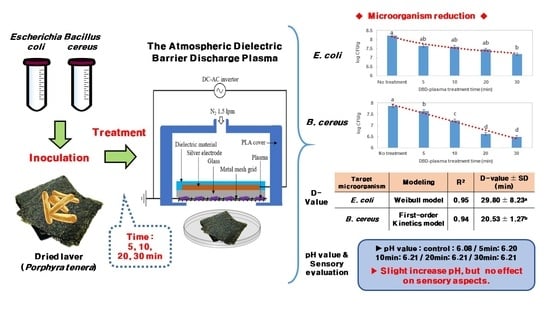
The Efficiency of Atmospheric Dielectric Barrier Discharge Plasma against Escherichia coli and Bacillus cereus on Dried Laver (Porphyra tenera)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strain
2.2. Inoculation of Dried Laver
2.3. Atmospheric DBD Plasma Treatment
2.4. Microbial Enumeration
2.5. Determination of D-Values
2.6. pH Value Analysis
2.7. Sensory Evaluation
2.8. Statistical Analysis
3. Results
3.1. Effects of Atmospheric DBD Plasma on the Reduction of E. coli and B. cereus in Dried Laver
3.2. pH and Sensory Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lee, E.J.; Kim, G.R.; Lee, H.J.; Kwon, J.H. Monitoring microbiological contamination, pre-decontamination, and irradiation status of commercial dried laver (Porphyra sp.) products. Korean J. Food Sci. Technol. 2017, 49, 20–27. [Google Scholar] [CrossRef] [Green Version]
- Cho, S.M.; Kim, B.M.; Han, K.J.; Seo, H.Y.; Han, Y.N.; Yang, E.H.; Kim, D.S. Current status of the domestic processed laver market and manufacturers. Food Sci. Ind. 2009, 42, 57–70. [Google Scholar]
- Choi, E.S.; Kim, N.H.; Lee, S.H.; Rhee, M.S. Seafood and Bacteria. Safe Food 2012, 7, 3–13. [Google Scholar]
- Noh, B.Y.; Hwang, S.H.; Cho, Y.S. Microbial Contamination levels in Porphyra sp. distributed in Korea. Korean J. Fish Aquat. Sci. 2019, 52, 180–184. [Google Scholar]
- Son, K.T.; Lach, T.; Jung, Y.J.; Kang, S.K.; Eom, S.H.; Lee, D.S.; Lee, M.S.; Kim, Y.M. Food hazard analysis during dried-laver processing. Korean J. Fish Aquat. Sci. 2014, 17, 197–201. [Google Scholar] [CrossRef]
- Ryu, Y.H.; Uhm, H.S.; Park, G.S.; Choi, E.H. Sterilization of Neurospora crassa by noncontacted low temperature atmospheric pressure surface discharged plasma with dielectric barrier structure. J. Korean Vac. Soc. 2013, 22, 55–65. [Google Scholar] [CrossRef] [Green Version]
- Pankaj, S.K.; Wan, Z.; Keener, K.M. Effects of cold plasma on food quality: A Review. Foods 2018, 7, 4. [Google Scholar] [CrossRef] [Green Version]
- Mok, C.K.; Lee, T.H. Operational properties and microbial inactivation performance of dielectric barrier discharge plasma treatment system. Food Eng. Progress 2011, 15, 398–403. [Google Scholar]
- Kim, J.E.; Kim, I.H.; Min, S.C. Microbial decontamination of vegetables and spices using cold plasma treatments. Korean J. Food Sci. Technol. 2013, 45, 735–741. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.J.; Song, Y.S.; Park, Y.R.; Ryu, S.M.; Jeon, H.Y.; Eom, S.H. Sterilization of Food-Borne Pathogenic Bacteria by Atmospheric Pressure Dielectric Barrier Discharge Plasma. J. Food Hyg. Saf. 2017, 32, 222–227. [Google Scholar] [CrossRef]
- Choi, M.S.; Kim, J.Y.; Jeon, E.B.; Park, S.Y. Antibacterial activity of dielectric barrier discharge plasma against main food-borne bacteria in suspensions. Korean J. Fish Aquat. Sci. 2019, 52, 617–624. [Google Scholar]
- Kim, K.Y.; Paik, N.W.; Kim, Y.H.; Yoo, K.H. Bactericidal efficacy of non-thermal DBD plasma on Staphylococcus aureus and Escherichia coli. J. Korean Soc. Occup. Environ. Hyg. 2018, 28, 61–79. [Google Scholar]
- Buzrul, S.; Alpas, H. Modeling inactivation kinetics of food borne pathogens at a constant temperature. LWT 2007, 40, 632–637. [Google Scholar] [CrossRef]
- Kwon, K.O. Microbial Zazard Analysis and Microbial Changes in Processing of Dried-Laver Manufacturing Facilities; Department of Food Science and Technology, Graduate School, Pukyong National University: Busan, Korea, 2018. [Google Scholar]
- Korea Agricultural Trade Information (KATI). Current State of Exports and Imports of Laver in the Last 5 Years in Korea. 2019. Available online: http://www.kati.net/product/basisInfo.do?lcdCode=MD178 (accessed on 12 May 2020).
