An Absorption and Plasma Kinetics Study of Monoterpenes Present in Mastiha Oil in Humans
Abstract
:1. Introduction
2. Experimental methods
2.1. Materials
2.2. Ethics
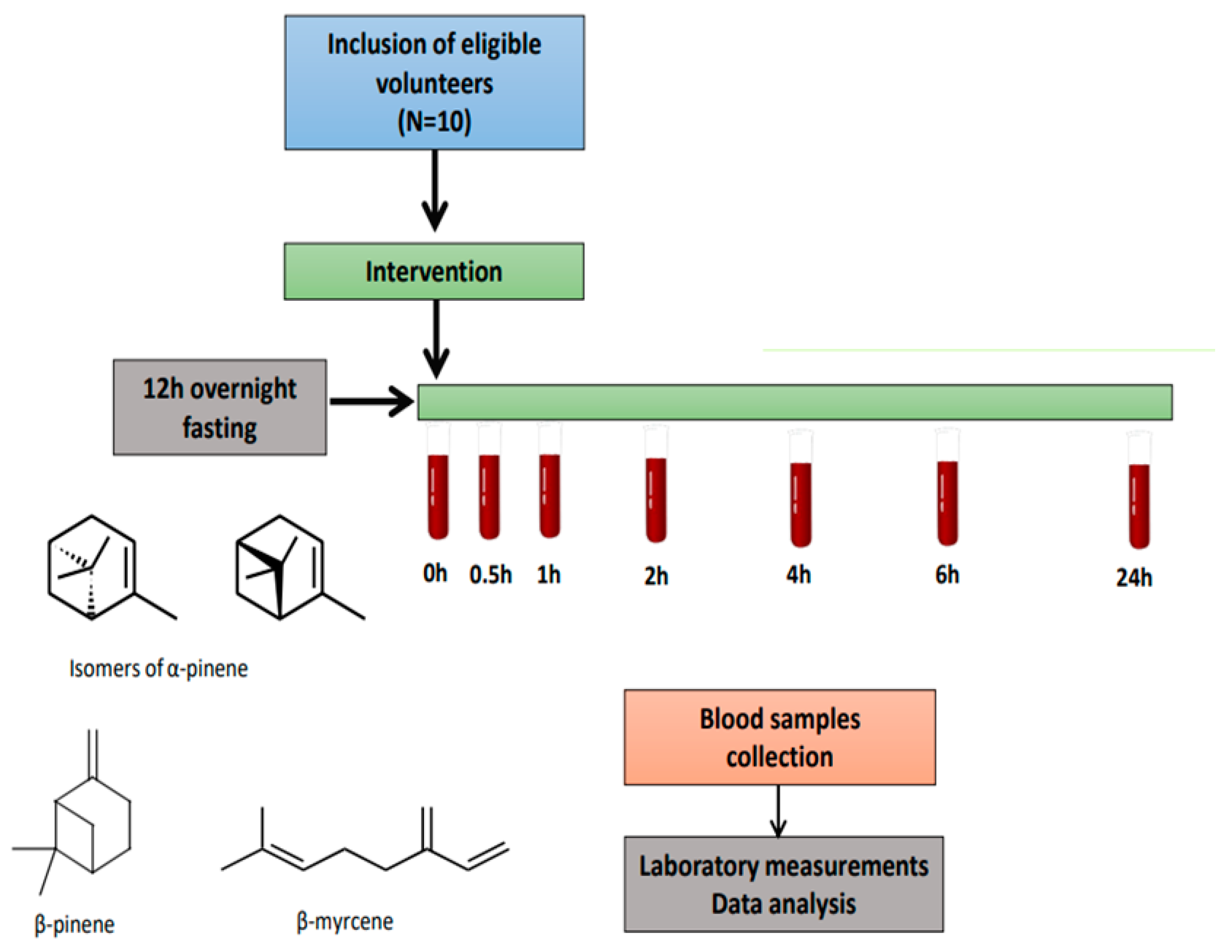
2.3. Study Design
2.4. Postprandial Study Protocol
2.4.1. Baseline Assessment
2.4.2. Intervention
2.5. Analytical Techniques and Assays
2.5.1. Detection and Quantification of Monoterpenes in Plasma Samples
2.5.2. Kinetics of Monoterpenes in Plasma
2.5.3. Antioxidant Capacity
2.6. Statistics
3. Results
3.1. Profiling of MO
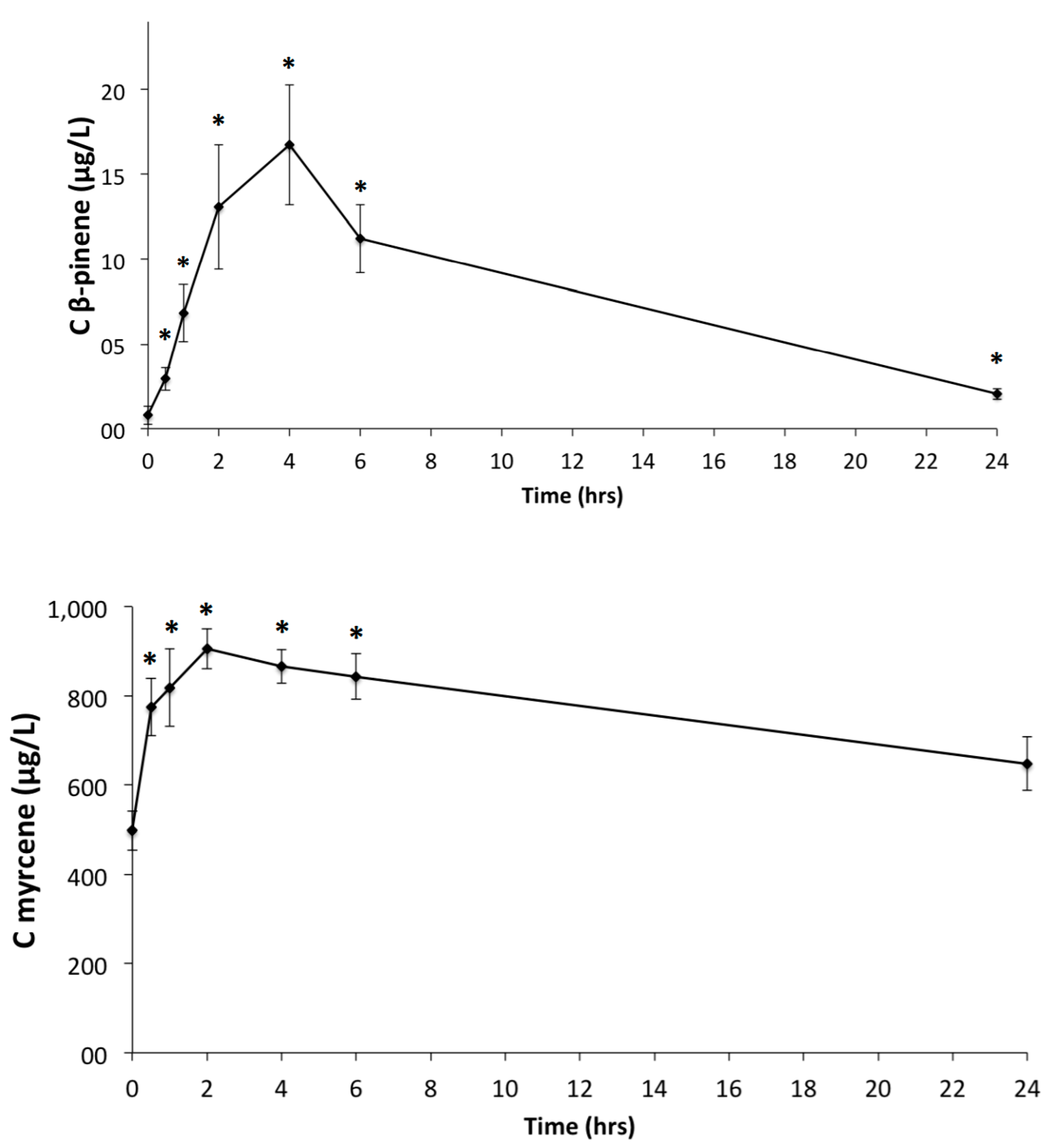
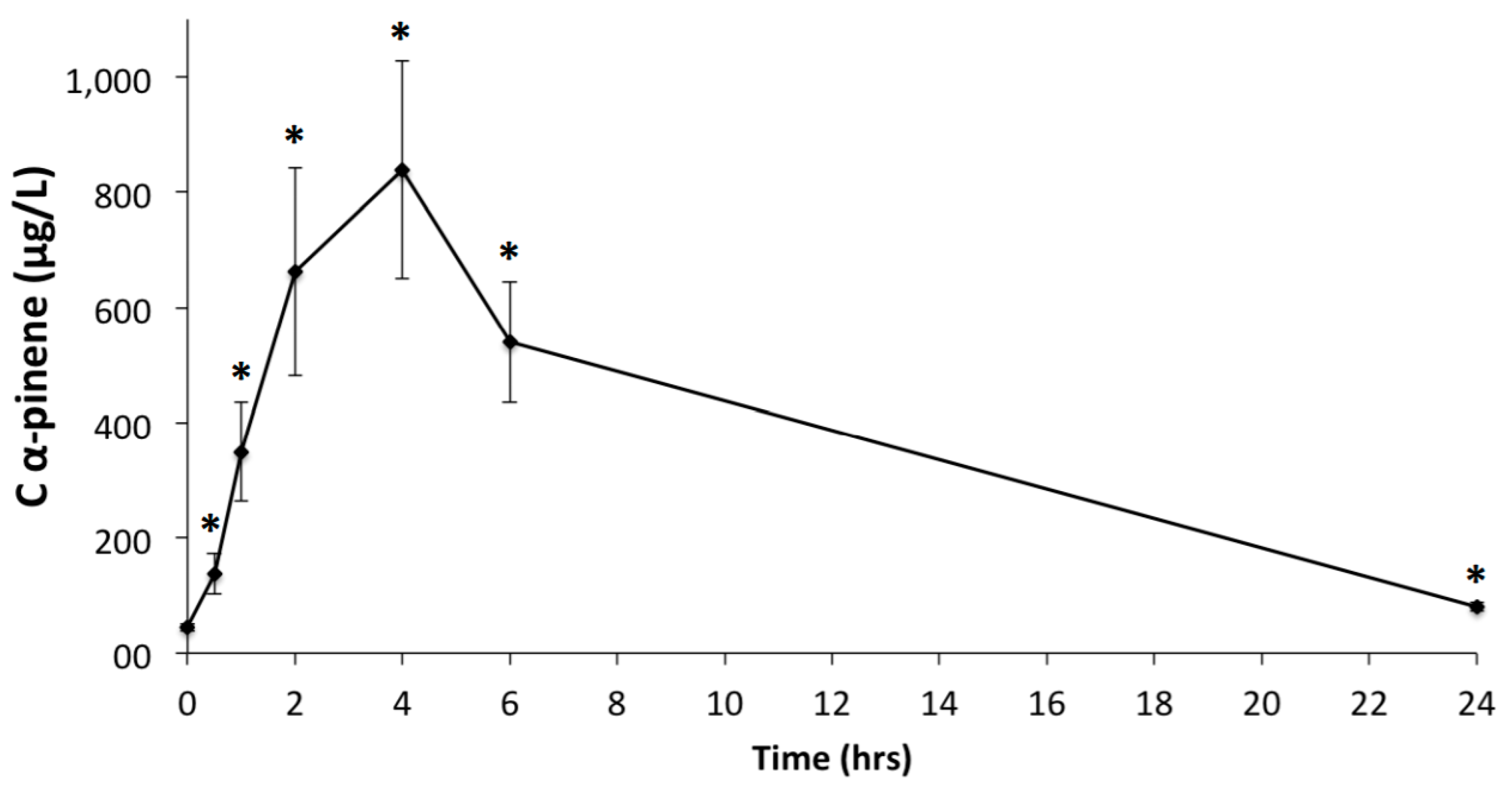
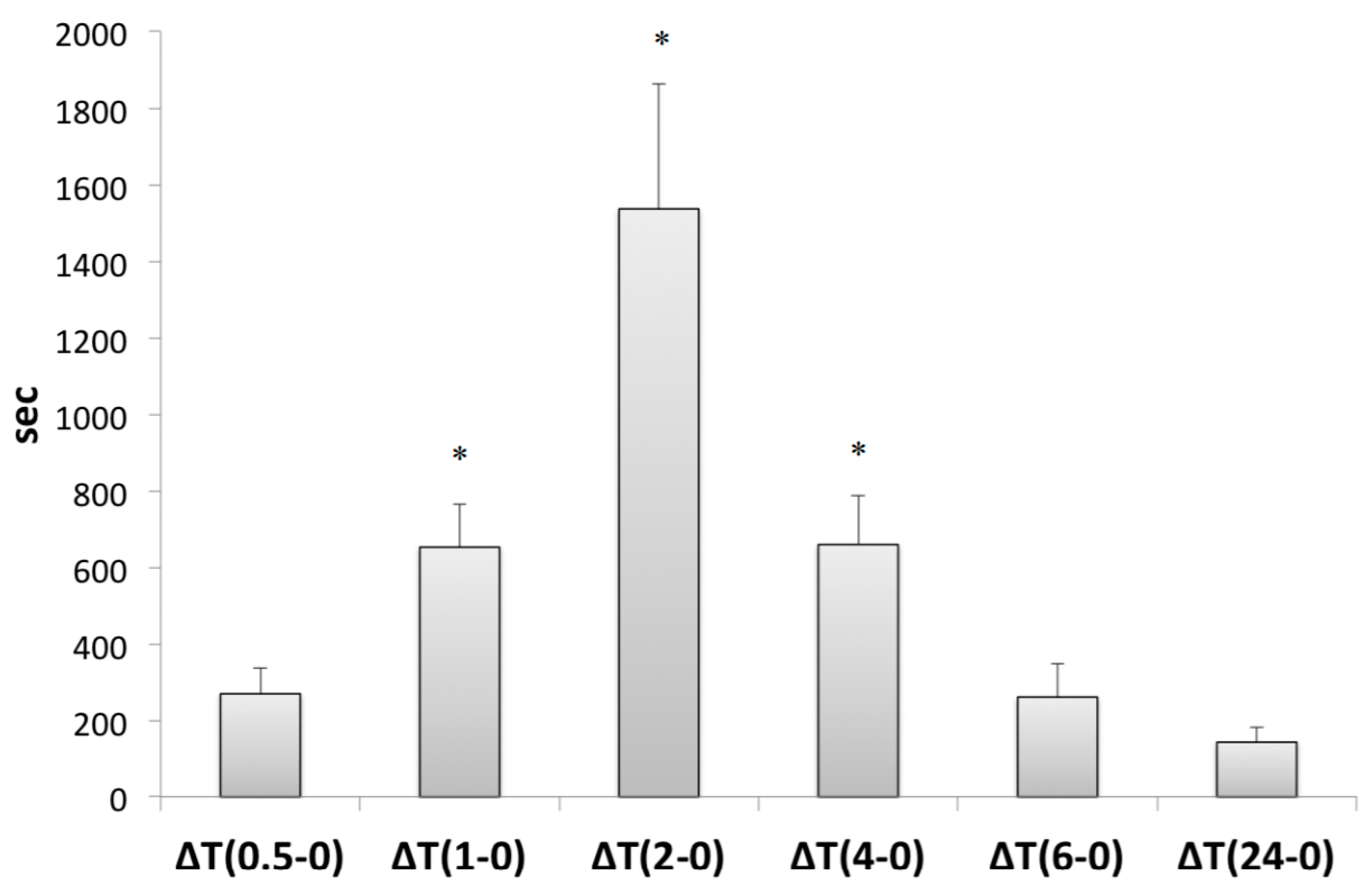
3.2. GC-MS-MS Analysis of Plasma Samples
3.3. Antioxidant Capacity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Diederich, M. Natural products target the hallmarks of chronic diseases. Biochem. Pharmacol. 2020, 173, 113828. [Google Scholar] [CrossRef] [PubMed]
- Paraschos, S.; Magiatis, P.; Mitakou, S.; Petraki, K.; Kalliaropoulos, A.; Maragkoudakis, P.; Mentis, A.; Sgouras, D.; Skaltsounis, A.-L. In Vitro and In Vivo Activities of Chios Mastic Gum Extracts and Constituents against Helicobacter pylori. Antimicrob. Agents Chemother. 