Residue Analysis of Insecticides in Potatoes by QuEChERS-dSPE/UHPLC-PDA
Abstract
:1. Introduction
2. Materials and Methods
2.1. Standards and Materials
2.2. Samples
2.3. Standard Solutions
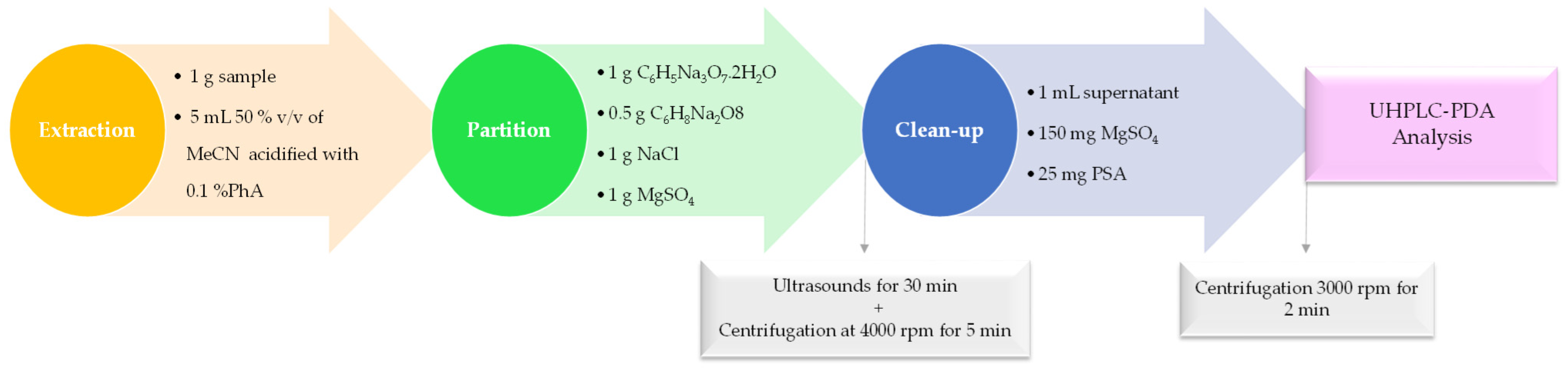
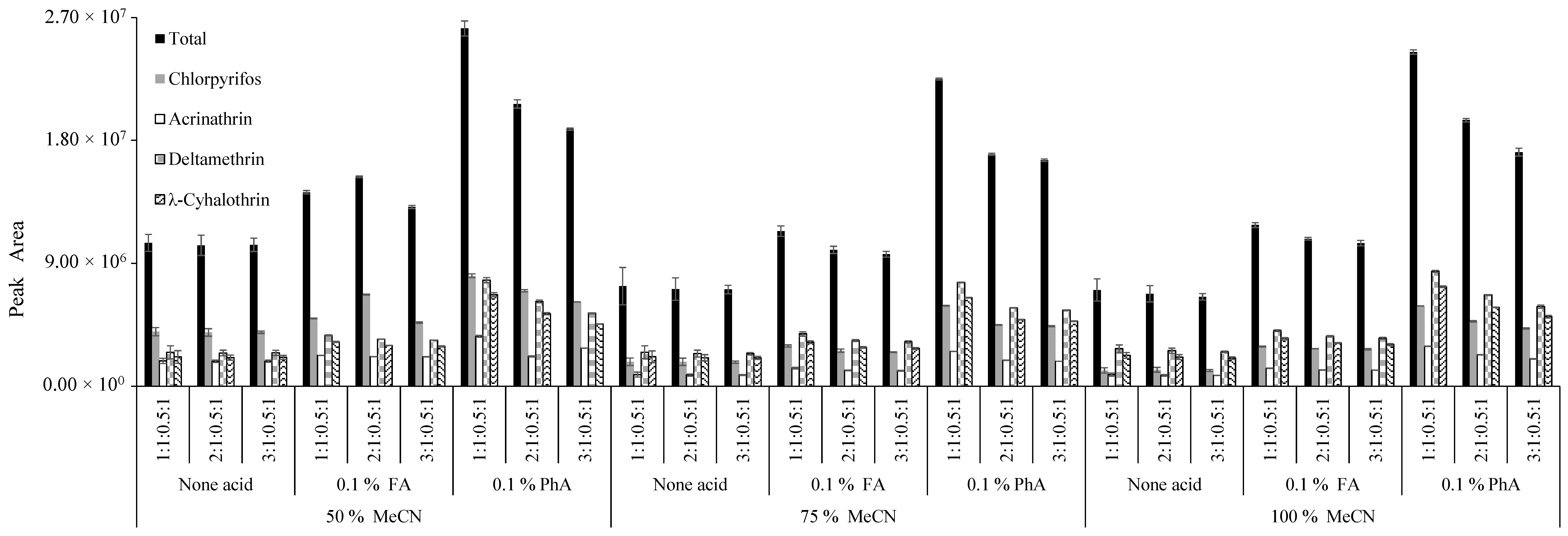
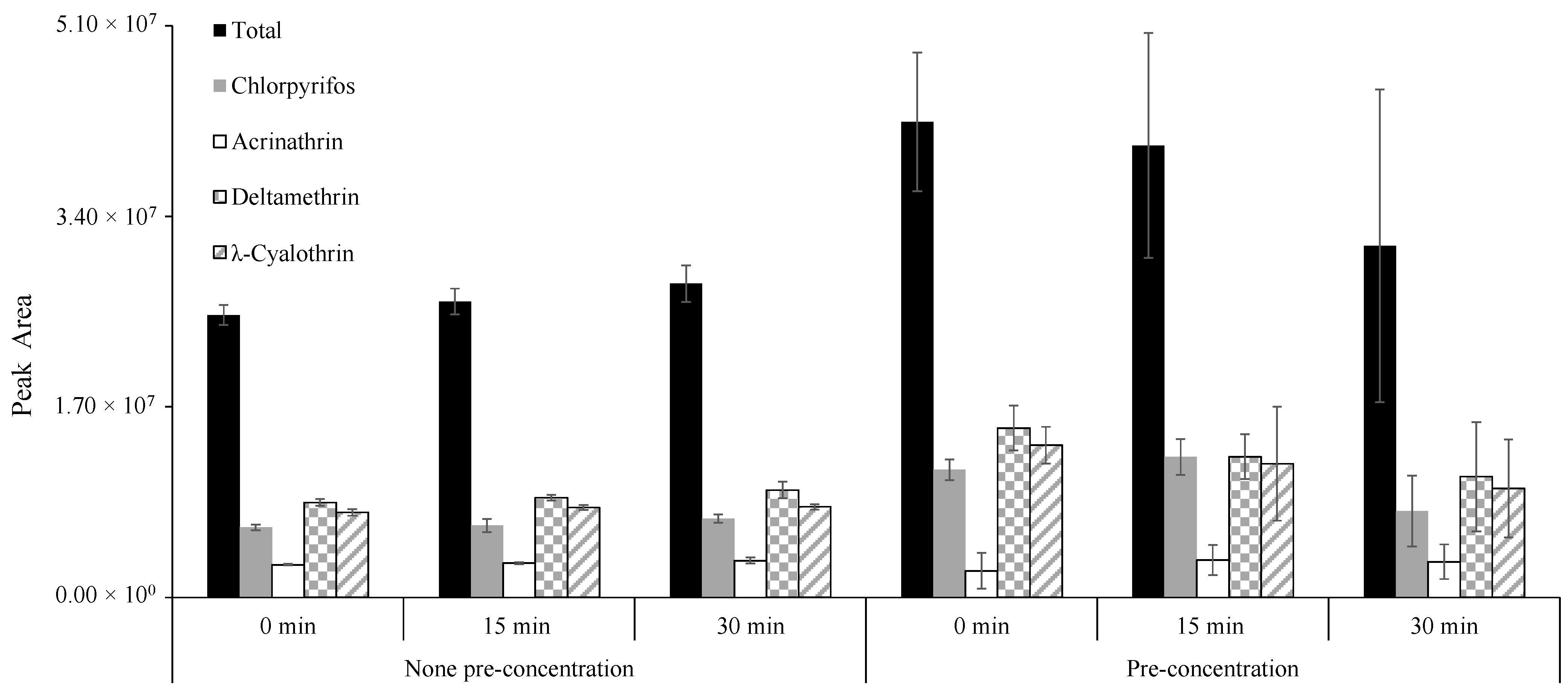
2.4. Optimization of the Modified QuEChERS-dSPE Extraction Procedure by Experimental Design
Optimized QuEChERS-dSPE Procedure
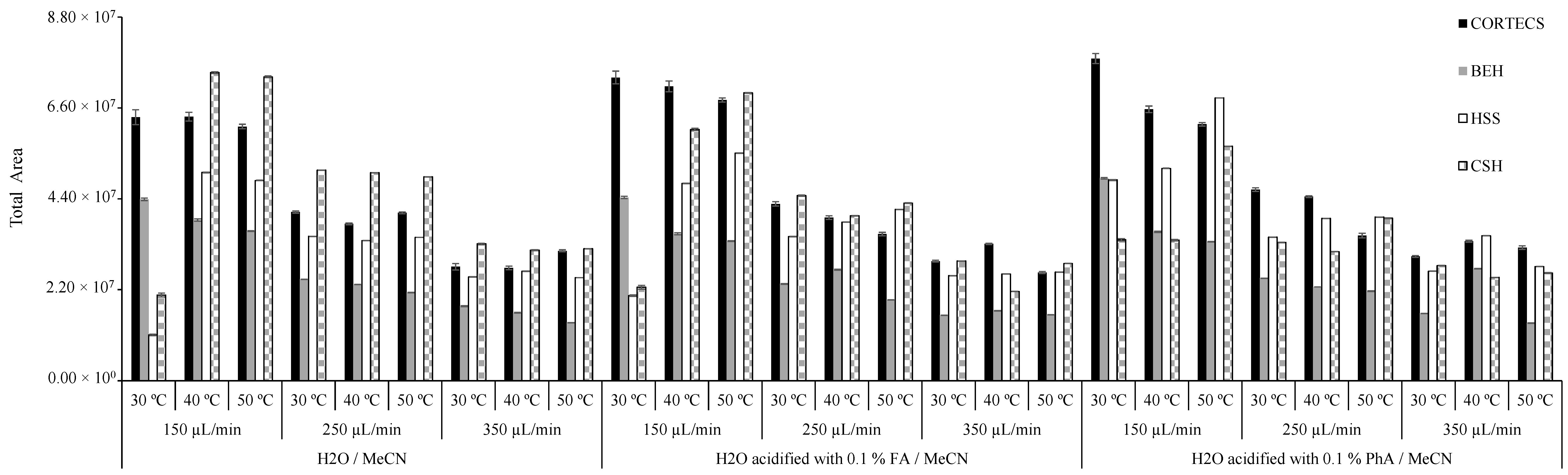
2.5. Optimization of UHPLC-PDA Conditions
Optimized UHPLC-PDA Conditions
2.6. Method Validation
3. Results and Discussion
3.1. Optimization of QuEChERS-dSPE Procedure
3.2. Optimization of UHPLC-PDA Conditions
3.3. Method Validation
3.4. Quantification of Insecticides in Potatoes
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chen, L.; Jia, C.; Li, F.; Jing, J.; Yu, P.; He, M.; Zhao, E. Dissipation and residues of fluazinam and dimethomorph in potatoes, potato plants, and soil, determined by QuEChERS ultra-performance liquid chromatography tandem mass spectrometry. Environ. Sci. Pollut. Res. 2018, 25, 32783–32790. [Google Scholar] [CrossRef]
- Song, S.; Huang, H.; Chen, Z.; Wei, J.; Deng, C.; Tan, H.; Li, X. Representative commodity for six leafy vegetables based on the determination of six pesticide residues by gas chromatography. Acta Chromatogr. 2019, 31, 49–56. [Google Scholar] [CrossRef]
- Bagheri, H.; Yamini, Y.; Safari, M.; Asiabi, H.; Karimi, M.; Heydari, A. Simultaneous determination of pyrethroids residues in fruit and vegetable samples via supercritical fluid extraction coupled with magnetic solid phase extraction followed by HPLC-UV. J. Supercrit. Fluids 2016, 107, 571–580. [Google Scholar] [CrossRef]
