Investigation of Raman Spectroscopy (with Fiber Optic Probe) and Chemometric Data Analysis for the Determination of Mineral Content in Aqueous Infant Formula
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Raman Spectral Data Acquisition of Aqueous INF Samples
2.3. Inductively Coupled Plasma Atomic Emission Spectroscopy (ICP-AES) Analysis
2.3.1. Preparation of Reagents and Standard Solutions for ICP-AES
2.3.2. Dry Digestion
2.3.3. ICP-AES Apparatus and Working Conditions
2.4. Accuracy Determination on ICP-AES Analysis
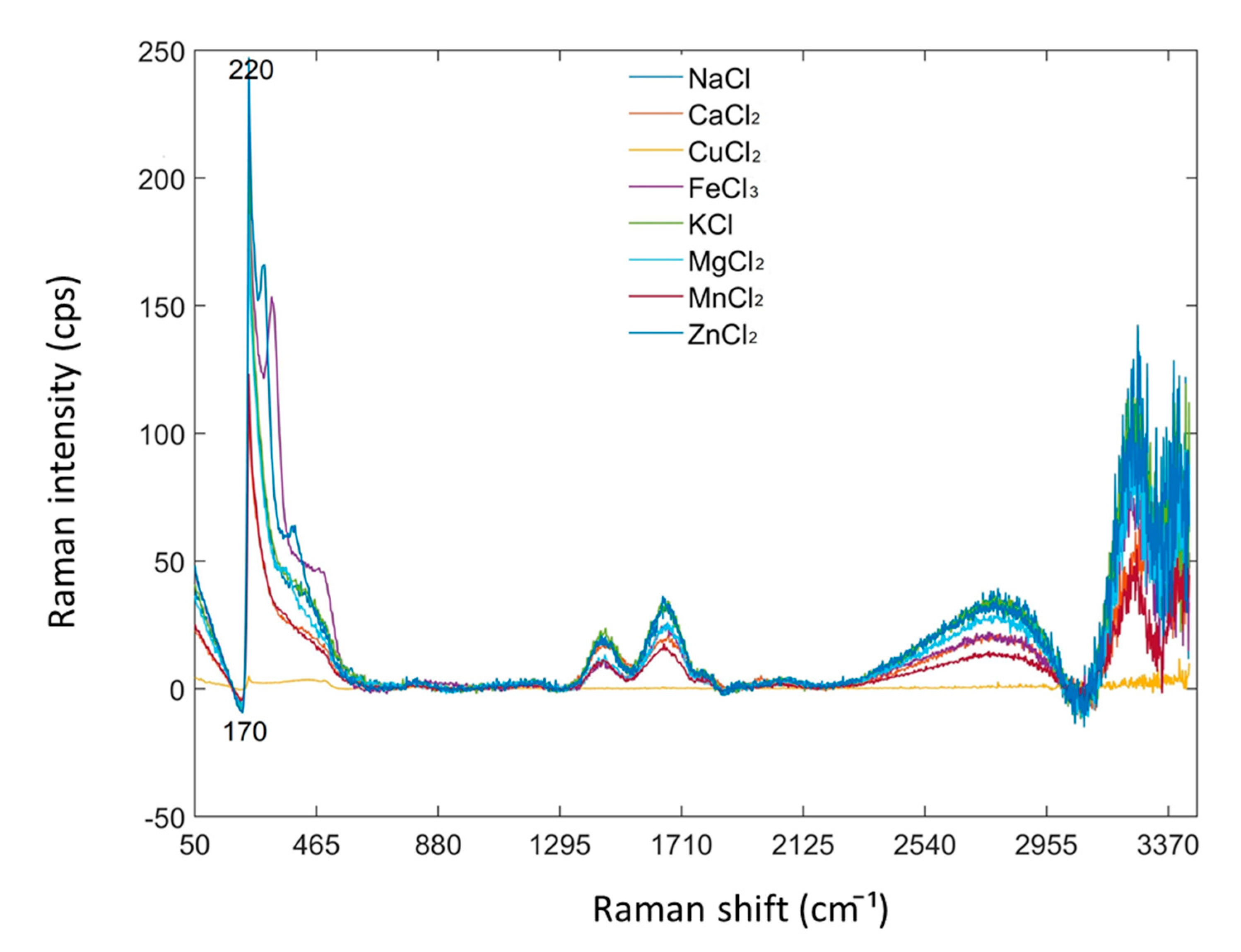
2.5. Control Experiments on Chloride Salts Using RS
2.6. Chemometric Analysis
3. Results and Discussion
3.1. Raman Spectra of Aqueous INF Samples
3.2. Results of ICP-AES Analysis
3.3. Accuracy of ICP-AES Analysis
3.4. Prediction of Mineral Elements Using PLSR Models
3.4.1. PLSR Models Based on All Aqueous INF Samples—Results and Discussion
3.4.2. PLSR Models Developed and Validated Using Calibration and Validation Data Sets—Results and Discussion
3.4.3. Discussion on Regression Coefficients of PLSR Models for Prediction Mineral Elements
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Federal Food, Drug, and Cosmetic Act (FFDCA). Guidance for Industry: Frequently Asked Questions about FDA’s Regulation of Infant Formula. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industry-frequently-asked-questions-about-fdas-regulation-infant-formula (accessed on 7 February 2020).
