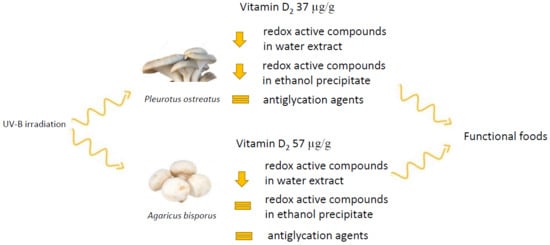
The Effect of UV Irradiation on Vitamin D2 Content and Antioxidant and Antiglycation Activities of Mushrooms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Mushrooms
2.3. Determination of Vitamin D2
2.4. Recovery of Bioactive Fractions
2.5. Antioxidant Activity
2.6. Antiglycation Activity Using Bovine Serum Albumin (BSA)/Fructose Model Systems
2.7. Statistical Analysis of Data
3. Results and Discussion
3.1. The Effect of Mushroom Irradiation on Vitamin D2 Content
3.2. The Effect of Mushroom Irradiation on Antioxidant Activity
3.3. The Effect of Mushroom Irradiation on Antiglycation Activity
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Holick, M.F.; Chen, T.C. Vitamin D deficiency: A worldwide problem with health consequences. Am. J. Clin. Nutr. 2008, 87, 1080S–1086S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganji, V.; Abu-Dbaa, R.; Othman, H.; Zewein, M.; Al-Abdi, T.; Zumin, S. Validation of vitamin D-specific food frequency questionnaire against food records for Qatari women. Foods 2020, 9, 195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Li, Z.; Wei, Y.; Fu, J.; Feng, Y.; Chen, D.; Xu, D. Status and influential factors of vitamin D among children aged 0 to 6 years in a Chinese population. BMC Public Health 2020, 20, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Roman Viñas, B.; Ribas Barba, L.; Ngo, J.; Gurinovic, M.; Novakovic, R.; Cavelaars, A.; de Groot, L.C.P.G.M.; Veer, P.v.; Matthys, C.; Majem, L.S. Projected prevalence of inadequate nutrient intakes in Europe. Ann. Nutr. Metab. 2011, 59, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Reider, C.A.; Chung, R.-Y.; Devarshi, P.P.; Grant, R.W.; Mitmesser, S.H. Inadequacy of immune health nutrients: Intakes in US adults, the 2005–2016 NHANES. Nutrients 2020, 12, 1735. [Google Scholar] [CrossRef]
- Galanakis, C.M. The food systems in the era of the coronavirus (COVID-19) pandemic crisis. Foods 2020, 9, 523. [Google Scholar] [CrossRef]
- McClements, D.J. Development of next-generation nutritionally fortified plant-based milk substitutes: Structural design principles. Foods 2020, 9, 421. [Google Scholar] [CrossRef] [Green Version]
- Taofiq, O.; Fernandes, A.; Barrosa, L.; Barreiro, M.F.; Ferreira, I.C.F.R. UV-irradiated mushrooms as a source of vitamin D2: A review. Trends Food Sci. Technol. 2017, 70, 82–94. [Google Scholar] [CrossRef]
- Morales, D.; Gil-Ramirez, A.; Smiderle, F.R.; Piris, A.J.; Ruiz-Rodriguez, A.; Soler-Rivas, C. Vitamin D-enriched extracts obtained from shiitake mushrooms (lentinula edodes) by supercritical fluid extraction and UV-irradiation. Inn. Food Sci. Emerg. Technol. 2017, 41, 330–333. [Google Scholar] [CrossRef] [Green Version]
- Carrasco-González, J.A.; Serna-Saldívar, S.O.; Gutiérrez-Uribe, J.A. Nutritional composition and nutraceutical properties of the Pleurotus fruiting bodies: Potential use as food ingredient. J. Food Compos. Anal. 2017, 58, 69–81. [Google Scholar] [CrossRef]