- Cabrera, L.D.; Caldas, S.S.; Prestes, O.D.; Primel, E.G.; Zanella, R. Evaluation of alternative sorbents for dispersive solid-phase extraction clean-up in the QuEChERS method for the determination of pesticide residues in rice by liquid chromatography with tandem mass spectrometry. J. Sep. Sci. 2016, 39, 1945–1954. [Google Scholar]
- Rai, S.; Singh, A.K.; Srivastava, A.; Yadav, S.; Siddiqui, M.H.; Mudiam, M.K.R. Comparative evaluation of QuEChERS method coupled to DLLME extraction for the analysis of multiresidue pesticides in vegetables and fruits by gas chromatography-mass spectrometry. Food Anal. Methods 2016, 9, 2656–2669. [Google Scholar] [CrossRef]
- Bordin, A.B.; Minetto, L.; do Nascimento Filho, I.; Beal, L.L.; Moura, S. Determination of pesticide residues in whole wheat flour using modified QuEChERS and LC–MS/MS. Food Anal. Methods 2017, 10, 1–9. [Google Scholar] [CrossRef]
- Neme, K.; Satheesh, N. Review on pesticide residue in plant food products: Health impacts and mechanisms to reduce the residue levels in food. Arch. Appl. Sci. Res. 2016, 8, 55–60. [Google Scholar]
- Lee, J.; Kim, L.; Shin, Y.; Lee, J.; Lee, J.; Kim, E.; Moon, J.K.; Kim, J.H. Rapid and simultaneous analysis of 360 pesticides in brown rice, spinach, orange, and potato using microbore GC-MS/MS. J. Agric. Food Chem. 2017, 65, 3387–3395. [Google Scholar] [CrossRef]
- Shi, X.; Liu, J.; Sun, A.; Li, D.; Chen, J. Group-selective enrichment and determination of pyrethroid insecticides in aquaculture seawater via molecularly imprinted solid phase extraction coupled with gas chromatography-electron capture detection. J. Chromatogr. A 2012, 1227, 60–66. [Google Scholar] [CrossRef]