2006, 51, 551–559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Assimopoulou, A.N.; Papageorgiou, V.P. GC-MS analysis of penta- and tetra-cyclic triterpenes from resins of Pistacia species. Part I. Pistacia lentiscus var. Chia. Biomed. Chromatogr. 2005, 19, 285–311. [Google Scholar] [CrossRef] [PubMed]
- Kaliora, A.C.; Mylona, A.; Chiou, A.; Petsios, D.G.; Andrikopoulos, N.K. Detection and Identification of Simple Phenolics in Pistacia lentiscus Resin. J. Liq. Chromatogr. Relat. Technol. 2004, 27, 289–300. [Google Scholar] [CrossRef]
- Boelens, M.H.; Jiménez, R. Chemical composition of the essential oils from the gum and from various parts of Pistacia lentiscus l. (mastic gum tree). Flavour Fragr. J. 1991, 6, 271–275. [Google Scholar] [CrossRef]
- Subramaniyan, S.D.; AshokKumar, N. Citral, A Monoterpene Protect Against High Glucose Induced Oxidative Injury in HepG2 Cell In Vitro-An Experimental Study. J. Clin. Diagn. Res. 2017, 11, BC10–BC15. [Google Scholar] [CrossRef]
- Kodikonda, M.; Naik, P.R. Modulatory effect of garcinol in streptozotocin-induced diabetic Wistar rats. Arch. Physiol. Biochem. 2017, 123, 322–329. [Google Scholar] [CrossRef]
- Salehi, B.; Upadhyay, S.; Orhan, I.E.; Jugran, A.K.; Jayaweera, S.L.D.; Dias, D.A.; Sharopov, F.; Taheri, Y.; Martins, N.; Baghalpour, N.; et al. Therapeutic Potential of α- and β-Pinene: A Miracle Gift of Nature. Biomolecules 2019, 9, 738. [Google Scholar] [CrossRef] [Green Version]