- McCance, W. The Composition of Foods; The Royal Society of Chemistry: London, UK, 1993; ISBN 978-1-84973-636-7. [Google Scholar]
- Molska, A.; Gutowska, I.; Baranowska-Bosiacka, I.; Nocen, I.; Chlubek, D. The content of elements in infant formulas and drinks against mineral requirements of children. Biol. Trace Elem. Res. 2014, 158, 422–427. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ruiz, C.; Alegria, A.; Barbera, R.; Farre, R.; Lagarda, M.J. Direct determination of calcium, magnesium, sodium, potassium and iron in infant formulas by atomic spectroscopy. Comparison with dry and wet digestions methods. Food Nahr. 1995, 39, 497–504. [Google Scholar] [CrossRef]
- Stürup, S.; Büchert, A. Direct determination of copper and iodine in milk and milk powder in alkaline solution by flow injection inductively coupled plasma mass spectrometry. Anal. Bioanal. Chem. 1996, 354, 323–326. [Google Scholar] [CrossRef] [PubMed]
- McKinstry, P.; Indyk, H.; Kim, N. The determination of major and minor elements in milk and infant formula by slurry nebulisation and inductively coupled plasma—Optical emission spectrometry (ICP-OES). Food Chem. 1999, 65, 245–252. [Google Scholar] [CrossRef]
- Ataro, A.; McCrindle, R.; Botha, B.; McCrindle, C.; Ndibewu, P. Quantification of trace elements in raw cow’s milk by inductively coupled plasma mass spectrometry (ICP-MS). Food Chem. 2008, 111, 243–248. [Google Scholar] [CrossRef]
- Bilandžić, N.; Đokić, M.; Sedak, M.; Solomun, B.; Varenina, I.; Knežević, Z.; Benić, M. Trace element levels in raw milk from northern and southern regions of Croatia. Food Chem. 2011, 127, 63–66. [Google Scholar] [CrossRef]
- Khan, N.; Jeong, I.S.; Hwang, I.M.; Kim, J.S.; Choi, S.H.; Nho, E.Y.; Choi, J.Y.; Park, K.S.; Kim, S.H. Analysis of minor and trace elements in milk and yogurts by inductively coupled plasma-mass spectrometry (ICP-MS). Food Chem. 2014, 147, 220–224. [Google Scholar] [CrossRef]
- Bakircioglu, D.; Topraksever, N.; Yurtsever, S.; Kizildere, M.; Kurtulus, Y.B. Investigation of macro, micro and toxic element concentrations of milk and fermented milks products by using an inductively coupled plasma optical emission spectrometer, to improve food safety in Turkey. Microchem. J. 2018, 136, 133–138. [Google Scholar] [CrossRef]
- Montagne, D.-H.; Van Dael, P.; Skanderby, M.; Hugelshofer, W. Infant Formulae—Powders and Liquids. In Dairy Powders and Concentrated Products; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2009; pp. 294–331. ISBN 9781444322729. [Google Scholar]
- Jiang, Y.J.; Guo, M. Processing technology for infant formula. In Human Milk Biochemistry and Infant Formula Manufacturing Technology; Guo, M., Ed.; Woodhead Publishing: Cambridge, UK, 2014; pp. 211–218. ISBN 9781845697242. [Google Scholar]