- Lavelli, V.; Proserpio, C.; Gallotti, F.; Laureati, M.; Pagliarini, E. Circular reuse of bio-resources: The role of Pleurotus spp. in the development of functional foods. Food Funct. 2018, 9, 1353–1372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cardwell, G.; Bornman, J.F.; James, A.P.; Black, L.J. A review of mushrooms as a potential source of dietary vitamin D. Nutrients 2018, 10, 1498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Redmond, R.W.; Kocheva, I.E. Symposium-in-print: Singlet oxygen invited review. Spatially resolved cellular responses to singlet oxygen. Photochem. Photobiol. 2006, 82, 1178–1186. [Google Scholar] [CrossRef] [PubMed]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [Green Version]
- Sri Harsha, P.S.C.; Lavelli, V. Use of grape pomace phenolics to counteract endogenous and exogenous advanced glycation end-product formation. Nutrients 2019, 11, 1917. [Google Scholar] [CrossRef] [Green Version]
- Ritota, M.; Manzi, P. Pleurotus spp. cultivation on different agri-food by-products: Example of biotechnological application. Sustainability 2019, 11, 5049. [Google Scholar] [CrossRef] [Green Version]
- Bekiaris, G.; Tagkouli, D.; Koutrotsios, G.; Kalogeropoulos, N.; Zervakis, G.I. Pleurotus mushrooms content in glucans and ergosterol assessed by ATR-FTIR spectroscopy and multivariate analysis. Foods 2020, 9, 535. [Google Scholar] [CrossRef]
- Morales, D.; Tabernero, M.; Largo, C.; Polo, G.; Piris, A.J.; Soler-Rivas, C. Effect of traditional and modern culinary processing, bioaccessibility, biosafety and bioavailability of eritadenine, a hypocholesterolemic compound from edible mushrooms. Food Funct. 2018, 9, 6360–6368. [Google Scholar] [CrossRef] [Green Version]
- Menga, G.; Zhua, H.; Yangb, S.; Wua, F.; Zheng, H.; Chen, E.; Xua, J. Attenuating effects of Ganoderma lucidum polysaccharides on myocardial collagen cross-linking relates to advanced glycation end product and antioxidant enzymes in high-fat-diet and streptozotocin-induced diabetic rats. Carbohydr. Polym. 2011, 84, 180–185. [Google Scholar] [CrossRef]
- Pedrali, D.; Gallotti, F.; Proserpio, C.; Pagliarini, E.; Lavelli, V. Kinetic study of vitamin D2 degradation in mushroom powder to improve its applications in fortified foods. LWT Food Sci. Technol. 2020, 125, 109248. [Google Scholar] [CrossRef]
- Sławińska, A.; Fornal, E.; Radzki, W.; Skrzypczak, K.; Zalewska-Korona, M.; Michalak-Majewska, M.; Parfieniuk, E.; Stachniuk, A. Study on vitamin D2 stability in dried mushrooms during drying and storage. Food Chem. 2016, 199, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Wittig, M.; Krings, U.; Berger, R.G. Single-run analysis of vitamin D photoproducts in oyster mushroom (Pleurotus ostreatus) after UV-B treatment. J. Food Compos. Anal. 2013, 31, 266–274. [Google Scholar] [CrossRef]
- Huang, S.J.; Lin, C.P.; Tsai, S.Y. Vitamin D2 content and antioxidant properties of fruit body and mycelia of edible mushrooms by UV-B irradiation. J. Food Compos. Anal. 2015, 42, 38–45. [Google Scholar] [CrossRef]
- Chen, P.; Weng, Y.; Yu, Z.; Koo, M.; Wang, B. Extraction temperature affects the activities of antioxidation, carbohydrate-digestion enzymes, and angiotensin-converting enzyme of Pleurotus citrinopileatus extract. J. Food Drug Anal. 2016, 24, 548–555. [Google Scholar] [CrossRef] [Green Version]
- Sarangi, I.; Gosh, D.; Sanjaya, S.; Maiti, K. Anti-tumor and immunomodulating effects of Pleurotus ostreatus mycelia-derived proteoglycans. Int. Immunopharmacol. 2008, 6, 1287–1297. [Google Scholar] [CrossRef] [PubMed]