- Sieniawska, E.; Swatko-Ossor, M.; Sawicki, R.; Skalicka-Woźniak, K.; Ginalska, G. Natural Terpenes Influence the Activity of Antibiotics against Isolated Mycobacterium tuberculosis. Med. Princ. Prac. 2016, 26, 108–112. [Google Scholar] [CrossRef]
- Matsuo, A.L.; Figueiredo, C.R.; Arruda, D.C.; Pereira, F.V.; Scutti, J.A.B.; Massaoka, M.H.; Travassos, L.R.; Sartorelli, P.; Lago, J.H.G. α-Pinene isolated from Schinus terebinthifolius Raddi (Anacardiaceae) induces apoptosis and confers antimetastatic protection in a melanoma model. Biochem. Biophys. Res. Commun. 2011, 411, 449–454. [Google Scholar] [CrossRef] [Green Version]
- Papada, E.; Gioxari, A.; Brieudes, V.; Amerikanou, C.; Halabalaki, M.; Skaltsounis, L.; Smyrnioudis, I.; Kaliora, A. Bioavailability of Terpenes and Postprandial Effect on Human Antioxidant Potential. An Open-Label Study in Healthy Subjects. Mol. Nutr. Food Res. 2017, 62, 1700751. [Google Scholar] [CrossRef] [PubMed]
- Aurrekoetxea, I.; Ruiz-Sanz, J.I.; Del Agua, A.R.; Navarro, R.; Hernandez, M.L.; Matorras, R.; Prieto, B.; Ruiz-Larrea, M.B. Serum oxidizability and antioxidant status in patients undergoing in vitro fertilization. Fertil. Steril. 2010, 94, 1279–1286. [Google Scholar] [CrossRef] [PubMed]