- Rösch, A.; Beck, B.; Hollender, J.; Singer, H. Picogram per liter quantification of pyrethroid and organophosphate insecticides in surface waters: A result of large enrichment with liquid–liquid extraction and gas chromatography coupled to mass spectrometry using atmospheric pressure chemical ionization. Anal. Bioanal. Chem. 2019, 411, 3151–3164. [Google Scholar]
- Liu, F.; Yang, X.; Wu, X.; Xi, X.; Gao, H.; Zhang, S.; Zhou, W.; Lu, R. A dispersive magnetic solid phase microextraction based on ionic liquid-coated and cyclodextrin-functionalized magnetic core dendrimer nanocomposites for the determination of pyrethroids in juice samples. Food Chem. 2018, 268, 485–491. [Google Scholar] [CrossRef]
- Wu, M.; Chen, G.; Liu, P.; Zhou, W.; Jia, Q. Polydopamine-based immobilization of a hydrazone covalent organic framework for headspace solid-phase microextraction of pyrethroids in vegetables and fruits. J. Chromatogr. A 2016, 1456, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zeng, F.; Dong, Q.; Cao, Y.; Fan, H.; Deng, C. Rapid determination method for 12 pyrethroid pesticide residues in tea by stir bar sorptive extraction-thermal desorption-gas chromatography. Phys. Procedia 2012, 25, 1776–1780. [Google Scholar] [CrossRef] [Green Version]
- Grande-Martínez, Á.; Arrebola-Liébanas, F.J.; Martínez-Vidal, J.L.; Hernández-Torres, M.E.; Garrido-Frenich, A. Optimization and validation of a multiresidue pesticide method in rice and wheat flour by modified QuECHERS and GS-MS/MS. Food Anal. Methods 2016, 9, 548–563. [Google Scholar] [CrossRef]
- Manav, Ö.G.; Dinç-Zor, Ş.; Alpdoğan, G. Optimization of a modified QuEChERS method by means of experimental design for multiresidue determination of pesticides in milk and dairy products by GC–MS. Microchem. J. 2019, 144, 124–129. [Google Scholar] [CrossRef]
