High-Value-Added Compound Recovery with High-Temperature Hydrothermal Treatment and Steam Explosion, and Subsequent Biomethanization of Residual Strawberry Extrudate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Residual Strawberry Extrudate
2.2. Hydrothermal Treatment Systems and Separation of Liquid and Solid Phases
2.3. Extraction of Phenolic Compounds
2.4. Anaerobic Digestion Experimental Procedure
2.5. Kinetic Study
2.6. Chemical Analyses
3. Results and Discussion
3.1. Effect of Hydrothermal Treatments on the Substrate Characteristics
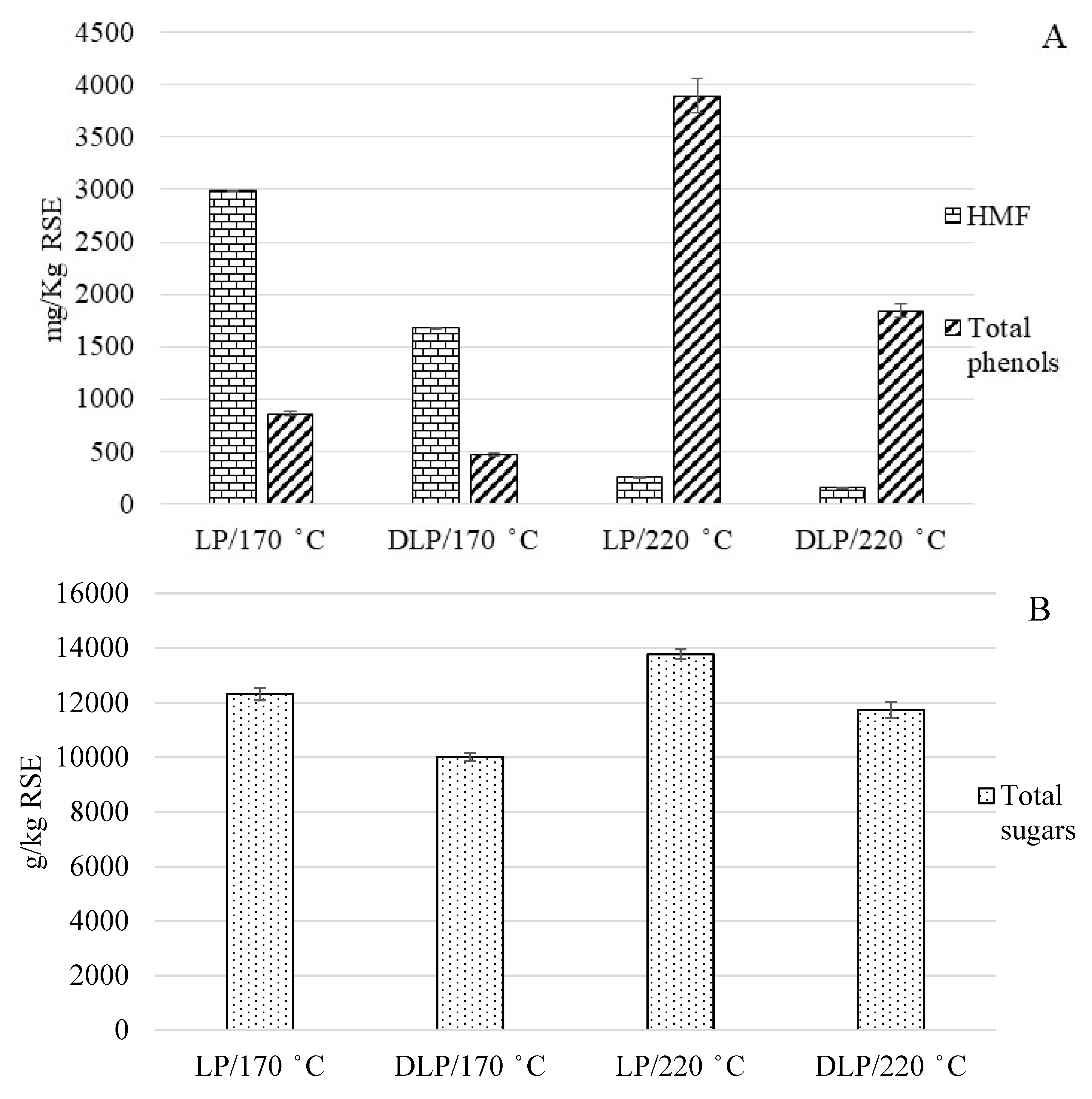
3.2. Effect of the Extraction of Phenolic Compounds in the Liquid Phase
3.3. Anaerobic Digestibility Study after the Application of Hydrothermal Treatments at High Temperatures and Subsequent Extraction of Phenolic Compounds
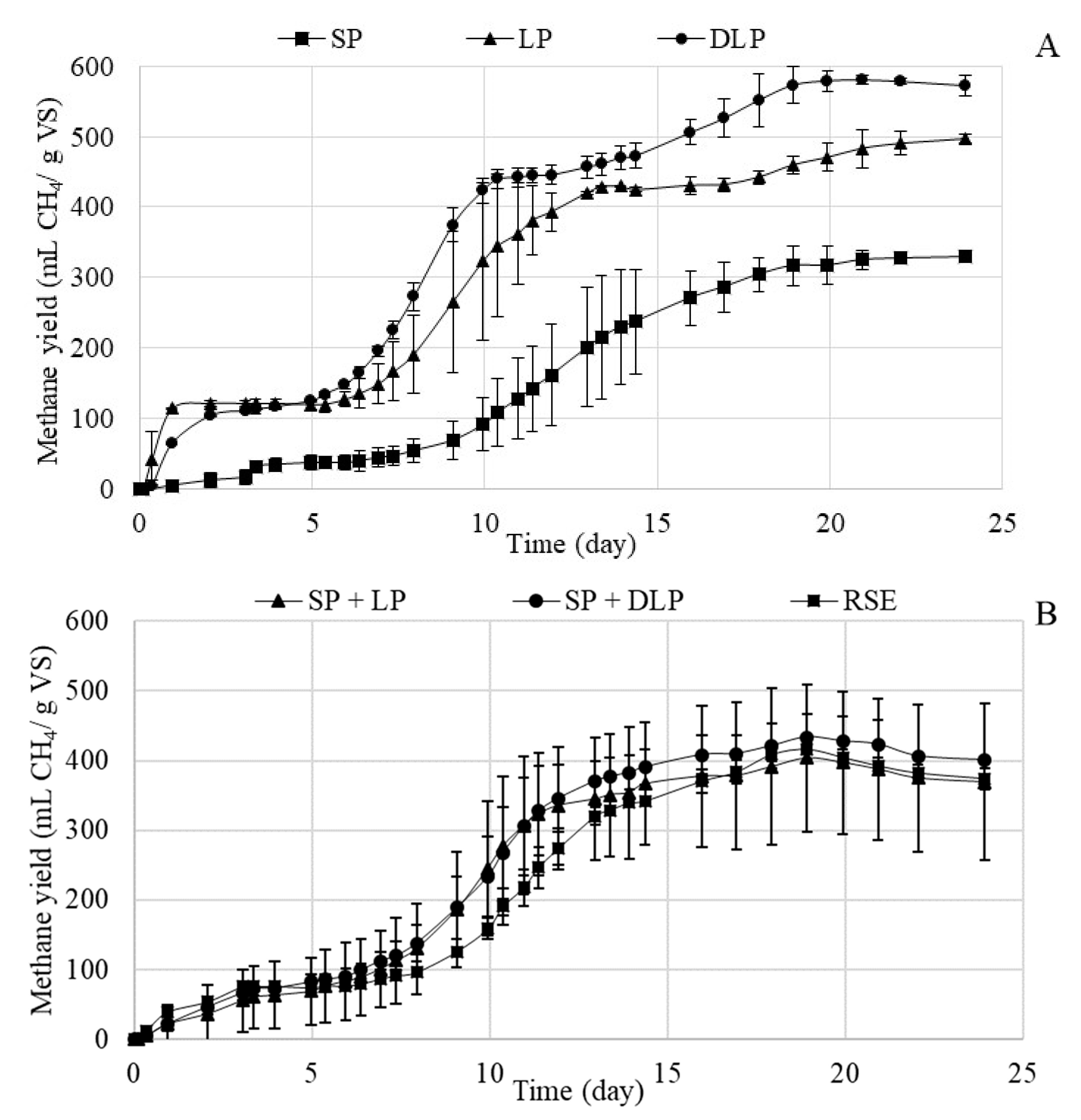
3.3.1. Methane Potential and Kinetic Study of the Anaerobic Process after Treatment at 170 °C
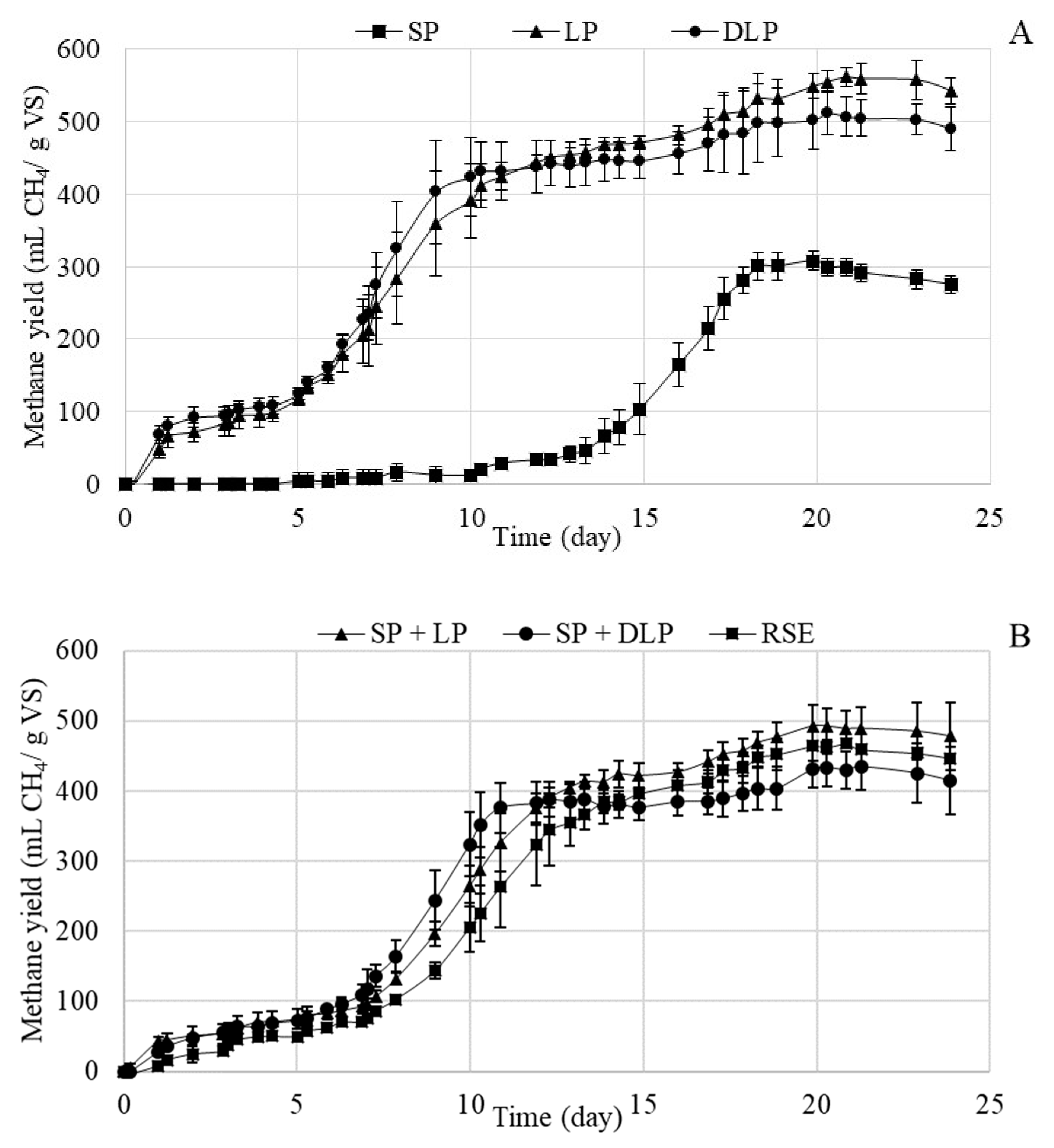
3.3.2. Methane Potential and Kinetic Study of the Anaerobic Digestion Process after Hydrotreatment at 220 °C
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
Appendix A.1. Hydrothermal Treatment Systems and Separation of Liquid and Solid Phases
Appendix A.2. Extraction of Phenolic Compounds
Appendix A.3. Anaerobic Digestion—Experimental Procedure
Appendix A.4. Kinetic Study
Appendix A.5. Chemical Analyses
References
- FAO. FAOSTAT: Food and Agriculture Organization of the United Nations; FAO: Rome, Italy, 2019. [Google Scholar]
- Gagneten, M.; Corfield, R.; Mattson, M.G.; Sozzi, A.; Leiva, G.; Salvatori, D.; Schebor, C. Spray-dried powders from berries extracts obtained upon several processing steps to improve the bioactive components content. Powder Technol. 2019, 342, 1008–1015. [Google Scholar] [CrossRef] [Green Version]