- Esterbauer, H.; Jiirgens, G. Mechanistic and genetic aspects of susceptibility of LDL to oxidation. Curr. Opin. Lipidol. 1993, 4, 114–124. [Google Scholar] [CrossRef]
- Lewis, C.A.; Palanker, A.L. Acute Toxicity Studies in Rats and Rabbits; Unpublished report; Research Institute of Fragrance Materials: Englewood Cliffs, NJ, USA, 1979. [Google Scholar]
- Li, W.; Hong, B.; Li, Z.; Li, Q.; Bi, K. GC-MS method for determination and pharmacokinetic study of seven volatile constituents in rat plasma after oral administration of the essential oil of Rhizoma Curcumae. J. Pharm. Biomed. Anal. 2018, 149, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Thao, N.T.; Kashiwagi, T.; Sawamura, M. Characterization by GC-MS of Vietnamese Citrus Species and Hybrids Based on the Isotope Ratio of Monoterpene Hydrocarbons. Biosci. Biotechnol. Biochem. 2007, 71, 2155–2161. [Google Scholar] [CrossRef] [Green Version]
- Furtado, N.A.J.C.; Pirson, L.; Edelberg, H.; Miranda, L.M.; Loira-Pastoriza, C.; Préat, V.; Larondelle, Y.; Andre, C.M. Pentacyclic Triterpene Bioavailability: An Overview of In Vitro and In Vivo Studies. Molecules 2017, 22, 400. [Google Scholar] [CrossRef] [Green Version]