- Narenderan, S.T.; Meyyanathan, S.N.; Karri, V.V.S.R.; Babu, B.; Chintamaneni, P. Multivariate response surface methodology assisted modified QuEChERS extraction method for the evaluation of organophosphate pesticides in fruits and vegetables cultivated in Nilgiris, South India. Food Chem. 2019, 300, 125188. [Google Scholar] [CrossRef] [PubMed]
- Nantia, E.A.; Moreno-González, D.; Manfo, F.P.T.; Gámiz-Gracia, L.; García-Campaña, A.M. QuEChERS-based method for the determination of carbamate residues in aromatic herbs by UHPLC-MS/MS. Food Chem. 2017, 216, 334–341. [Google Scholar] [CrossRef]
- González-Curbelo, M.Á.; Socas-Rodríguez, B.; Herrera-Herrera, A.V.; González-Sálamo, J.; Hernández-Borges, J.; Rodríguez-Delgado, M.Á. Evolution and applications of the QuEChERS method. TrAC Trends Anal. Chem. 2015, 71, 169–185. [Google Scholar] [CrossRef]
- Anastassiades, M.; Lehotay, S.J.; Stajnbaher, D.; Schenck, F.J. Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “dispersive solid-phase extraction” for the determination of pesticide residues in produce. J. AOAC Int. 2003, 86, 412–431. [Google Scholar] [CrossRef] [Green Version]
- Correia-Sá, L.; Fernandes, V.C.; Carvalho, M.; Calhau, C.; Domingues, V.F.; Delerue-Matos, C. Optimization of QuEChERS method for the analysis of organochlorine pesticides in soils with diverse organic matter. J. Sep. Sci. 2012, 35, 1521–1530. [Google Scholar] [CrossRef]
- Frenich, A.G.; Martínez Vidal, J.L.; Pastor-Montoro, E.; Romero-González, R. High-throughput determination of pesticide residues in food commodities by use of ultra-performance liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2008, 390, 947–959. [Google Scholar] [CrossRef] [PubMed]