- Tsenkova, R.; Atanassova, S.; Toyoda, K.; Ozaki, Y.; Itoh, K.; Fearn, T. Near-Infrared Spectroscopy for Dairy Management: Measurement of Unhomogenized Milk Composition. J. Dairy Sci. 1999, 82, 2344–2351. [Google Scholar] [CrossRef]
- Tsenkova, R.; Atanassova, S.; Kawano, S.; Toyoda, K. Somatic cell count determination in cow’s milk by near-infrared spectroscopy: A new diagnostic tool. J. Anim. Sci. 2001, 79, 2550–2557. [Google Scholar] [CrossRef]
- Melfsen, A.; Hartung, E.; Haeussermann, A. Accuracy of in-line milk composition analysis with diffuse reflectance near-infrared spectroscopy. J. Dairy Sci. 2012, 95, 6465–6476. [Google Scholar] [CrossRef] [PubMed]
- Bonfatti, V.; Degano, L.; Menegoz, A.; Carnier, P. Short communication: Mid-infrared spectroscopy prediction of fine milk composition and technological properties in Italian Simmental. J. Dairy Sci. 2016, 99, 8216–8221. [Google Scholar] [CrossRef] [PubMed]
- Visentin, G.; Penasa, M.; Gottardo, P.; Cassandro, M.; De Marchi, M. Predictive ability of mid-infrared spectroscopy for major mineral composition and coagulation traits of bovine milk by using the uninformative variable selection algorithm. J. Dairy Sci. 2016, 99, 8137–8145. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, M.-P.; Ferrand, M.; Gelé, M.; Pourchet, D.; Miranda, G.; Martin, P.; Brochard, M.; Boichard, D. Short communication: Genetic parameters for milk protein composition predicted using mid-infrared spectroscopy in the French Montbéliarde, Normande, and Holstein dairy cattle breeds. J. Dairy Sci. 2017, 100, 6371–6375. [Google Scholar] [CrossRef] [PubMed]
- Moros, J.; Garrigues, S.; De La Guardia, M. Evaluation of nutritional parameters in infant formulas and powdered milk by Raman spectroscopy. Anal. Chim. Acta 2007, 593, 30–38. [Google Scholar] [CrossRef] [PubMed]
- McGoverin, C.; Clark, A.; Holroyd, S.; Gordon, K.C. Raman spectroscopic quantification of milk powder constituents. Anal. Chim. Acta 2010, 673, 26–32. [Google Scholar] [CrossRef]
- Júnior, P.H.R.; Oliveira, K.D.S.; De Almeida, C.E.R.; De Oliveira, L.F.C.; Stephani, R.; Pinto, M.D.S.; De Carvalho, A.F.; Perrone, Í.T. FT-Raman and chemometric tools for rapid determination of quality parameters in milk powder: Classification of samples for the presence of lactose and fraud detection by addition of maltodextrin. Food Chem. 2016, 196, 584–588. [Google Scholar] [CrossRef]
- Stephani, R.; Oliveira, K.D.S.; De Almeida, C.E.R.; Perrone, Í.T.; De Carvalho, A.F.; De Oliveira, L.F.C.; Almeida, M.R. Raman spectroscopy as a tool to identify modification of whey protein concentrate (WPC) during shelf life. Food Packag. Shelf Life 2017, 11, 1–9. [Google Scholar] [CrossRef]