- Kanellos, P.T.; Kaliora, A.; Gioxari, A.; Christopoulou, G.O.; Kalogeropoulos, N.; Karathanos, V.T. Absorption and Bioavailability of Antioxidant Phytochemicals and Increase of Serum Oxidation Resistance in Healthy Subjects Following Supplementation with Raisins. Plant Foods Hum. Nutr. 2013, 68, 411–415. [Google Scholar] [CrossRef]
- González-Burgos, E.; Gómez-Serranillos, M.P. Terpene compounds in nature: A review of their potential antioxidant activity. Curr. Med. Chem. 2012, 19, 5319–5341. [Google Scholar] [CrossRef]
- Oboh, G.; Ademosun, A.; Odubanjo, O.V.; Akinbola, I.A. Antioxidative Properties and Inhibition of Key Enzymes Relevant to Type-2 Diabetes and Hypertension by Essential Oils from Black Pepper. Adv. Pharmacol. Sci. 2013, 926047. [Google Scholar] [CrossRef]
- Domínguez-Perles, R.; Auñón, D.; Ferreres, F.; Gil-Izquierdo, A. Gender differences in plasma and urine metabolites from Sprague-Dawley rats after oral administration of normal and high doses of hydroxytyrosol, hydroxytyrosol acetate, and DOPAC. Eur. J. Nutr. 2015, 56, 215–224. [Google Scholar] [CrossRef]
- García-Villalba, R.; Larrosa, M.; Possemiers, S.; Tomás-Barberán, F.A.; Espín, J.C. Bioavailability of phenolics from an oleuropein-rich olive (Olea europaea) leaf extract and its acute effect on plasma antioxidant status: Comparison between pre- and postmenopausal women. Eur. J. Nutr. 2013, 53, 1015–1027. [Google Scholar] [CrossRef] [PubMed]
