Effect of L-cysteine, Boswellia serrata, and Whey Protein on the Antioxidant and Physicochemical Properties of Pork Patties
Abstract
1. Introduction
2. Materials and Methods
2.1. Pork Patty Formulation and Processing
2.2. Proximate Composition
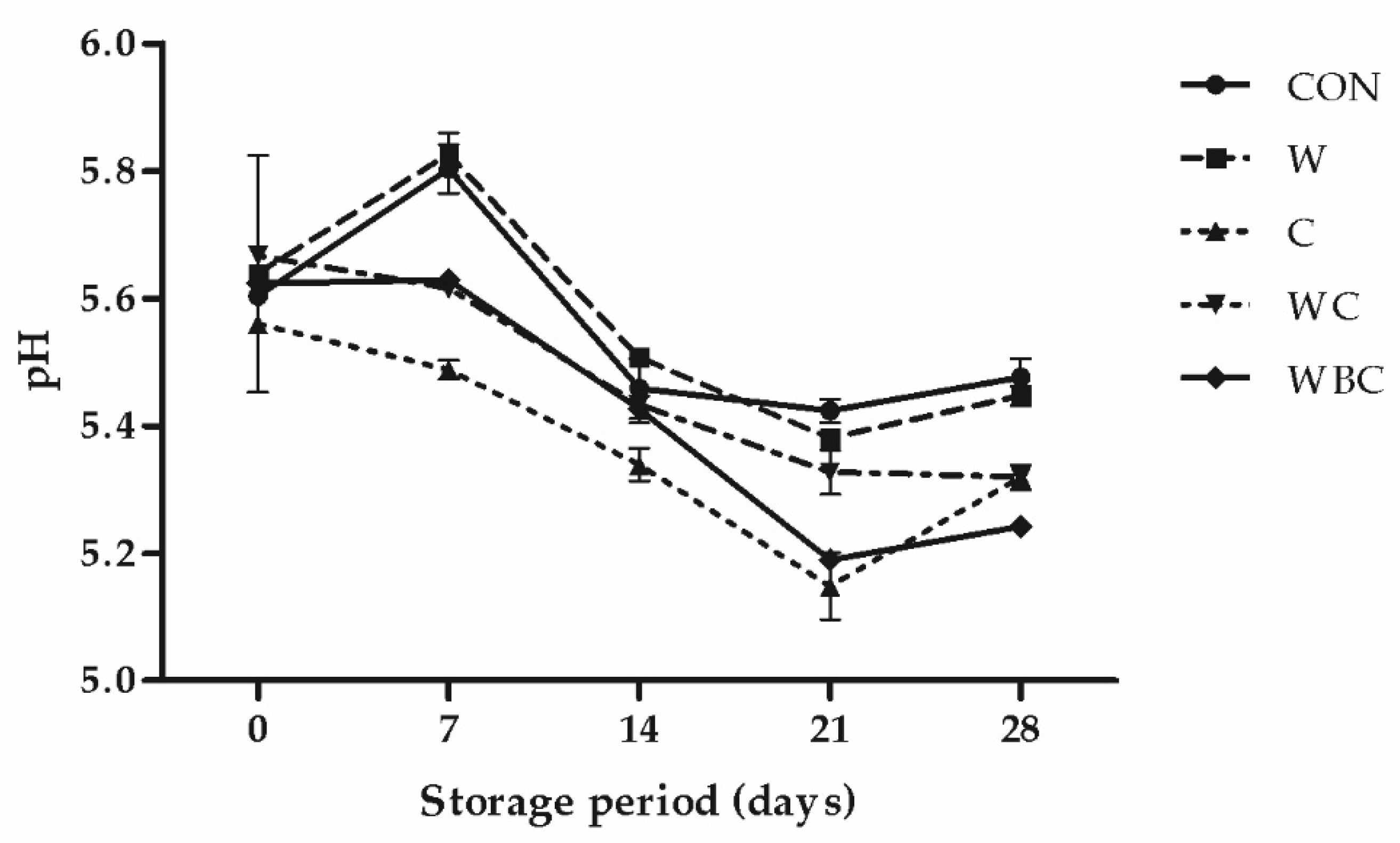
2.3. pH
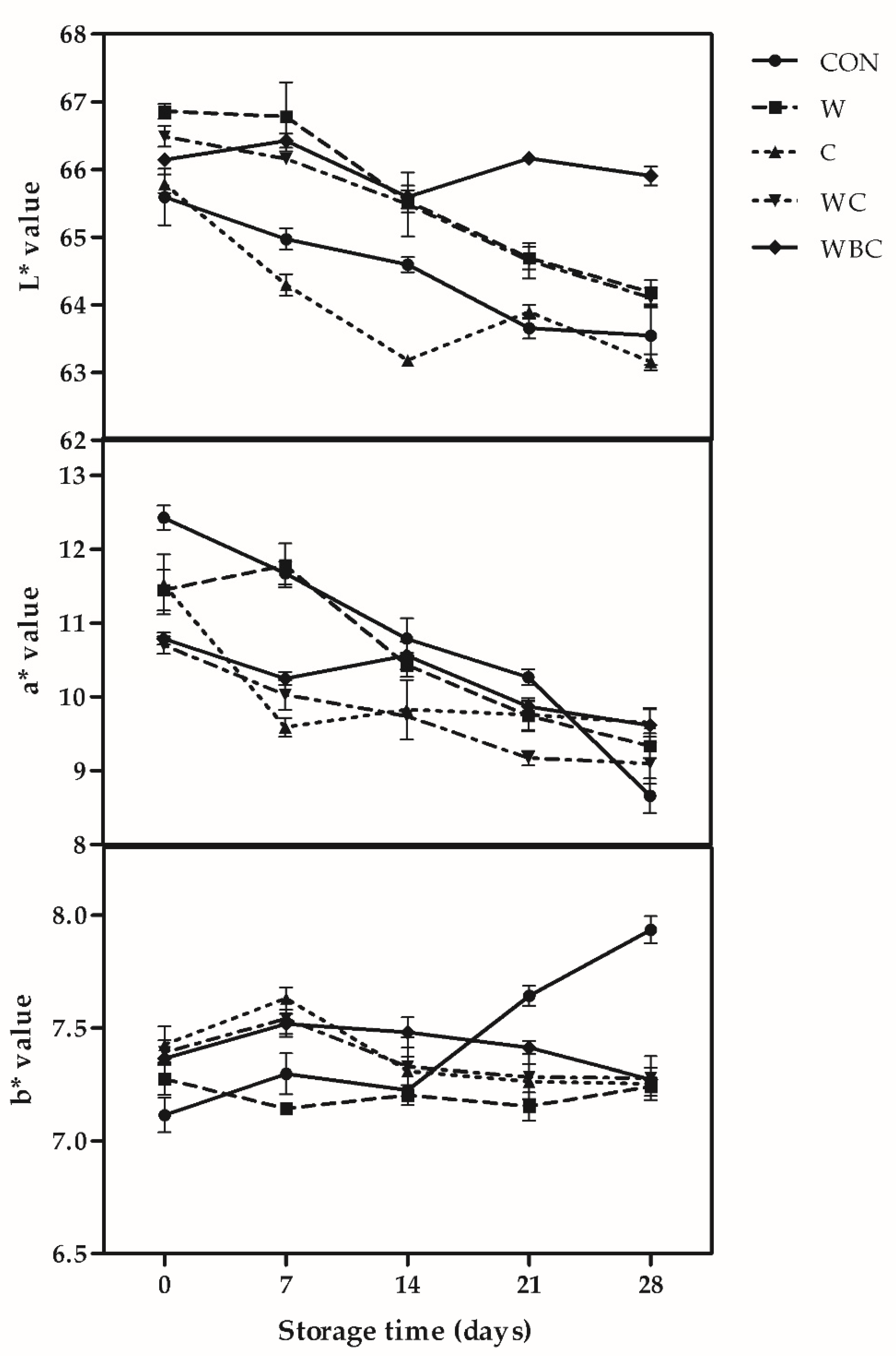
2.4. Color
2.5. Water-Holding Capacity (WHC)
2.6. Cooking Loss and Texture Profile Analysis (TPA)
2.7. Sensory Evaluation
2.8. Thiobarbituric Acid-Reactive Substances (TBARS)
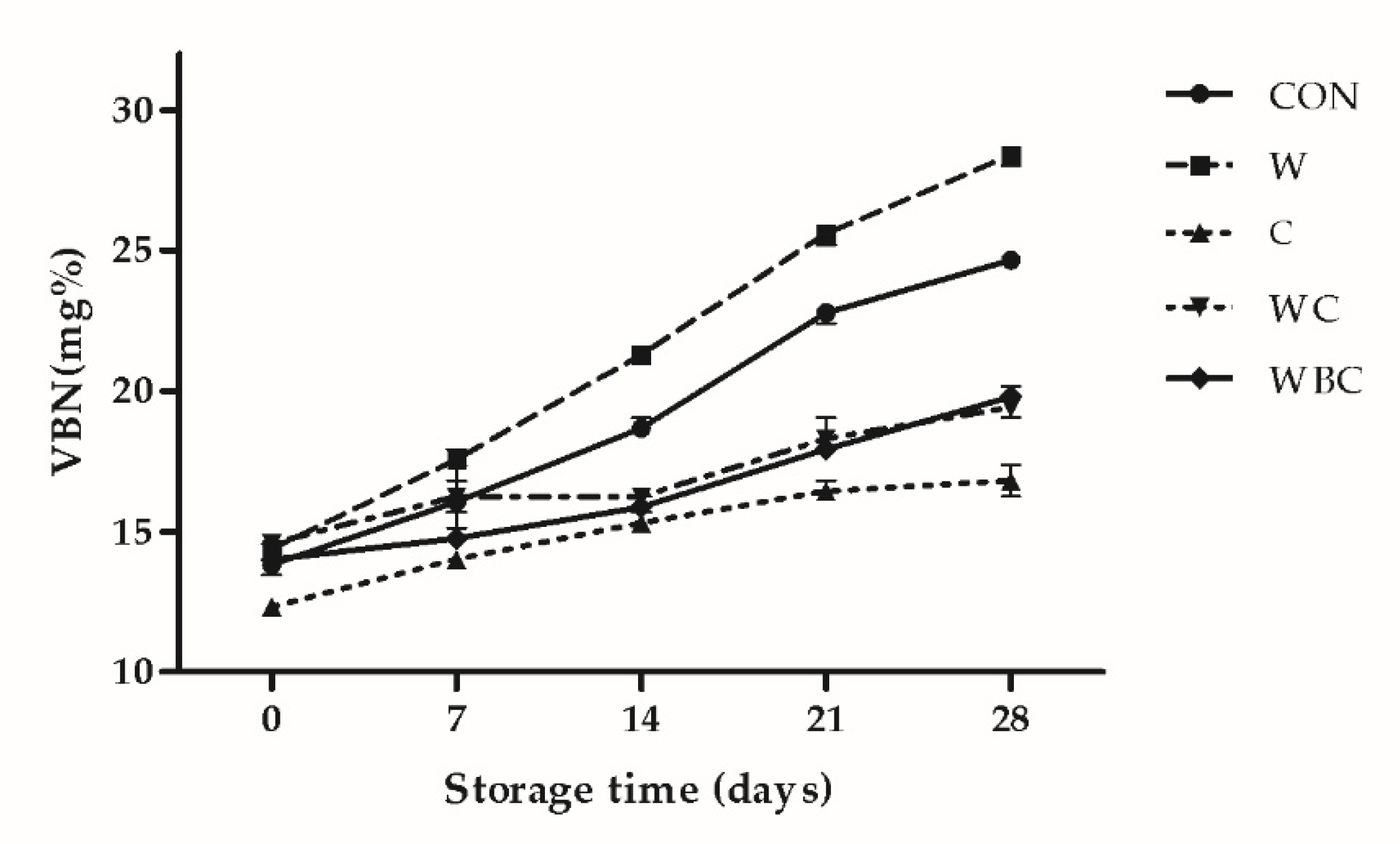
2.9. Volatile Basic Nitrogen (VBN)
2.10. 2-Diphenyl-1-Picrylhydrazyl (DPPH) Radical-Scavenging Activity
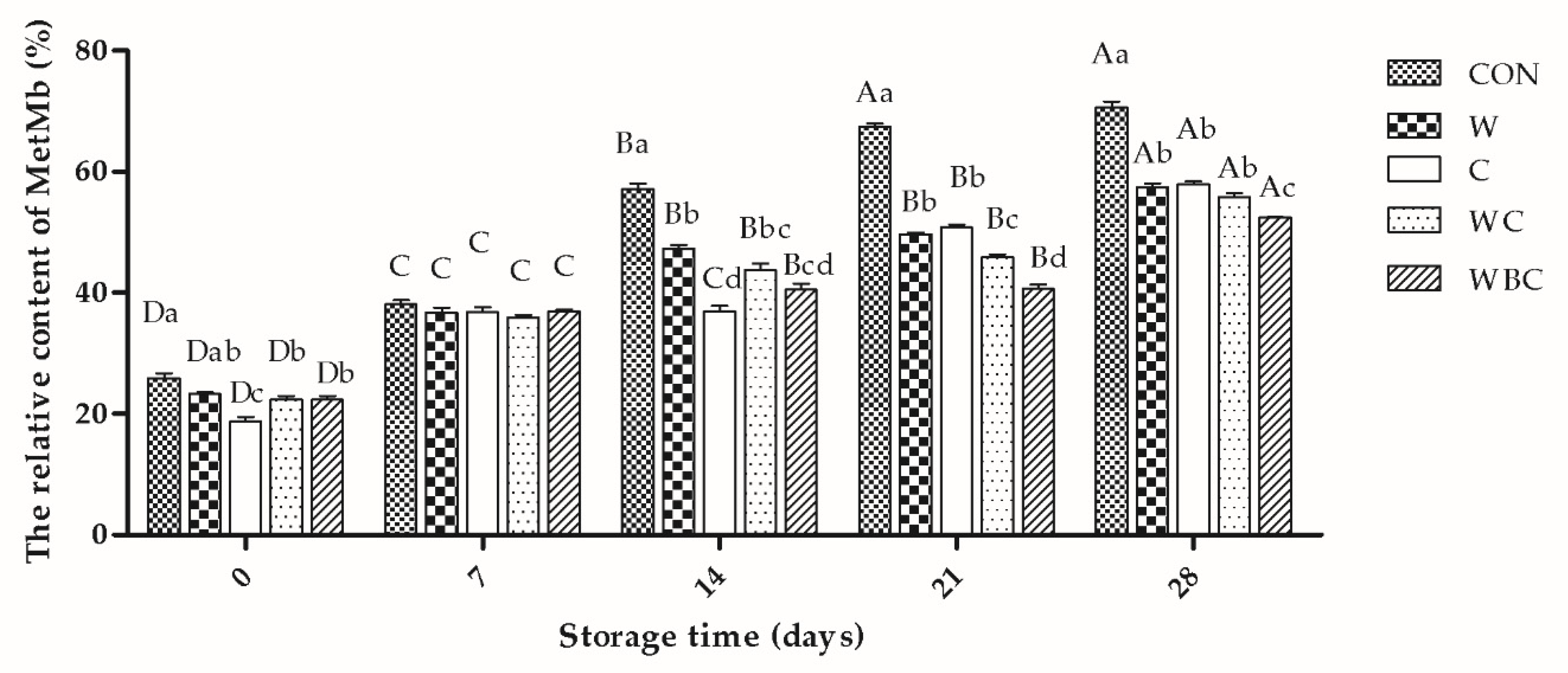