- Aaby, K.; Ekeberg, D.; Skrede, G. Characterization of phenolic compounds in strawberry (Fragaria x ananassa) fruits by different HPLC detectors and contribution of individual compounds to total antioxidant capacity. J. Agric. Food Chem. 2007, 55, 4395–4406. [Google Scholar] [CrossRef] [PubMed]
- Giampieri, F.; Tulipani, S.; Alvarez-Suarez, J.M.; Quiles, J.L.; Mezzetti, B.; Battino, M. The strawberry: Composition, nutritional quality, and impact on human health. Nutrition 2012, 28, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Ariza, M.T.; Reboredo-Rodríguez, P.; Mazzoni, L.; Forbes-Hernández, T.Y.; Giampieri, F.; Afrin, S.; Gasparrini, M.; Soria, C.; Martínez-Ferri, E.; Battino, M.; et al. Strawberry achenes are an important source of bioactive compounds for human health. Int. J. Mol. Sci. 2016, 17, 1103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, X.; Zhang, Y.; Zhang, F.; Wang, Y.; Yi, J.; Liao, X. Effects of high hydrostatic pressure on enzymes, phenolic compounds, anthocyanins, polymeric color and color of strawberry pulps. J. Sci. Food Agric. 2011, 91, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Turk, M.; Perino, S.; Cendres, A.; Petitcolas, E.; Soubrat, T.; Chemat, F. Alternative process for strawberry juice processing: Microwave hydrodiffusion and gravity. Lwt Food Sci. Technol. 2017, 84, 626–633. [Google Scholar] [CrossRef]
- Odriozola-Serrano, I.; Soliva-Fortuny, R.; Martín-Belloso, O. Phenolic acids, flavonoids, vitamin C and antioxidant capacity of strawberry juices processed by high-intensity pulsed electric fields or heat treatments. Eur. Food Res. Technol. 2008, 228, 239–248. [Google Scholar] [CrossRef]
- Odriozola-Serrano, I.; Soliva-Fortuny, R.; Martín-Belloso, O. Impact of high-intensity pulsed electric fields variables on vitamin C, anthocyanins and antioxidant capacity of strawberry juice. Lwt Food Sci. Technol. 2009, 42, 93–100. [Google Scholar] [CrossRef]