- El-Abassy, R.; Eravuchira, P.; Donfack, P.; Von Der Kammer, B.; Materny, A. Fast determination of milk fat content using Raman spectroscopy. Vib. Spectrosc. 2011, 56, 3–8. [Google Scholar] [CrossRef]
- Cheng, Y.; Dong, Y.; Wu, J.; Yang, X.; Bai, H.; Zheng, H.; Ren, N.; Zou, Y.; Li, M. Screening melamine adulterant in milk powder with laser Raman spectrometry. J. Food Compos. Anal. 2010, 23, 199–202. [Google Scholar] [CrossRef]
- Rajapandiyan, P.; Tang, W.-L.; Yang, J.; Panneerselvam, R. Rapid detection of melamine in milk liquid and powder by surface-enhanced Raman scattering substrate array. Food Control 2015, 56, 155–160. [Google Scholar] [CrossRef]
- Nieuwoudt, M.; Holroyd, S.; McGoverin, C.; Simpson, M.; Williams, D. Rapid, sensitive, and reproducible screening of liquid milk for adulterants using a portable Raman spectrometer and a simple, optimized sample well. J. Dairy Sci. 2016, 99, 7821–7831. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.; Gordon, K.C.; Holroyd, S. Raman spectroscopic quantification of calcium carbonate in spiked milk powder samples. Vib. Spectrosc. 2013, 67, 87–91. [Google Scholar] [CrossRef]
- Cama-Moncunill, R.; Casado-Gavalda, M.P.; Cama-Moncunill, X.; Markiewicz-Keszycka, M.; Dixit, Y.; Cullen, P.J.; Sullivan, C. Quantification of trace metals in infant formula premixes using laser-induced breakdown spectroscopy. Spectrochim. Acta Part B At. Spectrosc. 2017, 135, 6–14. [Google Scholar] [CrossRef]
- Cama-Moncunill, X.; Markiewicz-Keszycka, M.; Dixit, Y.; Cama-Moncunill, R.; Casado-Gavalda, M.P.; Cullen, P.J.; Sullivan, C. Feasibility of laser-induced breakdown spectroscopy (LIBS) as an at-line validation tool for calcium determination in infant formula. Food Control 2017, 78, 304–310. [Google Scholar] [CrossRef]
- Wang, Q.; Lonergan, S.M.; Yu, C. Rapid determination of pork sensory quality using Raman spectroscopy. Meat Sci. 2012, 91, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.K.; Lucey, P.G.; Ghosh, M.; Hubble, H.W.; Horton, K.A. Stand-off Raman spectroscopic detection of minerals on planetary surfaces. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2003, 59, 2391–2407. [Google Scholar] [CrossRef]
- Markovski, C.; Byrne, J.; Lalla, E.; Lozano-Gorrin, A.; Klingelhöfer, G.; Rull, F.; Kappler, A.; Hoffmann, T.; Schröder, C. Abiotic versus biotic iron mineral transformation studied by a miniaturized backscattering Mössbauer spectrometer (MIMOS II), X-ray diffraction and Raman spectroscopy. Icarus 2017, 296, 49–58. [Google Scholar] [CrossRef]
- Morris, M.D.; Mandair, G.S. Raman Assessment of Bone Quality. Clin. Orthop. Relat. Res. 2010, 469, 2160–2169. [Google Scholar] [CrossRef]
