70 ± 0.00 a | 0.70 ± 0.05 b | 0.80 ± 0.05 c |
| 4 | 0.70 ± 0.05 a | 0.80 ± 0.05 b | 0.90 ± 0.05 c |
| 8 | 0.90 ± 0.10 a | 0.95 ± 0.15 b | 1.25 ± 0.10 c |
| 12 | 1.20 ± 0.10 a | 1.30 ± 0.10 b | 1.55 ± 0.15 c |
| 18 | 1.45 ± 0.05 a | 1.60 ± 0.05 b | 1.85 ± 0.00 c |
| 24 | 1.60 ± 0.05 a | 1.70 ± 0.00 b | 1.90 ± 0.05 c |
| 48 | 1.60 ± 0.00 a | 1.70 ± 0.05 b | 1.90 ± 0.05 c |
| Time (h) | LAB | LAB+ Algae 0.1% | LAB+ Algae 1.5% |
|---|---|---|---|
| Total Acidity (mL 0.1-M NaOH/1 mL) | |||
| 0 | 0.70 ± 0.05 a | 0.70 ± 0.05 b | 0.85 ± 0.05 c |
| 4 | 0.75 ± 0.05 a | 0.80 ± 0.05 b | 1.00 ± 0.10 c |
| 8 | 0.95 ± 0.10 a | 1.05 ± 0.10 b | 1.40 ± 0.05 c |
| 12 | 1.30 ± 0.15 a | 1.45 ± 0.10 b | 1.80 ± 0.15 c |
| 18 | 1.70 ± 0.05 a | 1.90 ± 0.05 b | 2.30 ± 0.05 c |
| 24 | 2.40 ± 0.05 a | 2.55 ± 0.05 b | 2.70 ± 0.00 c |
| 48 | 2.45 ± 0.05 a | 2.60 ± 0.05 b | 2.70 ± 0.05 c |
| Time (h) | LAB | LAB+ Algae 0.1% | LAB+ Algae 1.5% |
|---|---|---|---|
| Total Acidity (mL 0.1-M NaOH/1 mL) | |||
| 0 | 0.70 ± 0.05 a | 0.70 ± 0.05 b | 0.90 ± 0.00 c |
| 4 | 0.75 ± 0.05 a | 0.75 ± 0.05 b | 0.95 ± 0.05 c |
| 8 | 0.80 ± 0.05 a | 0.90 ± 0.10 b | 1.10 ± 0.15 c |
| 12 | 0.85 ± 0.05 a | 1.10 ± 0.10 b | 1.30 ± 0.10 c |
| 18 | 0.90 ± 0.10 a | 1.30 ± 0.15 b | 1.45 ± 0.00 c |
| 24 | 1.40 ± 0.05 a | 1.60 ± 0.05 b | 1.80 ± 0.05 c |
| 48 | 1.45 ± 0.05 a | 1.65 ± 0.05 b | 1.80 ± 0.05 c |
| Enzymes | 0944 | 0944 + Algae | 0980 | 0980 + Algae | 0992 | 0992 + Algae | MG 451814 | MG 451814 + Algae |
|---|---|---|---|---|---|---|---|---|
| Control | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Alkaline phosphatase | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Esterase (C 4) | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 |
| Esterase lipase (C 8) | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 |
| Lipase (C 14) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Leucine arylamidase | 3 | 3 | 5 | 5 | 3 | 3 | 5 | 5 |
| Valine arylamidase | 2 | 2 | 3 | 4 | 2 | 2 | 5 | 4 |
| Cystine arylamidase | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Trypsin | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| α-chymotrypsin | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Acid phosphatase | 2 | 2 | 5 | 5 | 1 | 1 | 2 | 1 |
| Naphthol-AS-bi-phosphohydrolase | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| α-galactosidase | 0 | 0 | 1 | 2 | 0 | 0 | 2 | 2 |
| β-galactosidase | 0 | 0 | 5 | 5 | 0 | 1 | 5 | 5 |
| β-glucuronidase | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| α-glucosidase | 1 | 0 | 1 | 4 | 0 | 0 | 2 | 2 |
| β-glucosidase | 4 | 3 | 5 | 5 | 3 | 4 | 5 | 5 |
| N-acetyl-β-glucosaminidase | 1 | 1 | 0 | 0 | 1 | 2 | 0 | 0 |
| α-mannosidase | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| α-fucosidase | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ścieszka, S.; Klewicka, E. Influence of the Microalga Chlorella vulgaris on the Growth and Metabolic Activity of Lactobacillus spp. Bacteria. Foods 2020, 9, 959. https://doi.org/10.3390/foods9070959
Ścieszka S, Klewicka E. Influence of the Microalga Chlorella vulgaris on the Growth and Metabolic Activity of Lactobacillus spp. Bacteria. Foods. 2020; 9(7):959. https://doi.org/10.3390/foods9070959
Chicago/Turabian StyleŚcieszka, Sylwia, and Elżbieta Klewicka. 2020. "Influence of the Microalga Chlorella vulgaris on the Growth and Metabolic Activity of Lactobacillus spp. Bacteria" Foods 9, no. 7: 959. https://doi.org/10.3390/foods9070959
APA StyleŚcieszka, S., & Klewicka, E. (2020). Influence of the Microalga Chlorella vulgaris on the Growth and Metabolic Activity of Lactobacillus spp. Bacteria. Foods, 9(7), 959. https://doi.org/10.3390/foods9070959