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Sex: Male | Obesity |
| Age: 20–40 years old | Alcohol or drug abuse |
| Medication, vitamins and inorganic supplements | |
| Vegan and macrobiotic diet before and during the trial | |
| Gastrointestinal diseases (i.e., atrophic gastritis, Inflammatory Bowel Disease, peptic ulcer, gastrointestinal cancer) |
| Anthropometric | |
|---|---|
| Age (year) | 25.8 ± 2.4 |
| Height (cm) | 178.0 ± 1.7 |
| Weight (kg) | 85.2 ±3.7 |
| BMI (kg/m2) | 26.9 ± 1.2 |
| Body fat (%) | 19.9 ± 2.9 |
| Total Body Water (%) | 57.1 ± 2.0 |
| Muscle mass (kg) | 64.2 ± 1.4 |
| Bone mass (kg) | 3.3 ± 0.0 |
| Biochemical | |
| Red Blood Cells (/μL) | 5,256,250.0 ± 111,624.9 |
| White Blood Cells (/μL) | 6923.8 ± 646.6 |
| Hemoglobin (g/dL) | 16.1 ± 0.4 |
| Hematocrit (g/dL) | 46.1 ± 1.0 |
| Mean Corpuscular Volume (fl) | 87.8 ± 1.1 |
| Mean Corpuscular Hemoglobin Concentration (g/dL) | 35.0 ± 0.3 |
| Mean Corpuscular Hemoglobin (pg/RBC) | 30.7 ± 0.3 |
| Glucose (mg/dL) | 89.5 ± 3.4 |
| Total Cholesterol (mg/dL) | 182.4 ± 11.6 |
| Triglycerides (mg/dL) | 86.7 ± 16.4 |
| High-Density Lipoprotein (mg/dL) | 45.3 ± 2.5 |
| Low-Density Lipoprotein (mg/dL) | 111.7 ± 7.4 |