- Kajdžanoska, M.; Petreska, J.; Stefova, M. Comparison of different extraction solvent mixtures for characterization of phenolic compounds in strawberries. J. Agric. Food Chem. 2011, 59, 5272–5278. [Google Scholar] [CrossRef]
- Rodríguez-Gutiérrez, G.; Cardoso, J.C.J.C.; Rubio-Senent, F.; Serrano, A.; Borja, R.; Fernández-Bolaños, J.; Fermoso, F.G.F.G. Thermally-treated strawberry extrudate: A rich source of antioxidant phenols and sugars. Innov. Food Sci. Emerg. Technol. 2019, 51, 186–193. [Google Scholar] [CrossRef] [Green Version]
- Garrote, G.; Domínguez, H.; Parajó, J.C. Hydrothermal processing of lignocellulosic materials. Holz Als Roh Und Werkst. 1999, 57, 191–202. [Google Scholar] [CrossRef]
- Hendriks, A.T.W.M.; Zeeman, G. Pretreatments to enhance the digestibility of lignocellulosic biomass. Bioresour. Technol. 2009, 100, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Zhao, J.; Xu, F.; Li, Y. Pretreatment of lignocellulosic biomass for enhanced biogas production. Prog. Energy Combust. Sci. 2014, 42, 35–53. [Google Scholar] [CrossRef]
- Rubio Senent, F. Recuperación de Compuestos Bioactivos a Partir de Subproductos del Aceite de Oliva. Ph.D. Thesis, Facultad de Farmacia, Universidad de Sevilla, Sevilla, Spain, 2011. [Google Scholar]
- Rubio-senent, F.; Rodríguez-gutiérrez, G.; Lama-muñoz, A.; Fernández-bolaños, J. Phenolic extract obtained from steam-treated olive oil waste: Characterization and antioxidant activity. Lwt Food Sci. Technol. 2013, 54, 114–124. [Google Scholar] [CrossRef]
- Trujillo-Reyes, Á.; Cubero-Cardoso, J.; Rodríguez-Gutiérrez, G.; García-Martín, J.F.; Rodríguez-Galán, M.; Borja, R.; Serrano, A.; Fermoso, F.G. Extraction of phenolic compounds and production of biomethane from strawberry and raspberry extrudates. Biochem. Eng. J. 2019, 147. [Google Scholar] [CrossRef]
- Serrano, A.; Newton, G.; Alonso-Fariñas, B.; Fermoso, F.G.; Villa-Gomez, D.K. pH-Controlled fermentation of strawberry waste as phenol solubilisation method. J. Clean. Prod. 2020, 266, 121924. [Google Scholar] [CrossRef]