- Rizzetti, T.M.; Kemmerich, M.; Martins, M.L.; Prestes, O.D.; Adaime, M.B.; Zanella, R. Optimization of a QuEChERS based method by means of central composite design for pesticide multiresidue determination in orange juice by UHPLC–MS/MS. Food Chem. 2016, 196, 25–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grimalt, S.; Dehouck, P. Review of analytical methods for the determination of pesticide residues in grapes. J. Chromatogr. A 2016, 1433, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Chemat, F.; Rombaut, N.; Sicaire, A.-G.; Meullemiestre, A.; Fabiano-Tixier, A.-S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef] [PubMed]
- Hanot, V.; Goscinny, S.; Deridder, M. A simple multi-residue method for the determination of pesticides in fruits and vegetables using a methanolic extraction and ultra-high-performance liquid chromatography-tandem mass spectrometry: Optimization and extension of scope. J. Chromatogr. A 2015, 1384, 53–66. [Google Scholar] [CrossRef]
- Peña, A.; Ruano, F.; Mingorance, M.D. Ultrasound-assisted extraction of pesticides from olive branches: A multifactorial approach to method development. Anal. Bioanal. Chem. 2006, 385, 918–925. [Google Scholar] [CrossRef]
- Da Costa Morais, E.H.; Collins, C.H.; Jardim, I.C.S.F. Pesticide determination in sweet peppers using QuEChERS and LC–MS/MS. Food Chem. 2018, 249, 77–83. [Google Scholar] [CrossRef]
- Wilkowska, A.; Biziuk, M. Determination of pesticide residues in food matrices using the QuEChERS methodology. Food Chem. 2011, 125, 803–812. [Google Scholar] [CrossRef]
- Bruzzoniti, M.C.; Checchini, L.; De Carlo, R.M.; Orlandini, S.; Rivoira, L.; Del Bubba, M. QuEChERS sample preparation for the determination of pesticides and other organic residues in environmental matrices: A critical review. Anal. Bioanal. Chem. 2014, 406, 4089–4116. [Google Scholar] [CrossRef]