- Lavelli, V.; Sri Harsha, P.S.C.; Spigno, G. Modelling the stability of maltodextrin-encapsulated grape skins phenolics used as a new ingredient in apple puree. Food Chem. 2016, 209, 323–331. [Google Scholar] [CrossRef]
- Lavelli, V.; Hidalgo, A.; Pompei, C.; Brandolini, A. Radical scavenging activity of einkorn (Triticum monococcum L. subsp. monococcum) wholemeal flour and its relationship to soluble phenolic and lipophilic antioxidant content. J. Cereal Sci. 2009, 49, 319–321. [Google Scholar] [CrossRef]
- Lavelli, V.; Sri Harsha, P.S.C. Microencapsulation of grape skin phenolics for pH controlled release of antiglycation agents. Food Res. Int. 2019, 119, 822–828. [Google Scholar] [CrossRef]
- Jasinghe, V.J.; Perera, C.O. Distribution of ergosterol in different tissues of mushrooms and its effect on the conversion of ergosterol to vitamin D2 by UV irradiation. Food Chem. 2005, 92, 541–546. [Google Scholar] [CrossRef]
- Merdivan, S.; Lindequist, U. Ergosterol peroxide: A mushroom-derived compound with promising biological activities-A review. Int. J. Med. Mushrooms 2017, 19, 93–105. [Google Scholar] [CrossRef]
- Calvo, M.S.; Whiting, S.J.; Barton, C.N. Vitamin D fortification in the United States and Canada: Current status and data needs. Am. J. Clin. Nutr. 2004, 80, 1710S–1716S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O′Mahony, L.; Stepien, M.; Gibney, M.J.; Nugent, A.P.; Brennan, L. The potential role of vitamin D enhanced foods in improving vitamin D status. Nutrients 2011, 3, 1023–1041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cashman, K.D.; O′Dea, R. Exploration of strategic food vehicles for vitamin D fortification in low/lower-middle income countries. J. Steroid Biochem. Mol. Biol. 2019, 195, 105479. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.; Huang, S.; Lo, S.H.; Wu, T.; Lian, P.; Mau, J. Flavor compounds and antioxidant properties of several cultivated mushrooms. Food Chem. 2009, 113, 578–584. [Google Scholar] [CrossRef]
- Puttaraju, N.G.; Yenkateshaiah, S.U.; Dharmesh, S.M.; Nanjarajurs, S.M.; Somasundaram, R. Antioxidant activity of indigenous edible mushrooms. J. Agric. Food Chem. 2006, 54, 9764–9772. [Google Scholar] [CrossRef]
- Reis, F.S.; Martins, A.; Barros, L.; Ferreira, I.C.F.R. Antioxidant properties and phenolic profile of the most widely appreciated cultivated mushrooms: A comparative study between in vivo and in vitro samples. Food Chem. Toxicol. 2012, 50, 1201–1207. [Google Scholar] [CrossRef]
- Carlsen, M.H.; Halvorsen, B.L.; Holte, K.; Bøhn, S.K.; Dragland, S.; Sampson, L. The total antioxidant content of more than 3100 foods, beverages, spices, herbs and supplements used worldwide. Nutr. J. 2010, 9, 3. [Google Scholar] [CrossRef]
- Wang, C.; Chen, Z.; Pan, Y.; Gao, X.; Chen, H. Anti-diabetic effects of Inonotus obliquus polysaccharides-chromium (III) complex in type 2 diabetic mice and its sub-acute toxicity evaluation in normal mice. Food Chem. Toxicol. 2017, 108, 498–509. [Google Scholar] [CrossRef]