- Korea Fish Information (KFI). Trade Statistics of Korean Laver. 2020. Available online: http://www.kfishinfo.com/info/view.do?no=450 (accessed on 12 May 2020).
- Kwon, K.; Ryu, D.G.; Jeong, M.C.; Kang, E.H.; Jang, Y.M.; Kwon, J.Y.; Kim, J.M.; Shin, I.S.; Kim, Y.M. Analysis of Microbial Contaminants and Microbial Changes during Dried-laver Pyropia spp. Processing. Korean J. Fish Aquat. Sci. 2018, 51, 8–14. [Google Scholar]
- Han, A. Effects of Microbial Reduction in Dried Laver and its Processing by Sterilizer and UV Irradiation; Department of Food Engineering, Graduate School, Mokpo National University: Mokpo, Korea, 2017. [Google Scholar]
- Kim, Y.J.; Oh, S.U.; Kim, M.J.; Kim, J.H.; Goh, J.B.; Choi, I.Y.; Park, M.K. Identification of electron beam-resistant bacteria in the microbial reduction of dried laver (Porphyra tenera) subjected to electron beam treatment. Korean J. Food Preserv. 2016, 23, 139–143. [Google Scholar] [CrossRef]
- Kim, A.J.; Shin, J.K. Nonthermal Sterilization of Dried Laver by Intense Pulsed Light with Batch System. Korean J. Food. Sci. Technol. 2014, 46, 778–781. [Google Scholar] [CrossRef] [Green Version]
- Wan, J.; Coventry, J.; Swiergon, P.; Sanguansri, P.; Versteeg, C. Advances in innovative processing technologies for microbial inactivation and enhancement of food safety-pulsed electric field and low-temperature plasma. Int. J. Food Sci. Technol. 2009, 20, 414–424. [Google Scholar] [CrossRef]
- Ahlfeld, B.; Li, Y.; Boulaaba, A.; Binder, A.; Schotte, U.; Zimmermann, J.L.; Morfill, G.; Klein, G. Inactivation of a foodborne norovirus outbreak strain with nonthermal atmospheric pressure plasma. mBio 2015, 6, 25587014. [Google Scholar] [CrossRef] [Green Version]
- Smet, C.; Baka, M.; Dickenson, A.; Walsh, J.L.; Valdramidis, V.P.; Van Impe, J.F. Antimicrobial efficacy of cold atmospheric plasma for different intrinsic and extrinsic parameters. Plasma Process Polym. 2017, 15, 1700048. [Google Scholar] [CrossRef]