- Frenich, A.G.; Romero-González, R.; del Mar Aguilera-Luiz, M. Comprehensive analysis of toxics (pesticides, veterinary drugs and mycotoxins) in food by UHPLC-MS. TrAC Trends Anal. Chem. 2014, 63, 158–169. [Google Scholar] [CrossRef]
- Wang, K.; Xie, X.; Zhang, Y.; Huang, Y.; Zhou, S.; Zhang, W.; Lin, Y.; Fan, H. Combination of microwave-assisted extraction and ultrasonic-assisted dispersive liquid-liquid microextraction for separation and enrichment of pyrethroids residues in Litchi fruit prior to HPLC determination. Food Chem. 2018, 240, 1233–1242. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.B.; Ding, J.; Zheng, S.J.; Yu, Q.W.; Yuan, B.F.; Feng, Y.Q. Magnetic “one-step” quick, easy, cheap, effective, rugged and safe method for the fast determination of pesticide residues in freshly squeezed juice. J. Chromatogr. A 2015, 1398, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Xiu-ping, Z.; Lin, M.; Lan-qi, H.; Jian-Bo, C.; Li, Z. The optimization and establishment of QuEChERS-UPLC–MS/MS method for simultaneously detecting various kinds of pesticides residues in fruits and vegetables. J. Chromatogr. B 2017, 1060, 281–290. [Google Scholar] [CrossRef]
- Li, J.; Li, Y.; Xu, D.; Zhang, J.; Wang, Y.; Luo, C. Determination of metrafenone in vegetables by matrix solid-phase dispersion and HPLC-UV method. Food Chem. 2017, 214, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Abd-Alrahman, S.H.; Almaz, M.M. Dissipation rate of different commercial formulations of propamocarb-hydrochloride applied to potatoes using HPLC–DAD. Arab. J. Chem. 2016, 9, S1402–S1405. [Google Scholar] [CrossRef] [Green Version]

| Structure | Chemical Family | MW (g/mol) | Water Solubility 25 °C (mg/L) | LD50 (mg/kg mouse) | ADI (µg/kg) bw/day | MRL (µg/kg) | Regulation (EC) Nº | ||
|---|---|---|---|---|---|---|---|---|---|