- Edwards, H.G.M.; Vandenabeele, P.; Jehlička, J.; Benoy, T.J. An analytical Raman spectroscopic study of an important english oil painting of the 18th Century. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 118, 598–602. [Google Scholar] [CrossRef]
- Wubshet, S.G.; Wold, J.P.; Böcker, U.; Sanden, K.W.; Afseth, N.K. Raman spectroscopy for quantification of residual calcium and total ash in mechanically deboned chicken meat. Food Control 2019, 95, 267–273. [Google Scholar] [CrossRef]
- Zhao, M.; Markiewicz-Keszycka, M.; Beattie, R.J.; Casado-Gavalda, M.P.; Cama-Moncunill, X.; O’Donnell, C.P.; Cullen, P.J.; Sullivan, C. Quantification of calcium in infant formula using laser-induced breakdown spectroscopy (LIBS), Fourier transform mid-infrared (FT-IR) and Raman spectroscopy combined with chemometrics including data fusion. Food Chem. 2020, 320, 126639. [Google Scholar] [CrossRef] [PubMed]
- Flynn, A.; Power, P. Nutritional Aspects of Minerals in Bovine and Human Milks. In Developments in Dairy Chemistry—3 Lactose and Minor Constituents; Fox, P.F., Ed.; Springer: Heidelberg, Germany, 1985; pp. 183–215. ISBN 978-94-009-4950-8. [Google Scholar]
- Mandel, J. Repeatability and Reproducibility. J. Qual. Technol. 1972, 4, 74–85. [Google Scholar] [CrossRef]
- Ikem, A.; Nwankwoala, A.; Odueyungbo, S.; Nyavor, K.; Egiebor, N. Levels of 26 elements in infant formula from USA, UK, and Nigeria by microwave digestion and ICP–OES. Food Chem. 2002, 77, 439–447. [Google Scholar] [CrossRef]
- Zhang, Z.-M.; Chen, S.; Liang, Y. Baseline correction using adaptive iteratively reweighted penalized least squares. Analyst 2010, 135, 1138. [Google Scholar] [CrossRef]
- Zhao, M.; Beattie, R.J.; Fearon, A.M.; O’Donnell, C.; Downey, G. Prediction of naturally-occurring, industrially-induced and total trans fatty acids in butter, dairy spreads and Cheddar cheese using vibrational spectroscopy and multivariate data analysis. Int. Dairy J. 2015, 51, 41–51. [Google Scholar] [CrossRef]
- Chong, I.-G.; Jun, C.-H. Performance of some variable selection methods when multicollinearity is present. Chemom. Intell. Lab. Syst. 2005, 78, 103–112. [Google Scholar] [CrossRef]
- Martens, H.; Martens, M. Modified Jack-knife estimation of parameter uncertainty in bilinear modelling by partial least squares regression (PLSR). Food Qual. Prefer. 2000, 11, 5–16. [Google Scholar] [CrossRef]
- Li-Chan, E. The applications of Raman spectroscopy in food science. Trends Food Sci. Technol. 1996, 7, 361–370. [Google Scholar] [CrossRef]
- Kirk, J.; Dann, S.; Blatchford, C. Lactose: A definitive guide to polymorph determination. Int. J. Pharm. 2007, 334, 103–114. [Google Scholar] [CrossRef]