- Choi, M.S.; Jeon, E.B.; Kim, J.Y.; Choi, E.H.; Lim, J.S.; Choi, J.S.; Park, S. Impact of non-thermal dielectric barrier discharge plasma on Staphylococcus aureus and Bacillus cereus quality of dried blackmouth angler (Lophiomus setigerus). J. Food Eng. 2020, 278, 109952. [Google Scholar] [CrossRef]
- Kim, J.W.; Puligundla, P.; Mok, C.K. Dielectric barrier discharge plasma for microbial decontamination of dried laver: Effects on physicochemical characteristics. Int. J. Food Sci. Technol. 2015, 50, 2630–2638. [Google Scholar] [CrossRef]
- Kim, H.J.; Woo, K.S.; Jo, C.; Lee, S.K.; Park, H.Y.; Sim, E.Y.; Won, Y.J.; Lee, S.B.; Oh, S.K. Effect of atmospheric pressure plasma on the quality of commercially available sunsik. J. Food Hyg. Saf. 2016, 31, 375–379. [Google Scholar] [CrossRef]
- Muhammad, A.I.; Li, Y.; Liao, X.; Liu, D.; Ye, X.; Chen, S.; Hu, Y.; Wang, J.; Ding, T. Effect of dielectric barrier discharge plasma on background microflora and physicochemical properties of tiger nut milk. Food Control. 2019, 96, 119–127. [Google Scholar] [CrossRef]
- Park, S.Y.; Kang, S.J.; Ha, S.D. Inactivation of murine norovirus-1 in the edible seaweeds Capsosiphon fulvescens and Hizikia fusiforme using gamma radiation. Food Microbiol. 2016, 56, 80–86. [Google Scholar] [CrossRef]
- Huang, K.; Yu, L.; Wang, W.; Gai, L.; Wang, J. Comparing the pulsed electric field resistance of the microorganisms in grape juice: Application of the Weibull model. Food Control. 2014, 35, 241–251. [Google Scholar] [CrossRef]
- Park, S.Y.; Ha, S.D. Application of cold oxygen plasma for the reduction of cladosporium cladosporioides and penicillium citrinum on the surface of dried filefish (Stephanolepis cirrhifer) fillets. Int. J. Food Sci. Technol. 2015, 50, 966–973. [Google Scholar] [CrossRef]
- Park, S.Y.; Ha, S.D. Influence of NaCl on the inactivation of murine norovirus-1 and hepatitis A virus in the Korean traditional salted oyster product “Eoriguljeot” during storage. Food Res. Int. 2014, 62, 382–387. [Google Scholar] [CrossRef]