2.11. Metmyoglobin (MetMb)
2.12. Statistical Analysis
3. Results and Discussion
3.1. Proximate Analysis
3.2. WHC, Cooking Loss, and Texture Profile Analysis (TPA)
3.3. Sensory Evaluation
3.4. pH Analysis
3.5. Color Stability
3.6. VBN Content
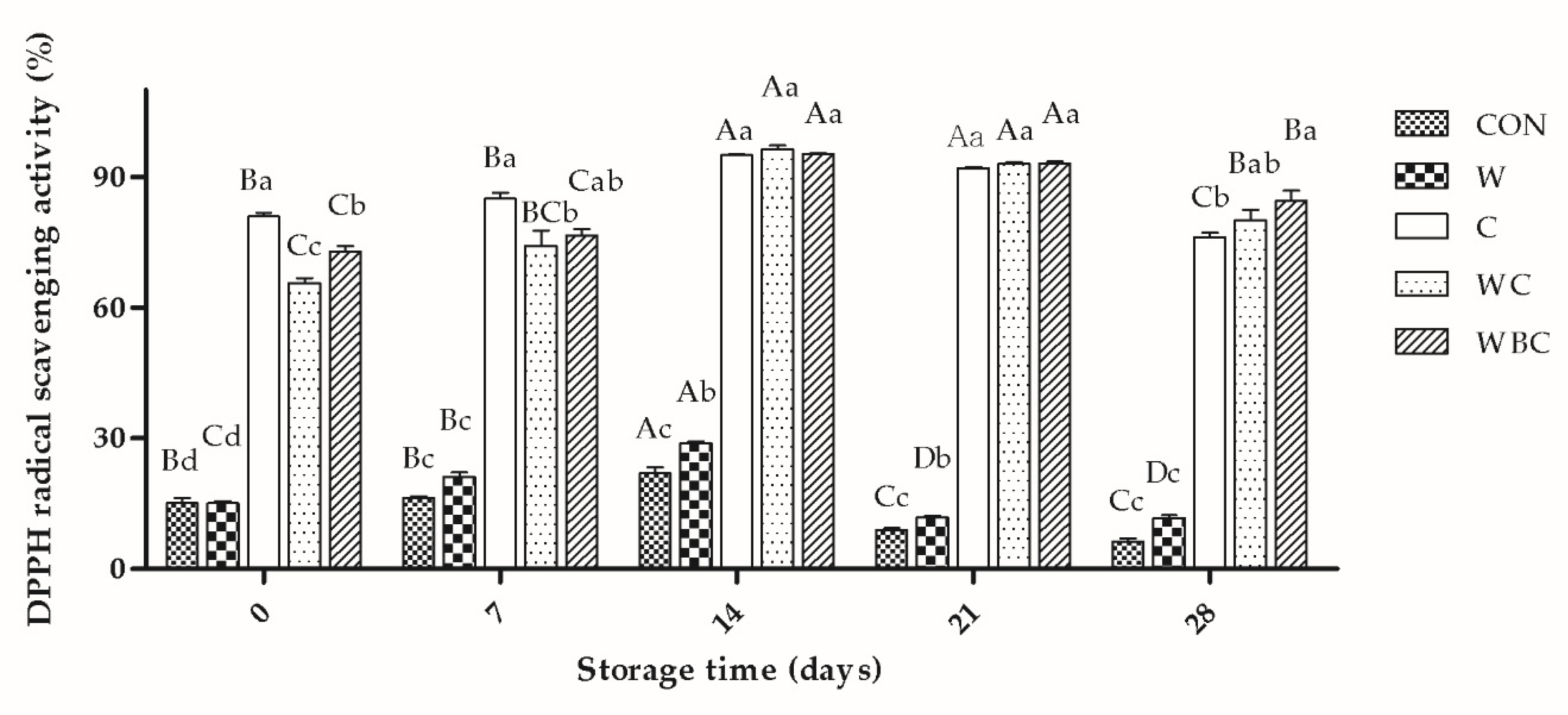
3.7. Antioxidant Activity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lantto, R.; Plathin, P.; Niemistö, M.; Buchert, J.; Autio, K. Effects of transglutaminase, tyrosinase and freeze-dried apple pomace powder on gel forming and structure of pork meat. LWT Food Sci. Technol. 2006, 39, 1117–1124. [Google Scholar] [CrossRef]