- Pereira-Pacheco, F.; Robledo, D.; Rodríguez-Carvajal, L.; Pelegrin, Y.F. Optimization of native agar extraction from Hydropuntia cornea from Yucatán, México. Bioresour. Technol. 2007, 98, 1278–1284. [Google Scholar] [CrossRef] [PubMed]
- Almeida, M.; Oliveira, K.D.S.; Stephani, R.; De Oliveira, L.F.C. Fourier-transform Raman analysis of milk powder: A potential method for rapid quality screening. J. Raman Spectrosc. 2011, 42, 1548–1552. [Google Scholar] [CrossRef]
- Beattie, R.J.; Bell, S.J.; Farmer, L.J.; Moss, B.; Patterson, D. Preliminary investigation of the application of Raman spectroscopy to the prediction of the sensory quality of beef silverside. Meat Sci. 2004, 66, 903–913. [Google Scholar] [CrossRef] [PubMed]
- Vegarud, G.E.; Langsrud, T.; Svenning, C. Mineral-binding milk proteins and peptides; occurrence, biochemical and technological characteristics. Br. J. Nutr. 2000, 84, 91–98. [Google Scholar] [CrossRef]
- Bermejo, P.; Peña, E.M.; Domı́nguez, R.; Bermejo, A.; Cocho, J.A.; Fraga, J.M. Iron and zinc in hydrolised fractions of human milk and infant formulas using an in vitro method. Food Chem. 2002, 77, 361–369. [Google Scholar] [CrossRef]
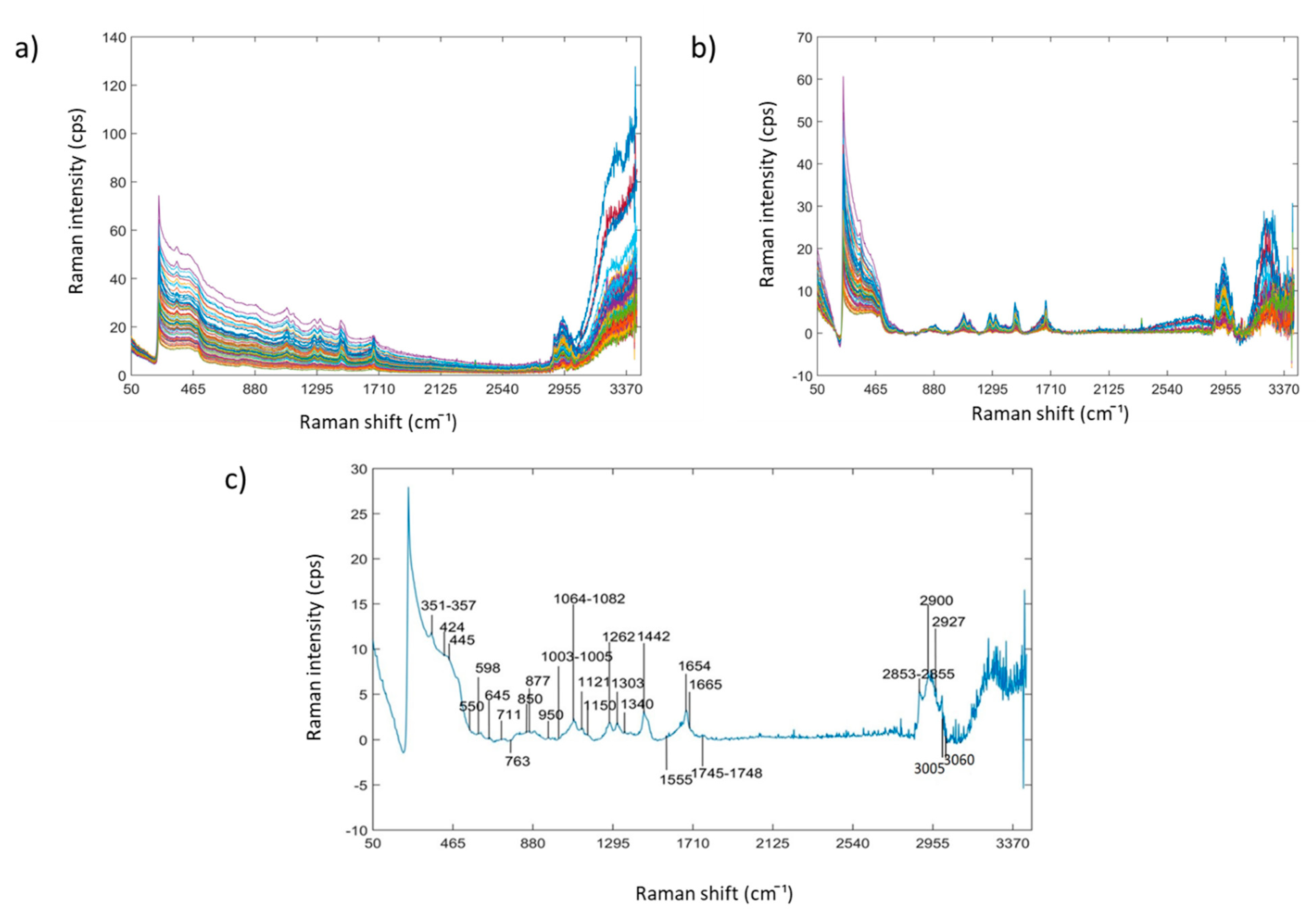
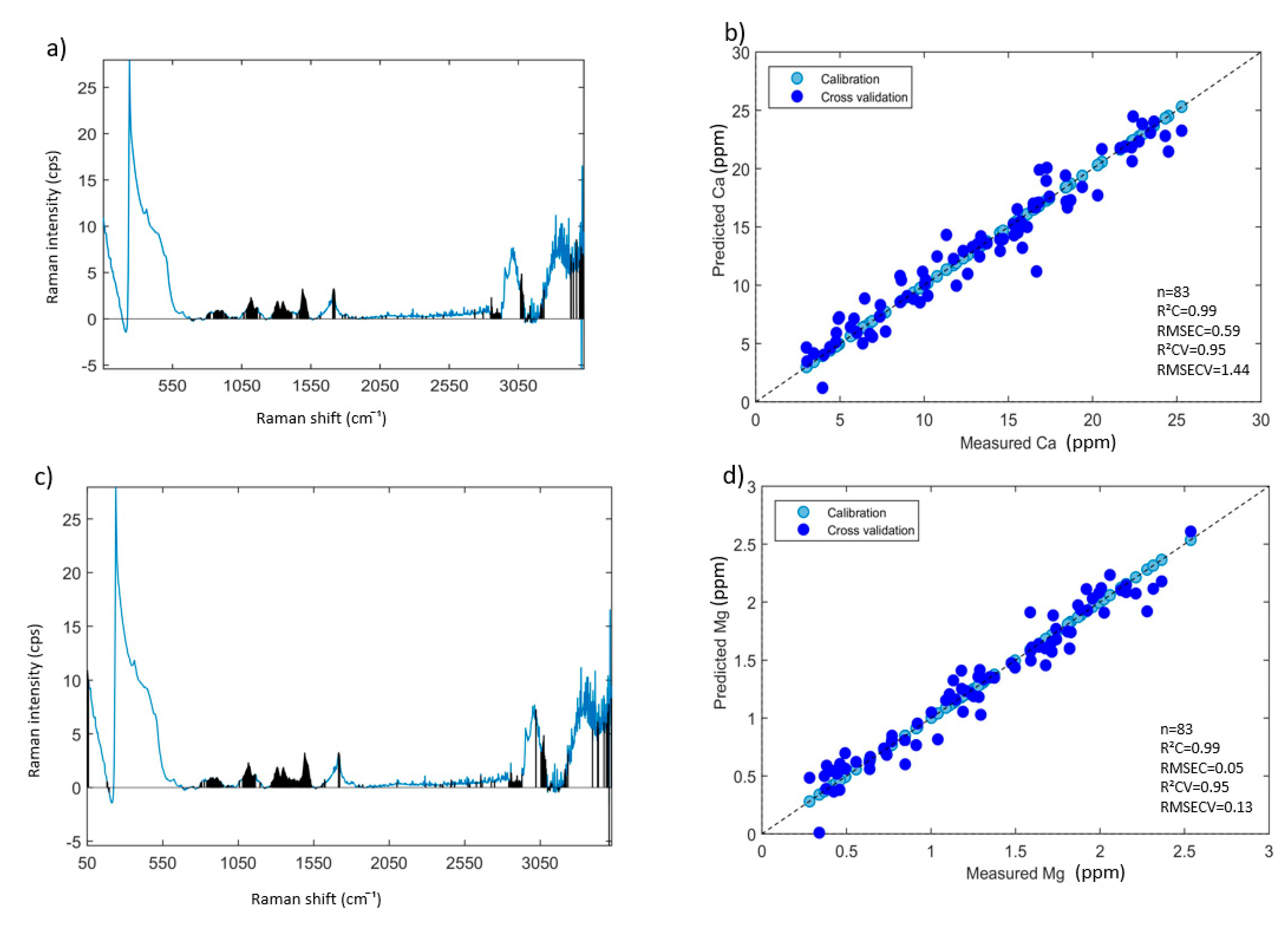
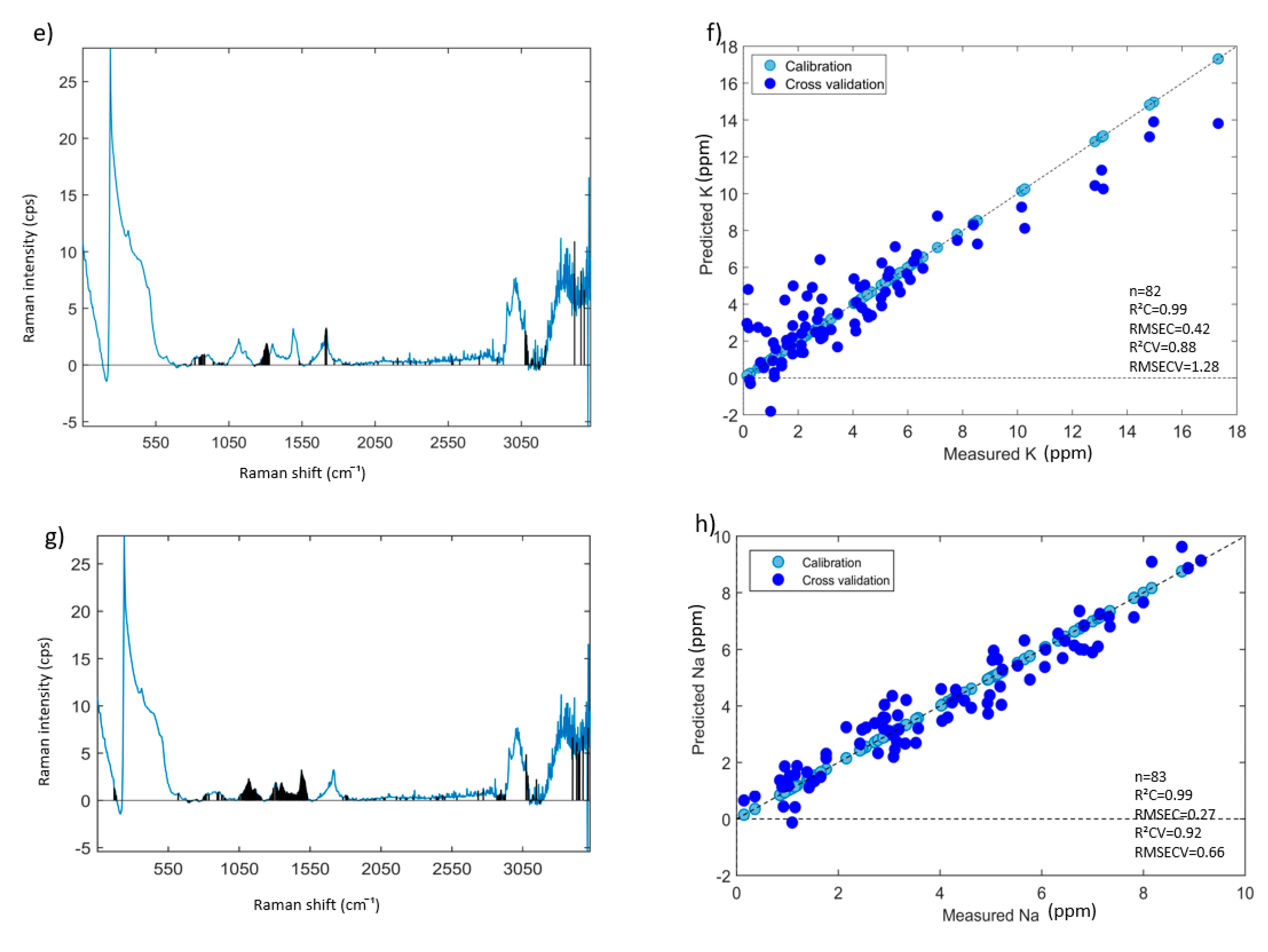
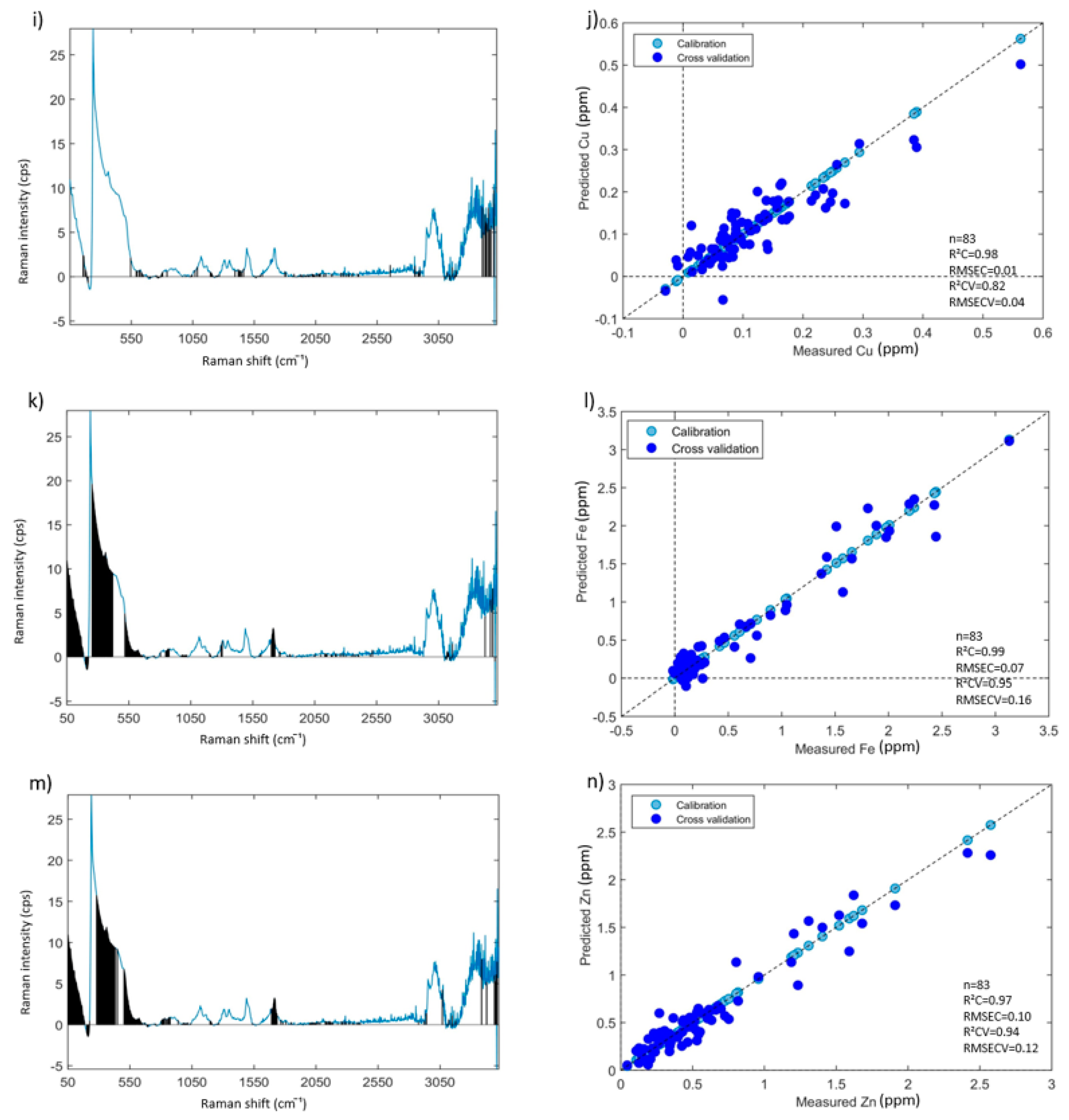
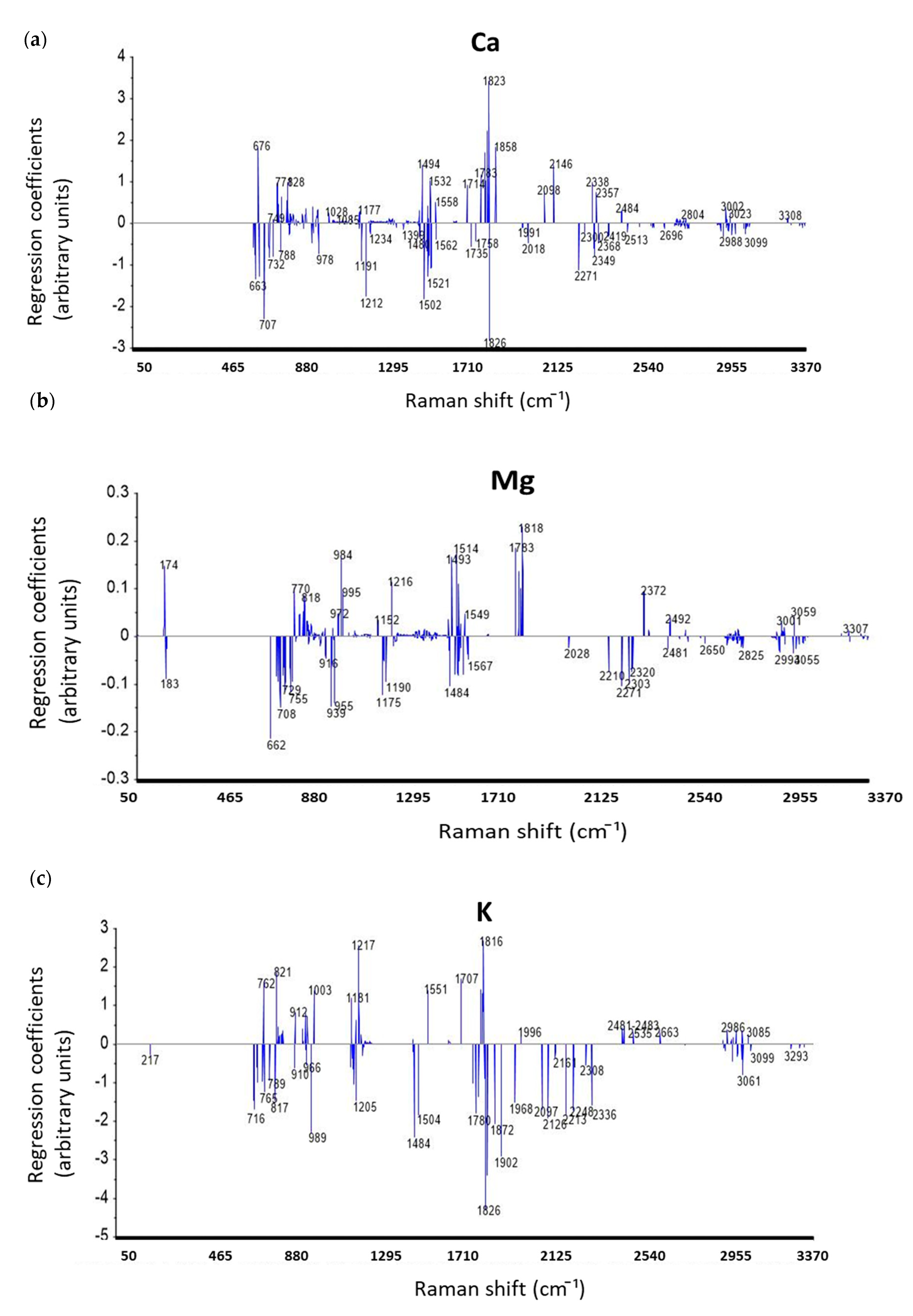
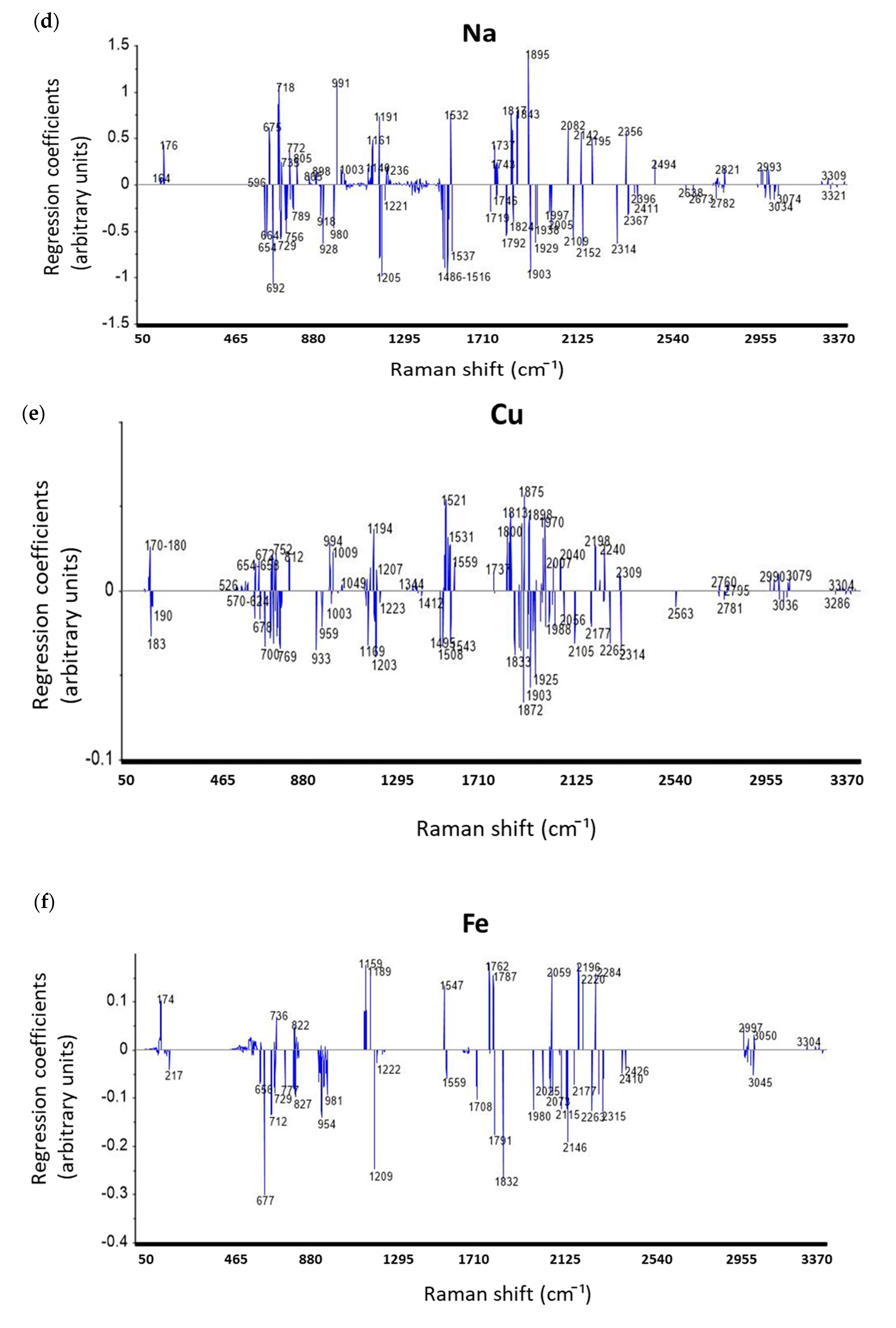
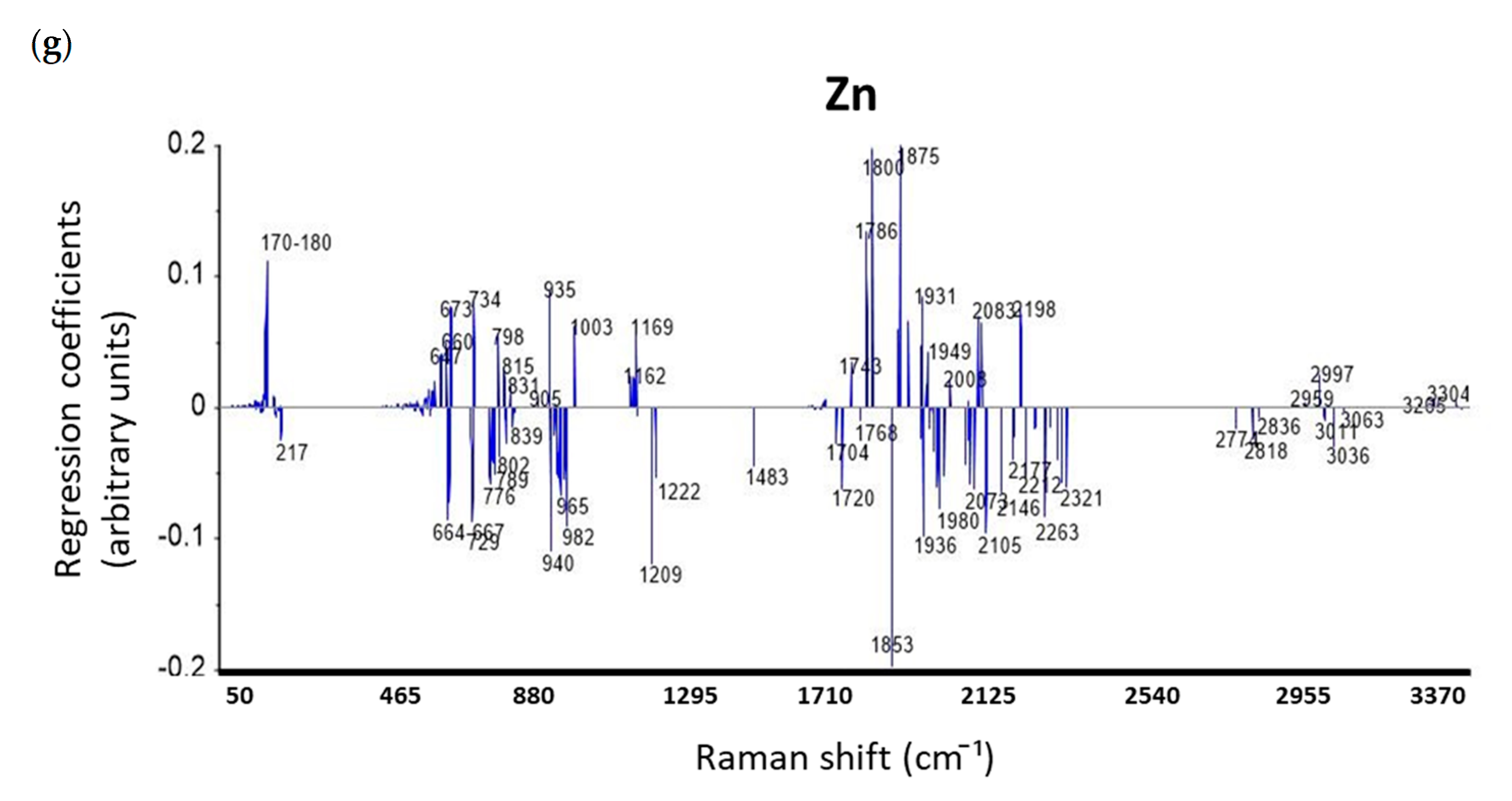
| Elements | ICP-AES Wavelength (nm) | Min (ppm) | Max (ppm) | Mean (ppm) | Median (ppm) | Repeatability (ppm) |
|---|---|---|---|---|---|---|
| Cu | 324.754 | 0.009 | 0.563 | 0.117 | 0.088 | 0.003 |
| Fe | 238.204 | 0.016 | 3.13 | 0.512 | 0.142 | 0.013 |
| Mn | 257.61 | −0.109 | 0.487 | 0 | −0.01 | 0.001 |
| Zn | 213.657 | 0.045 | 2.575 | 0.573 | 0.426 | 0.009 |
| Mg | 279.553 | 0.281 | 2.537 | 1.275 | 1.28 | 0.034 |
| Ca | 422.673 | 3.002 | 25.295 | 13.413 | 13.522 | 0.032 |
| Na | 588.995 | 0.144 | 9.133 | 4.055 | 3.571 | 0.034 |
| K | 766.491 | 0.129 | 17.319 | 4.227 | 2.919 | 0.017 |
| Element | Certified Value 1 | Measured Value 2 | Mean Recovery (%) |
|---|---|---|---|
| Cu (mg/kg) | 1.08 ± 0.06 | 1.94 ± 0.29 | 181.69 |
| Fe (mg/kg) | 4.6 ± 0.5 | 3.91 ± 0.76 | 87.86 |
| Mn(mg/kg) | 0.289 ± 0.018 | −0.56 ± 0.04 | −206.64 |
| Zn (mg/kg) | 44.8 ± 2.0 | 49.90 ± 4.82 | 112.09 |
| Mg (g/kg) | 1.26 ± 0.1 | 1.32 ± 0.12 | 106.19 |
| Ca (g/kg) | 13.9 ± 0.8 | 13.32 ± 0.71 | 96.44 |
| Na (g/kg) | 4.18 ± 0.19 | 1.87 ± 0.49 | 45.37 |
| K (g/kg) | 17 ± 0.7 | 14.62 ± 0.40 | 86.25 |
| Data Type | Raman Frequency (cm−1) | Calibration Samples | Spectral Variables | # PLS Loadings | R2C | RMSEC | R2CV | RMSECV | Bias | |
|---|---|---|---|---|---|---|---|---|---|---|