- Bunz, O.; Mese, K.; Zhang, W.; Piwowarczyk, A.; Ehrhardt, A. Effect of cold atmospheric plasma (CAP) on human adenoviruses is adenovirus type dependent. PLoS ONE. 2018, 13, 0202352. [Google Scholar] [CrossRef] [PubMed] [Green Version]

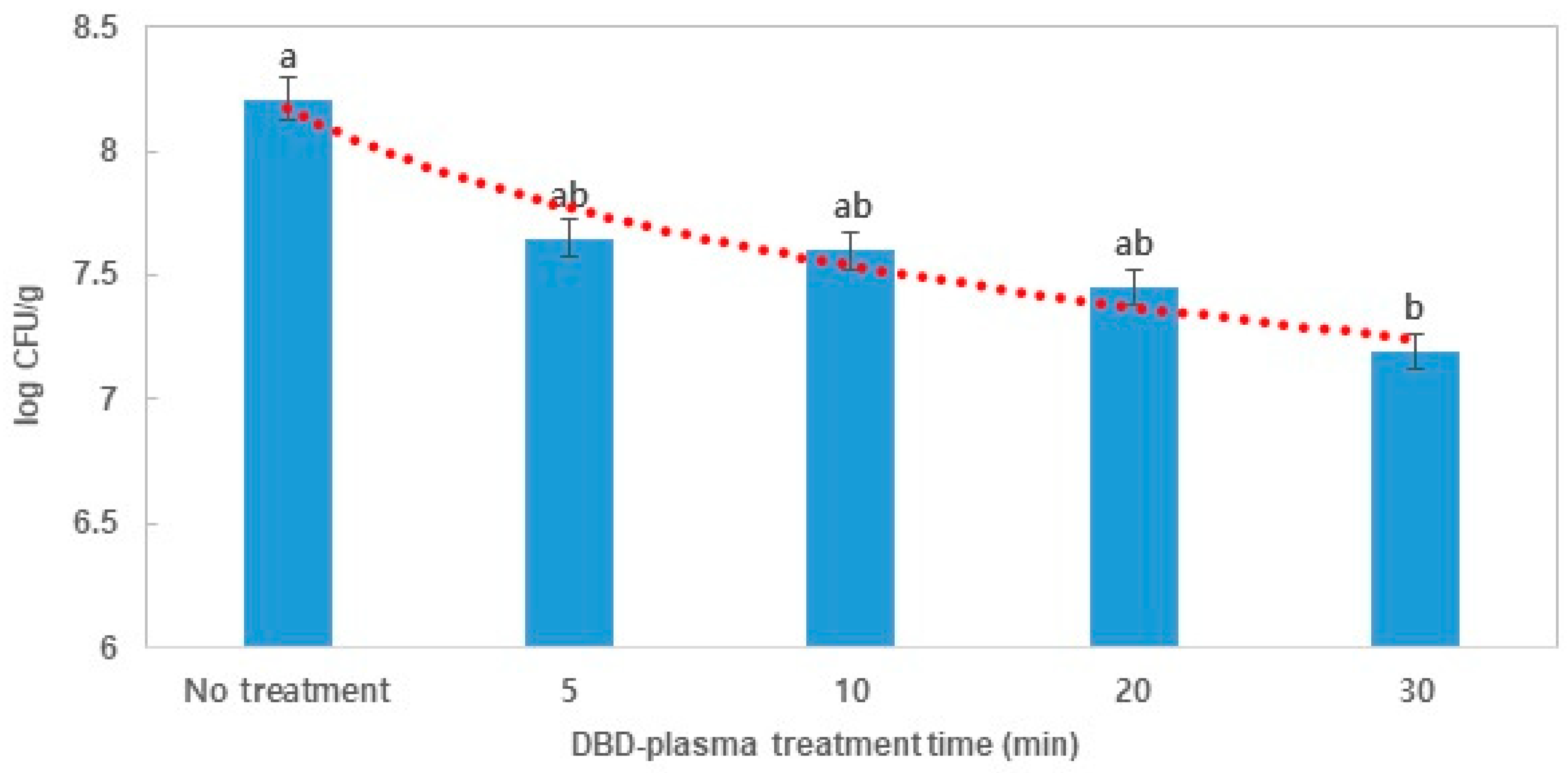

| Target Microorganism | Model Parameters | R2 | D-Value ± SD (min) | |
|---|---|---|---|---|
| E. coli | b ± SD | 0.316 ± 0.059 | 0.95 | 29.80 ± 8.23 a |
| n ± SD | 0.338 ± 0.064 | |||
| B. cereus | Decay slope | 0.049 ± 0.003 | 0.94 | 20.53 ± 1.27 b |
| Treatment Time (min) | pH |
|---|---|
| Control | 6.08 ± 0.02 b |
| 5 | 6.20 ± 0.01 a |
| 10 | 6.21 ± 0.01 a |
| 20 | 6.21 ± 0.03 a |
| 30 | 6.21 ± 0.01 a |
| Treatment Time (min) | Sensory Evaluation | |||
|---|---|---|---|---|
| Color | Flavor | Smell | Overall Acceptability | |
| Control | 6.67 ± 0.52 a | 6.25 ± 0.42 a | 6.33 ± 0.52 a | 6.83 ± 0.41 a |
| 5 | 6.50 ± 0.55 a | 6.17 ± 0.41 a | 6.25 ± 0.42 a | 6.75 ± 0.42 a |
| 10 | 6.33 ± 0.52 a | 6.08 ± 0.20 a | 6.17 ± 0.75 a | 6.58 ± 0.49 a |
| 20 | 6.25 ± 0.42 a | 6.00 ± 0.55 a | 6.25 ± 0.42 a | 6.58 ± 0.49 a |
| 30 | 6.17 ± 0.41 a | 6.08 ± 0.20 a | 6.08 ± 0.20 a | 6.50 ± 0.45 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.Y.; Jeon, E.B.; Choi, M.-S.; Choi, E.H.; Lim, J.S.; Choi, J.; Park, S.Y. The Efficiency of Atmospheric Dielectric Barrier Discharge Plasma against Escherichia coli and Bacillus cereus on Dried Laver (Porphyra tenera). Foods 2020, 9, 1013. https://doi.org/10.3390/foods9081013
Kim JY, Jeon EB, Choi M-S, Choi EH, Lim JS, Choi J, Park SY. The Efficiency of Atmospheric Dielectric Barrier Discharge Plasma against Escherichia coli and Bacillus cereus on Dried Laver (Porphyra tenera). Foods. 2020; 9(8):1013. https://doi.org/10.3390/foods9081013
Chicago/Turabian StyleKim, Ji Yoon, Eun Bi Jeon, Man-Seok Choi, Eun Ha Choi, Jun Sup Lim, Jinsung Choi, and Shin Young Park. 2020. "The Efficiency of Atmospheric Dielectric Barrier Discharge Plasma against Escherichia coli and Bacillus cereus on Dried Laver (Porphyra tenera)" Foods 9, no. 8: 1013. https://doi.org/10.3390/foods9081013