- Terefe, N.S.; Kleintschek, T.; Gamage, T.; Fanning, K.J.; Netzel, G.; Versteeg, C.; Netzel, M. Comparative effects of thermal and high pressure processing on phenolic phytochemicals in different strawberry cultivars. Innov. Food Sci. Emerg. Technol. 2013, 19, 57–65. [Google Scholar] [CrossRef]
- Siles, J.A.; Serrano, A.; Martín, A.; Martín, M.A. Biomethanization of waste derived from strawberry processing: Advantages of pretreatment. J. Clean. Prod. 2013, 42, 190–197. [Google Scholar] [CrossRef]
- Ward, A.J.; Hobbs, P.J.; Holliman, P.J.; Jones, D.L. Optimisation of the anaerobic digestion of agricultural resources. Bioresour. Technol. 2008, 99, 7928–7940. [Google Scholar] [CrossRef]
- Serrano, A.; Fermoso, F.G.; Alonso-Fariñas, B.; Rodríguez-Gutierrez, G.; Fernandez-Bolaños, J.; Borja, R. Olive mill solid waste biorefinery: High-temperature thermal pre-treatment for phenol recovery and biomethanization. J. Clean. Prod. 2017, 148, 314–323. [Google Scholar] [CrossRef] [Green Version]
- Millati, R.; Wikandari, R.; Ariyanto, T.; Putri, R.U.; Taherzadeh, M.J. Pretreatment Technologies for Anaerobic Digestion of Lignocelluloses and Toxic Feedstocks; Elsevier Ltd: Amsterdam, The Netherlands, 2020; Volume 304, p. 122998. [Google Scholar]
- Donoso-Bravo, A.; Pérez-Elvira, S.I.; Fdz-Polanco, F. Application of simplified models for anaerobic biodegradability tests. Evaluation of pre-treatment processes. Chem. Eng. J. 2010, 160, 607–614. [Google Scholar] [CrossRef]
- Thompson, W.; Leege, P.B.; Millner, P.D.; Watson, M.E. Test Methods for the Examination of Composting and Compos, Composting Council Reserach and Education Foundation. Available online: https://www.compostingcouncil.org/page/tmecc (accessed on 23 July 2020).