| Ca | AsLs | 50–3398 | 83 | 618 | 6 | 0.99 | 0.59 | 0.95 | 1.44 | 0 |
| air-PLS | 50–3398 | 83 | 605 | 4 | 0.92 | 1.77 | 0.86 | 2.36 | 0.074 | |
| BOC | 50–3398 | 83 | 782 | 4 | 0.86 | 2.37 | 0.84 | 2.53 | 0.041 | |
| S.G. 1st der. 7sm | 50–3398 | 83 | 708 | 5 | 0.96 | 1.2 | 0.87 | 2.29 | 0.033 | |
| Raw | 50–3398 | 83 | 1856 | 5 | 0.91 | 1.93 | 0.86 | 2.39 | 0.031 | |
| Mg | AsLs | 50–3398 | 83 | 683 | 6 | 0.99 | 0.05 | 0.95 | 0.13 | −0.002 |
| air-PLS | 50–3398 | 83 | 725 | 5 | 0.93 | 0.15 | 0.88 | 0.21 | 0.002 | |
| BOC | 50–3398 | 83 | 2611 | 4 | 0.86 | 0.23 | 0.83 | 0.25 | −0.002 | |
| S.G. 1st der. 7sm | 50–3398 | 83 | 707 | 3 | 0.86 | 0.23 | 0.82 | 0.26 | 0.007 | |
| Raw | 50–3398 | 83 | 736 | 4 | 0.89 | 0.2 | 0.87 | 0.22 | 0.005 | |
| K | AsLs | 50–3398 | 82 | 195 | 6 | 0.99 | 0.42 | 0.88 | 1.28 | −0.029 |
| air-PLS | 50–3398 | 82 | 1329 | 3 | 0.56 | 2.44 | 0.44 | 2.78 | 0.027 | |
| BOC | 50–3398 | 82 | 3473 | 4 | 0.55 | 2.46 | 0.41 | 2.86 | 0.021 | |
| S.G. 1st der. 7sm | 50–3398 | 82 | 525 | 2 | 0.54 | 2.48 | 0.47 | 2.7 | 0.001 | |
| Raw | 50–3398 | 82 | 311 | 4 | 0.47 | 2.67 | 0.36 | 2.96 | 0.027 | |
| Na | AsLs | 50–3398 | 83 | 463 | 6 | 0.99 | 0.27 | 0.92 | 0.66 | −0.038 |
| air-PLS | 50–3398 | 83 | 302 | 3 | 0.71 | 1.26 | 0.66 | 1.36 | 0.012 | |
| BOC | 50–3398 | 83 | 2611 | 4 | 0.87 | 0.22 | 0.85 | 0.24 | 0 | |
| S.G. 1st der. 7sm | 50–3398 | 83 | 622 | 2 | 0.74 | 1.18 | 0.71 | 1.27 | 0.012 | |
| Raw | 50–3398 | 83 | 794 | 4 | 0.76 | 1.14 | 0.67 | 1.36 | 0.003 | |
| Cu | AsLs | 50–3398 | 83 | 262 | 6 | 0.98 | 0.013 | 0.82 | 0.04 | −0.001 |
| air-PLS | 50–3398 | 83 | 1433 | 5 | 0.75 | 0.05 | 0.31 | 0.08 | 0 | |
| BOC | 50–3398 | 83 | 2681 | 2 | 0.38 | 0.07 | 0.34 | 0.08 | 0 | |
| S.G. 1st der. 7sm | 50–3398 | 83 | 778 | 3 | 0.61 | 0.06 | 0.29 | 0.08 | 0.002 | |
| Raw | 50–3398 | 83 | 3356 | 2 | 0.38 | 0.07 | 0.33 | 0.08 | 0 | |
| Fe | AsLs | 50–3398 | 83 | 643 | 5 | 0.99 | 0.07 | 0.95 | 0.16 | 0.003 |
| air-PLS | 50–3398 | 83 | 410 | 3 | 0.91 | 0.22 | 0.89 | 0.24 | −0.001 | |
| BOC | 50–3398 | 83 | 3473 | 4 | 0.9 | 0.22 | 0.87 | 0.26 | 0.001 | |
| S.G. 1st der. 7sm | 50–3398 | 83 | 372 | 4 | 0.95 | 0.16 | 0.91 | 0.22 | 0.006 | |
| Raw | 50–3398 | 83 | 3356 | 4 | 0.9 | 0.22 | 0.87 | 0.26 | 0.003 | |
| Zn | AsLs | 50–3398 | 83 | 683 | 4 | 0.97 | 0.1 | 0.94 | 0.12 | −0.001 |
| air-PLS | 50–3398 | 83 | 569 | 3 | 0.83 | 0.21 | 0.78 | 0.24 | 0.003 | |
| BOC | 50–3398 | 83 | 715 | 5 | 0.99 | 0.05 | 0.94 | 0.12 | −0.009 | |
| S.G. 1st der. 7sm | 50–3398 | 83 | 686 | 5 | 0.92 | 0.14 | 0.87 | 0.18 | 0.003 | |
| Raw | 50–3398 | 83 | 192 | 2 | 0.76 | 0.25 | 0.7 | 0.28 | −0.005 |