- Rysman, T.; Van Heckee, T.; Van Poucke, C.; De Smet, S.; Van Royen, G. Protein oxidation and proteolysis during storage and in vitro digestion of pork and beef patties. Food Chem. 2016, 209, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.L. Protein oxidation and implications for muscle food quality. In Antioxidants in Muscle Foods; Decker, E.A., Faustman, C., Lopez-Bote, C., Eds.; Wiley: New York, NY, USA, 2000; pp. 85–111. [Google Scholar]
- Shang, X.; Zhou, Z.; Jiang, S.; Guo, H.; Lu, Y. Interrelationship between myoglobin oxidation and lipid oxidation during the processing of Cantonese sausage with d-sodium erythorbate. J. Sci. Food Agric. 2020, 100, 1022–1029. [Google Scholar] [CrossRef]
- Ferreira, V.C.S.; Morcuende, D.; Madruga, M.S.; Silva, F.A.P.; Estevez, M. Role of protein oxidation in the nutritional loss and texture changes in ready-to-eat chicken patties. Int. J. Food Sci. Technol. 2018, 53, 1518–1526. [Google Scholar] [CrossRef]
- Nieto, G.; Jongberg, S.; Andersen, M.L.; Skibsted, L.H. Thiol oxidation and protein cross-link formation during chill storage of pork patties added essential oil of oregano, rosemary, or garlic. Meat Sci. 2013, 95, 177–184. [Google Scholar] [CrossRef]
- Barber, L.I.; Obinna-Echem, P.C.; Hart, B.E. Proximate composition, thiobarbituric acid and sensory properties of chicken-breadfruit patties wth piper guineense and monodora myristica oleoresins. Am. J. Food Sci. Tech. 2020, 8, 56–62. [Google Scholar]