| High Risk Rate | 4.0 ± 0.4 |
| Terpenes | Cmax (μg/L) | Tmax (h) | Area Under Curve (μg·h/L) |
|---|---|---|---|
| Myrcene | 966.6 ± 89.7 | 2.2 ± 1.7 | 15318.0 ± 7313.3 |
| α-Pinene | 914.8 ± 551.2 | 3.8 ± 1.2 | 7865.2 ± 5547.3 |
| β-Pinene | 18.0 ± 10.7 | 3.6 ± 0.9 | 164.0 ± 110.8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papada, E.; Gioxari, A.; Amerikanou, C.; Galanis, N.; Kaliora, A.C. An Absorption and Plasma Kinetics Study of Monoterpenes Present in Mastiha Oil in Humans. Foods 2020, 9, 1019. https://doi.org/10.3390/foods9081019
Papada E, Gioxari A, Amerikanou C, Galanis N, Kaliora AC. An Absorption and Plasma Kinetics Study of Monoterpenes Present in Mastiha Oil in Humans. Foods. 2020; 9(8):1019. https://doi.org/10.3390/foods9081019
Chicago/Turabian StylePapada, Efstathia, Aristea Gioxari, Charalampia Amerikanou, Nikolaos Galanis, and Andriana C. Kaliora. 2020. "An Absorption and Plasma Kinetics Study of Monoterpenes Present in Mastiha Oil in Humans" Foods 9, no. 8: 1019. https://doi.org/10.3390/foods9081019
APA StylePapada, E., Gioxari, A., Amerikanou, C., Galanis, N., & Kaliora, A. C. (2020). An Absorption and Plasma Kinetics Study of Monoterpenes Present in Mastiha Oil in Humans. Foods, 9(8), 1019. https://doi.org/10.3390/foods9081019