| Raman Frequency (cm−1) | Spectral Variables | Calibration Samples | #PLS Loadings | R2C | RMSEC | Bias | R2CV | RMSECV | Bias | Validation Samples | R2P | RMSEP | Bias | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ca | 50–3398 | 910 | 42 | 5 | 0.99 | 0.62 | 0.000 | 0.93 | 1.75 | −0.186 | 41 | 0.76 | 3.05 | −0.456 |
| 50–1800 | 322 | 42 | 4 | 0.98 | 0.96 | 0.000 | 0.93 | 1.68 | −0.017 | 41 | 0.75 | 3.17 | −0.205 | |
| Mg | 50–3398 | 450 | 42 | 3 | 0.98 | 0.10 | 0.000 | 0.95 | 0.15 | 0.006 | 41 | 0.75 | 0.29 | −0.027 |
| 50–1800 | 476 | 42 | 4 | 0.98 | 0.08 | 0.000 | 0.94 | 0.15 | −0.007 | 41 | 0.77 | 0.29 | −0.035 | |
| K | 50–3398 | 87 | 41 | 5 | 0.95 | 0.87 | 0.000 | 0.75 | 1.95 | 0.025 | 41 | 0.22 | 3.80 | 0.800 |
| 50–1800 | 125 | 41 | 7 | 0.99 | 0.30 | 0.000 | 0.91 | 1.15 | −0.099 | 41 | 0.31 | 3.45 | 1.120 | |
| Na | 50–3398 | 317 | 42 | 4 | 0.99 | 0.24 | 0.000 | 0.93 | 0.63 | 0.005 | 41 | 0.57 | 1.54 | 0.241 |
| 50–1800 | 211 | 42 | 6 | 0.99 | 0.20 | 0.000 | 0.90 | 0.74 | 0.014 | 41 | 0.6 | 1.51 | 0.235 | |
| Cu | 50–3398 | 620 | 42 | 5 | 0.98 | 0.01 | 0.000 | 0.75 | 0.04 | −0.029 | 41 | 0.08 | 0.11 | −0.029 |
| 50–1800 | 181 | 42 | 6 | 0.99 | 0.01 | 0.000 | 0.85 | 0.03 | −0.001 | 41 | 0.04 | 0.11 | −0.025 | |
| Fe | 50–3398 | 466 | 42 | 4 | 0.99 | 0.07 | 0.000 | 0.97 | 0.12 | 0.004 | 41 | 0.84 | 0.30 | 0.008 |
| 50–1800 | 211 | 42 | 6 | 0.99 | 0.20 | 0.000 | 0.90 | 0.74 | 0.014 | 41 | 0.6 | 1.51 | 0.235 | |
| Zn | 50–3398 | 1465 | 42 | 5 | 0.99 | 0.03 | 0.000 | 0.94 | 0.12 | 0.000 | 41 | 0.8 | 0.25 | 0.019 |
| 50–1800 | 869 | 42 | 6 | 1.00 | 0.03 | 0.000 | 0.93 | 0.12 | −0.006 | 41 | 0.76 | 0.26 | 0.014 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, M.; Shaikh, S.; Kang, R.; Markiewicz-Keszycka, M. Investigation of Raman Spectroscopy (with Fiber Optic Probe) and Chemometric Data Analysis for the Determination of Mineral Content in Aqueous Infant Formula. Foods 2020, 9, 968. https://doi.org/10.3390/foods9080968
Zhao M, Shaikh S, Kang R, Markiewicz-Keszycka M. Investigation of Raman Spectroscopy (with Fiber Optic Probe) and Chemometric Data Analysis for the Determination of Mineral Content in Aqueous Infant Formula. Foods. 2020; 9(8):968. https://doi.org/10.3390/foods9080968
Chicago/Turabian StyleZhao, Ming, Saif Shaikh, Renxi Kang, and Maria Markiewicz-Keszycka. 2020. "Investigation of Raman Spectroscopy (with Fiber Optic Probe) and Chemometric Data Analysis for the Determination of Mineral Content in Aqueous Infant Formula" Foods 9, no. 8: 968. https://doi.org/10.3390/foods9080968
APA StyleZhao, M., Shaikh, S., Kang, R., & Markiewicz-Keszycka, M. (2020). Investigation of Raman Spectroscopy (with Fiber Optic Probe) and Chemometric Data Analysis for the Determination of Mineral Content in Aqueous Infant Formula. Foods, 9(8), 968. https://doi.org/10.3390/foods9080968