- Singh, J.; Suhag, M.; Dhaka, A. Augmented digestion of lignocellulose by steam explosion, acid and alkaline pretreatment methods: A review. Carbohydr. Polym. 2015, 117, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, F.; Silva, E.L.; Varesche, M.B.A. Hydrothermal processing of biomass for anaerobic digestion—A review. Renew. Sustain. Energy Rev. 2018, 98, 108–124. [Google Scholar] [CrossRef]
- Song, C.; Zhang, Y.; Xia, X.; Qi, H.; Li, M.; Pan, H.; Xi, B. Effect of inoculation with a microbial consortium that degrades organic acids on the composting efficiency of food waste. Microb. Biotechnol. 2018, 11, 1124–1136. [Google Scholar] [CrossRef] [Green Version]
- Jönsson, L.J.; Martín, C. Pretreatment of lignocellulose: Formation of inhibitory by-products and strategies for minimizing their effects. Bioresour. Technol. 2016, 199, 103–112. [Google Scholar] [CrossRef] [Green Version]
- Song, X.; Wachemo, A.C.; Zhang, L.; Bai, T.; Li, X.; Zuo, X.; Yuan, H. Effect of hydrothermal pretreatment severity on the pretreatment characteristics and anaerobic digestion performance of corn stover. Bioresour. Technol. 2019, 289, 121646. [Google Scholar] [CrossRef]
- Ghasimi, D.S.M.; Aboudi, K.; De Kreuk, M.; Zandvoort, M.H.; Van Lier, J.B. Impact of lignocellulosic-waste intermediates on hydrolysis and methanogenesis under thermophilic and mesophilic conditions. Chem. Eng. J. 2016, 295, 181–191. [Google Scholar] [CrossRef]
- Serrano, A.; Fermoso, F.G.; Alonso-Fariñas, B.; Rodríguez-Gutiérrez, G.; López, S.; Fernandez-Bolaños, J.; Borja, R. Performance evaluation of mesophilic semi-continuous anaerobic digestion of high-temperature thermally pre-treated olive mill solid waste. Waste Manag. 2019, 87, 250–257. [Google Scholar] [CrossRef]
- Kowalski, S.; Lukasiewicz, M.; Duda-Chodak, A.D.A.; Zięć, G. 5-Hydroxymethyl-2-Furfural (HMF)–Heat-Induced Formation, Occurrence in Food and Biotransformation—A Review. Pol. J. Food Nutr. Sci. 2013, 63. [Google Scholar] [CrossRef] [Green Version]
- Arfan, M.; Khan, R.; Rybarczyk, A.; Amarowicz, R. Antioxidant Activity of Mulberry Fruit Extracts. Int. J. Mol. Sci. 2012, 13, 2472–2480. [Google Scholar] [CrossRef] [PubMed]