| Insecticides | Chlorpyrifos C9H11Cl3NO3PS |  | Organophosphorus | 350.57 | 2 | 32–1000 | 1 | 50 | 839/2008 |
| Acrinathrin C26H21F6NO5 | 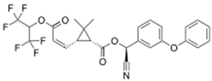 | Pyrethroid | 541.45 | 0.02 | 5000 | 10 | 50 | 839/2008 | |
| Deltamethrin C22H19Br2NO3 | 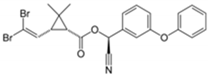 | 505.21 | <0.002 | 5000 | 10 | 300 | 2016/1822 | ||
| λ-Cyhalothrin C23H19ClF3NO3 | 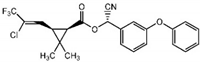 | 449.85 | 0.005 | 56 | 2.5 | 20 | 834/2013 | ||
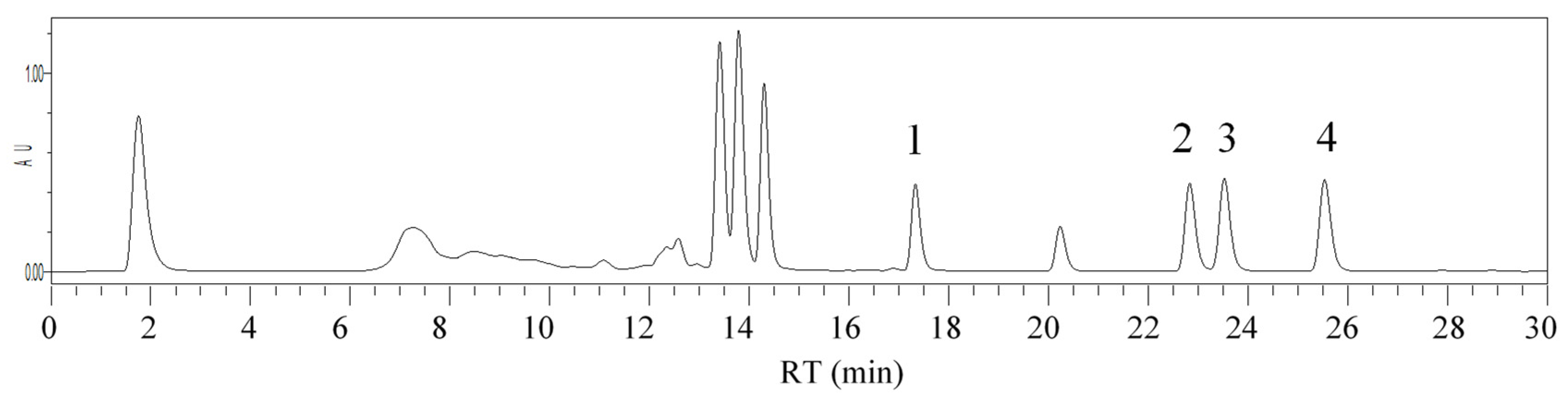
| RT (min) | Insecticides | λmax (nm) | Concentration Range (µg/kg) | Regression Equation | R2 | LOD (µg/kg) | LOQ (µg/kg) | ME (%) |
|---|---|---|---|---|---|---|---|---|
| 17.55 | Chlorpyrifos | 197 | 2.5–50 | y = 980.04x − 92.661 | 0.994 | 0.47 | 1.58 | 93 |
| 23.25 | λ-Cyhalothrin | 190 | 2.5–50 | y = 17,590x − 24,725 | 0.998 | 0.17 | 0.57 | 110 |
| 23.99 | Deltamethrin | 191.4 | 2.5–50 | y = 176,281x − 779,412 | 0.992 | 0.02 | 0.06 | 100 |
| 26.28 | Acrinathrin | 192 | 2.5–50 | y = 1159x + 1021.3 | 0.998 | 0.13 | 0.44 | 86 |
| Insecticides | Concentration Range (µg/kg) | Precision (% RSD) | Accuracy | ||
|---|---|---|---|---|---|
| Theoretical | Experimental | Intraday | Interday | Rec (%) ± SD | |
| Chlorpyrifos | 2.50 | 2.7 | 15.0 | 14.6 | 106 ± 3.46 |
| 25.0 | 23.5 | 7.65 | 2.80 | 94.1 ± 3.82 | |
| 50.0 | 52.1 | 7.62 | 2.10 | 104 ± 4.19 | |
| λ-Cyhalothrin | 2.50 | 2.6 | 12.7 | 7.89 | 105 ± 3.14 |
| 25.0 | 25.7 | 2.87 | 5.99 | 103 ± 4.08 | |
| 50.0 | 49.7 | 0.92 | 1.49 | 99.4 ± 4.56 | |
| Deltamethrin | 2.50 | 2.80 | 10.8 | 13.6 | 112 ± 2.43 |
| 25.0 | 26.7 | 6.72 | 3.38 | 107 ± 3.84 | |
| 50.0 | 49.7 | 0.90 | 1.02 | 99.4 ±3.37 | |
| Acrinathrin | 2.50 | 2.20 | 10.6 | 9.47 | 87.2 ± 4.26 |
| 25.0 | 23.6 | 15.7 | 17.1 | 94.4 ± 1.81 | |