- Yap, H.-Y.Y.; Tan, N.-H.; Ng, S.-T.; Tan, C.-S.; Fung, S.-Y. Inhibition of protein glycation by tiger milk mushroom [Lignosus rhinocerus (cooke) ryvarden] and search for potential anti-diabetic activity-related metabolic pathways by genomic and transcriptomic data mining. Front. Pharmacol. 2018, 9, 103. [Google Scholar] [CrossRef] [Green Version]
- Liping, S.; Xuejiao, S.; Yongliang, Z. Preparation, characterization and antiglycation activities of the novel polysaccharides from Boletus snicus. Int. J. Biol. Macromol. 2016, 92, 607–614. [Google Scholar] [CrossRef]

| Mushroom | Vitamin D2 μg/g d.w | Ergosterol μg/g d.w. |
|---|---|---|
| P. ostreatus | 3.1 c ± 0.1 | 3600 c ± 400 |
| P. ostreatusUV | 37 b ± 0.1 | 3200 c ± 300 |
| A. bisporus | 0.31 d ± 0.1 | 5700 a ± 300 |
| A. bisporusUV | 57 a ± 0.1 | 4800 b ± 300 |
| Food Category | Target Level for Vitamin D μg/100 g Food | Reference | Amount of UV-Irradiated Mushroom Powder g/100 Food |
|---|---|---|---|
| Ready-to-eat breakfast cereals | 8.75 | [31] | 0.22 |
| Milk | 1.05 | [31] | 0.03 |
| Yogurt | 2.225 | [31] | 0.06 |
| Margarine | 8.275 | [31] | 0.21 |
| Edible oil | 15 | [32] | 0.38 |
| Milk | 2 | [32] | 0.05 |
| Wheat flour | 2.8 | [32] | 0.07 |
| Milk | 1 | [33] | 0.03 |
| Orange juice | 10.5 | [33] | 0.26 |
| Mushroom and Treatment | Fraction Yield g/100 g | Antioxidant Activity | ||
|---|---|---|---|---|
| FC mg GAE/g fraction | DPPH µmol TE/g fraction | FRAP µmol FeII/g fraction | ||
| P. ostreatus WE | 54 b ± 3 | 24.6 c ± 1.3 | 56 c ± 4 | 82 d ± 5 |
| P. ostreatusUV WE | 54 b ± 3 | 19.6 d ± 1.5 (20%) | 48 d ± 1 (14%) | 58 e ± 14 (29%) |
| A. bisporus WE | 67 a ± 1 | 16.2 e ± 0.5 | 48 d ± 6 | 208 b ± 7 |
| A. bisporusUV WE | 70 a ± 1 | 15.0 f ± 0.2 (7%) | 37 e ± 7 (23%) | 131 c ± 28 (37%) |
| P. ostreatus EP | 7.2 c ± 0.1 | 32.1 b ± 0.6 | 71 b ± 1 | 222 b ± 1 |
| P. ostreatusUV EP | 7.3 c ± 0.1 | 25.8 c ± 0.7 (20%) | 49 d ± 3 (31%) | 127 c ± 14 (43%) |
| A. bisporus EP | 3.4 d ± 0.1 | 53.4 a ± 1.6 | 119 a ± 14 | 473 a ± 43 |
| A. bisporusUV EP | 3.2 d ± 0.1 | 53.4 a ± 0.8 | 103 a ± 17 | 505 a ± 14 |
| Mushroom and Treatment | Antiglycation Agents mg AG/g fraction |
|---|---|
| P. ostreatus WE | 113 c ± 22 |
| P. ostreatusUV WE | 131 c ± 25 |
| A. bisporus WE | 83 d ± 9 |
| A. bisporusUV WE | 89 d ± 10 |
| P. ostreatus EP | 177 b ± 10 |
| P. ostreatusUV EP | 170 b ± 6 |
| A. bisporus EP | 555 a ± 80 |
| A. bisporusUV EP | 500 a ± 89 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallotti, F.; Lavelli, V. The Effect of UV Irradiation on Vitamin D2 Content and Antioxidant and Antiglycation Activities of Mushrooms. Foods 2020, 9, 1087. https://doi.org/10.3390/foods9081087
Gallotti F, Lavelli V. The Effect of UV Irradiation on Vitamin D2 Content and Antioxidant and Antiglycation Activities of Mushrooms. Foods. 2020; 9(8):1087. https://doi.org/10.3390/foods9081087
Chicago/Turabian StyleGallotti, Francesca, and Vera Lavelli. 2020. "The Effect of UV Irradiation on Vitamin D2 Content and Antioxidant and Antiglycation Activities of Mushrooms" Foods 9, no. 8: 1087. https://doi.org/10.3390/foods9081087
APA StyleGallotti, F., & Lavelli, V. (2020). The Effect of UV Irradiation on Vitamin D2 Content and Antioxidant and Antiglycation Activities of Mushrooms. Foods, 9(8), 1087. https://doi.org/10.3390/foods9081087