- Tilay, A.; Bule, M.; Kishenkumar, J.; Annapure, U. Preparation of Ferulic Acid from Agricultural Wastes: Its Improved Extraction and Purification. J. Agric. Food Chem. 2008, 56, 7644–7648. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Siddique, J.A.; Laskar, M.A.; Kumar, R.; Mohd-Setapar, S.H.; Khatoon, A.; Shiekh, R.A. New generation Amberlite XAD resin for the removal of metal ions: A review. J. Eenviron. Sci. 2015, 31, 104–123. [Google Scholar] [CrossRef] [PubMed]
- Wheatley, A. Anaerobic Digestion: A Waste Treatment Technology. Elservier Appl. Sci. 1990. [Google Scholar]
- Borja, R.; Alba, J.; Banks, C.J. Impact of the main phenolic compounds of olive mill wastewater (OMW) on the kinetics of acetoclastic methanogenesis. Process Biochem. 1997, 32, 121–133. [Google Scholar] [CrossRef]
- Borja, R.; Banks, C.J.; Maestro-Durán, R.; Alba, J. The effects of the most important phenolic constituents of olive mill wastewater on batch anaerobic methanogenesis. Eenviron. Technol. 1996, 17, 167–174. [Google Scholar] [CrossRef]
- Ware, A.; Power, N. Modelling methane production kinetics of complex poultry slaughterhouse wastes using sigmoidal growth functions. Renew. Energy 2017, 104, 50–59. [Google Scholar] [CrossRef]
- Soto, M.L.; Moure, A.; Domínguez, H.; Parajó, J.C. Recovery, concentration and purification of phenolic compounds by adsorption: A review. J. Food Eng. 2011, 105, 1–27. [Google Scholar] [CrossRef]
- Fernández-Bolaños Guzmán, J.; Rodríguez-Gutiérrez, G.; Lama-Muñoz, A.; Fernández-Bolaños, J.M.; Maya-Castilla, I.; Rubio-Senent, F.; Marset-Castro, A. Method for Obtaining Hydroxytyrosol Extract, Mixture of Hydroxytyrosol and 3,4-dihydroxyphenylglycol Extract, and Hydroxytyrosyl Acetate Extract from By-Products of the Olive Tree and the Purification of Thereof. Available online: https://digital.csic.es/handle/10261/92556 (accessed on 23 July 2020).