| 50.0 | 51.5 | 5.10 | 3.20 | 103 ± 0.97 | |
| Sample (Amount, g) | Extraction Procedure | Analytical Method | LOD (µg/kg) | LOQ (µg/kg) | Rec (%) | Ref |
|---|---|---|---|---|---|---|
| Vegetables (2) | QuEChERS | GC-MS | - | 2–49.6 | 70–114 | [32] |
| Fruits/Vegetables (10) | QuEChERS-DLLME | GC-MS | 1.0–10 | 5.0–34 | 87–106 | [5] |
| Vegetables (5) | QuEChERS-dSPE | GC-MS/MS | - | <10 | 70–120 | [8] |
| Fruits/Vegetables (5) | HS-SPME | GC-ECD | 0.11–0.23 | 0.37–0.77 | 81–106 | [12] |
| Fruits/Vegetables (10) | QuEChERS-dSPE | LC-MS/MS | 0.1–1 | 0.5–5 | 77–110 | [16] |
| Fruits/Vegetables (10) | QuEChERS-dSPE | UHLC-MS/MS | 0.003–2.0 | 0.01–6.67 | 73–134 | [33] |
| Potatoes (10) | QuEChERS-dSPE | UHLC-MS/MS | 0.4–1.0 | 2.0–5.0 | 81–113 | [1] |
| Fruits (0.5) | UADLLME | HPLC-UV | 1.15–2.46 | 4.38–6.16 * | 83–91 | [31] |
| Fruits/Vegetables (1) | SFE-MSPE | HPLC-UV | - | 100 | 91–99 | [3] |
| Vegetables (2) | MSPD | HPLC-UV | 20 | 70 | 87–105 | [34] |
| Potatoes (10) | QuEChERS-dSPE | HPLC-DAD | 0.9 | 2.7 | 86–90 | [35] |
| Potatoes (1) | QuEChERS-dSPE | UHPLC-PDA | 0.02–0.47 | 0.44–1.58 | 94–112 | This work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reis, D.; Silva, P.; Perestrelo, R.; Câmara, J.S. Residue Analysis of Insecticides in Potatoes by QuEChERS-dSPE/UHPLC-PDA. Foods 2020, 9, 1000. https://doi.org/10.3390/foods9081000
Reis D, Silva P, Perestrelo R, Câmara JS. Residue Analysis of Insecticides in Potatoes by QuEChERS-dSPE/UHPLC-PDA. Foods. 2020; 9(8):1000. https://doi.org/10.3390/foods9081000
Chicago/Turabian StyleReis, Débora, Pedro Silva, Rosa Perestrelo, and José S. Câmara. 2020. "Residue Analysis of Insecticides in Potatoes by QuEChERS-dSPE/UHPLC-PDA" Foods 9, no. 8: 1000. https://doi.org/10.3390/foods9081000