- APHA. Standard Methods for Examination of Water and Wastewater, 23rd ed.; American Public Health Association, American Water Works Association, and Water Environment Federation: Washington, DC, USA, 2017. [Google Scholar]
| RSE | 170 °C, 60 min, 5 kg/cm2 | 220 °C, 5 min, 32 kg/cm2 | ||||
|---|---|---|---|---|---|---|
| RSE | SP | LP | SP | LP | ||
| pH | 3.7 ± 0.1 | 4.3 ± 0.1 | 3.8 ± 0.1 | 3.6 ± 0.1 | 3.8 ± 0.1 | |
| TS | (mg/kg RSE) | 144,681 ± 3986 | 94,280 ± 871 | 32,044 ± 1024 | 58,092 ± 1268 | 47,120 ± 1515 |
| VS | (mg/kg RSE) | 139,336 ± 4423 | 90,801 ± 3405 | 29,430 ± 606 | 56,162 ± 2106 | 43,296 ± 891 |
| CODt | (mg O2/kg RSE) | 200,366 ± 6312 | 116,027 ± 1918 | 40,516 ± 373 | 89,775 ± 3220 | 63,910 ± 915 |
| CODS | (mg O2/kg RSE) | 47,237 ± 317 | 14,423 ± 463 | 43,927 ± 352 | 14,279 ± 316 | 82,027 ± 1194 |
| Total Phenols | (mg gallic acid eq./kg RSE) | 2185 ± 64 | 1141 ± 30 | 858 ± 0 | 2200 ± 3 | 3895 ± 165 |
| Total Sugars | (mg glucose eq./kg RSE) | 2023 ± 99 | 875 ± 23 | 12,322 ± 222 | 1644 ± 34 | 13,769 ± 178 |
| Acids Sugars | (mg galacturonic acid eq./kg RSE) | 6.28 ± 0.12 | 2.11 ± 0.06 | 2.93 ± 0.06 | 0.29 ± 0.00 | 0.38 ± 0.01 |
| HMF | (mg/kg RSE) | n.d. | 73 ± 1 | 2993 ± 29 | 5 ± 1 | 155 ± 2 |
| RSE | SP | LP | DLP | SP + LP | SP + DLP | |
|---|---|---|---|---|---|---|
| pH | 7.7 ± 0.1 | 7.8 ± 0.1 | 7.8 ± 0.1 | 7.8 ± 0.1 | 7.7 ± 0.1 | 7.8 ± 0.1 |
| Alkalinity (mg CaCO3/L) | 5768 ± 278 | 5902 ± 47 | 6174 ± 145 | 6590 ± 145 | 6049 ±51 | 6028 ± 46 |
| TS (mg/kg) | 15,391 ± 442 | 15,570 ± 156 | 15,272 ± 118 | 15,057 ± 327 | 15,104 ± 265 | 15,610 ± 655 |
| VS (mg/kg) | 9918 ± 369 | 9961 ± 235 | 9250 ± 173 | 9226 ± 226 | 9837 ± 367 | 9981 ± 403 |
| CODS (mg O2/L) | 1181 ± 48 | 1184 ± 56 | 1448 ± 59 | 1464 ± 17 | 1224 ± 79 | 1196 ± 65 |
| Total phenols (mg gallic acid eq./L) | 158 ± 8 | 163 ± 1 | 172 ± 6 | 171 ± 9 | 159 ± 14 | 157 ± 4 |
| Experimental methane production (mL CH4/g VS) | 416 ± 8 | 329 ± 7 | 497 ± 6 | 580 ± 7 | 403 ± 105 | 434 ± 32 |
| Biodegradability (based on VS) (%) | 74 | 67 | 94 | 125 | 81 | 90 |
| Substrates | P (mL CH4/g VS) | Rm (mL CH4/g VS·d) | ʎ (d) | B0 (mL CH4/g VS) | R2 | Error (%) | S.E.E. |
|---|---|---|---|---|---|---|---|
| RSE | 345 ± 6 | 50.6 ± 4.4 | 11.45 ± 0.09 | 66 ± 4 | 0.9964 | 1.2 | 8.88 |
| SP | 305 ± 4 | 35.6 ± 1.5 | 12.37 ± 0.07 | 22 ± 3 | 0.9987 | 0.4 | 4.34 |
| LP | 351 ± 10 | 59.8 ± 9.3 | 9.5 ± 0.1 | 107 ± 8 | 0.9899 | 6.3 | 11.61 |
| DLP | 540 ± 54 | 52.4 ± 15.1 | 8.3 ± 0.5 | 22 ± 47 | 0.9755 | 3.0 | 10.9 |
| SP + LP | 341 ± 7 | 55.3 ± 5.6 | 9.5 ± 0.1 | 51 ± 6 | 0.9952 | 2.6 | 10.16 |
| SP + DLP | 364 ± 5 | 54.8 ± 4.1 | 10.02 ± 0.08 | 63 ± 4 | 0.9977 | 1.5 | 7.45 |
| RSE | SP | LP | DLP | SP + LP | SP + DLP | |
|---|---|---|---|---|---|---|
| pH | 7.7 ± 0.1 | 7.8 ± 0.1 | 7.7 ± 0.1 | 7.7 ± 0.1 | 7.7 ± 0.1 | 7.6 ± 0.1 |
| Alkalinity (mg CaCO3/L) | 5617 ± 18 | 5328 ± 185 | 5482 ± 141 | 5333 ± 142 | 5328 ± 204 | 5264 ± 59 |
| TS (mg/Kg) | 12,987 ± 180 | 13,412 ± 667 | 12,533 ± 237 | 12,702 ± 211 | 13,082 ± 354 | 13,401 ± 290 |
| MS (mg/Kg) | 5478 ± 246 | 5062 ± 440 | 4988 ± 142 | 5343 ± 412 | 5331 ± 279 | 5276 ± 351 |
| VS (mg/Kg) | 7685 ± 256 | 8425 ± 180 | 7370 ± 307 | 7171 ± 276 | 7848 ± 345 | 7975 ± 572 |
| CODS (mg O2/L) | 767 ± 33 | 1973 ± 89 | 1438 ± 39 | 1020 ± 62 | 1144 ± 63 | 937 ± 10 |
| Total phenols (mg gallic acid eq./L) | 134 ± 1 | 159 ± 2 | 201 ± 7 | 158 ± 9 | 176 ± 3 | 165 ± 5 |
| Theoretical methaneproduction (mL CH4/g VS) | 559 | 661 | 564 | 481 | 590 | 554 |
| Experimental methane production (mL CH4/g VS) | 468± 4 | 299 ± 12 | 562 ± 13 | 512 ± 30 | 493 ± 30 | 434 ± 27 |
| Biodegradability (based on VS) (%) | 84 | 45 | 100 | 106 | 84 | 78 |
| Substrates | P (mL CH4/g VS) | Rm (mL CH4/g VS·d) | ʎ (d) | B0 (mL CH4/g VS) | R2 | Error (%) | S.E.E. |
|---|---|---|---|---|---|---|---|
| RSE | 429 ± 9 | 54.7 ± 6.3 | 10.6 ± 0.1 | 27 ± 6 | 0.9998 | 2.2 | 11.56 |
| SP | 310 ± 4 | 62.3 ± 4.9 | 15.72 ± 0.07 | 6 ± 1 | 0.9962 | 2.8 | 7.92 |
| LP | 552 ± 34 | 49.3 ± 9.3 | 7.8 ± 0.3 | 8.3 ± 1.5 | 0.9886 | 0.1 | 10.92 |
| DLP | 412 ± 17 | 68.2 ± 14.3 | 7.4 ± 0.1 | 70 ± 14 | 0.9838 | 5.7 | 11.10 |
| SP + LP | 431 ± 8 | 60.2 ± 8.6 | 10.0 ± 0.1 | 44 ± 6 | 0.9942 | 3.3 | 12.43 |
| SP + DLP | 346 ± 8 | 78.7 ± 12.8 | 8.7 ± 0.1 | 59 ± 7 | 0.9893 | 6.5 | 10.31 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cubero-Cardoso, J.; Trujillo-Reyes, Á.; Serrano, A.; Rodríguez-Gutiérrez, G.; Borja, R.; Fermoso, F.G. High-Value-Added Compound Recovery with High-Temperature Hydrothermal Treatment and Steam Explosion, and Subsequent Biomethanization of Residual Strawberry Extrudate. Foods 2020, 9, 1082. https://doi.org/10.3390/foods9081082
Cubero-Cardoso J, Trujillo-Reyes Á, Serrano A, Rodríguez-Gutiérrez G, Borja R, Fermoso FG. High-Value-Added Compound Recovery with High-Temperature Hydrothermal Treatment and Steam Explosion, and Subsequent Biomethanization of Residual Strawberry Extrudate. Foods. 2020; 9(8):1082. https://doi.org/10.3390/foods9081082
Chicago/Turabian StyleCubero-Cardoso, Juan, Ángeles Trujillo-Reyes, Antonio Serrano, Guillermo Rodríguez-Gutiérrez, Rafael Borja, and Fernando G. Fermoso. 2020. "High-Value-Added Compound Recovery with High-Temperature Hydrothermal Treatment and Steam Explosion, and Subsequent Biomethanization of Residual Strawberry Extrudate" Foods 9, no. 8: 1082. https://doi.org/10.3390/foods9081082
APA StyleCubero-Cardoso, J., Trujillo-Reyes, Á., Serrano, A., Rodríguez-Gutiérrez, G., Borja, R., & Fermoso, F. G. (2020). High-Value-Added Compound Recovery with High-Temperature Hydrothermal Treatment and Steam Explosion, and Subsequent Biomethanization of Residual Strawberry Extrudate. Foods, 9(8), 1082. https://doi.org/10.3390/foods9081082