- Lara, M.S.; Gutierrez, J.I.; Timon, M.; Andres, A.I. Evaluation of two natural extracts (Rosmarinus officinalis L. and Melissa officinalis L.) as antioxidants in cooked pork patties packed in MAP. Meat Sci. 2011, 88, 481–488. [Google Scholar] [CrossRef]
- Lucera, A.; Costa, C.; Conte, A.; Del Nobile, M.A. Food applications of natural antimicrobial compounds. Front. Microbiol. 2012, 3, 1–13. [Google Scholar] [CrossRef]
- Shah, M.A.; Bosco, S.J.D.; Mir, S.A. Plant extracts as natural antioxidants in meat and meat products. Meat Sci. 2014, 98, 21–33. [Google Scholar] [CrossRef]
- Rodriguez-Carpena, J.G.; Morcuende, D.; Estevez, M. Avocado by-products as inhibitors of color deterioration and lipid and protein oxidation in raw porcine patties subjected to chilled storage. Meat Sci. 2011, 89, 166–173. [Google Scholar] [CrossRef]
- Jia, N.; Kong, B.; Liu, Q.; Diao, X.; Xia, X. Antioxidant activity of black currant (Ribes nigrum L.) extract and its inhibitory effect on lipid and protein oxidation of pork patties during chilled storage. Meat Sci. 2012, 91, 533–539. [Google Scholar] [CrossRef]
- Grandhi, R.R.; Cliplef, R.L. Effects of selection for lower backfat, and increased levels of dietary amino acids to digestible energy on growth performance, carcass merit and meat quality in boars, gilts, and barrows. Can. J. Anim. Sci. 1997, 77, 487–496. [Google Scholar] [CrossRef]
- Zhou, C.; Li, J.; Tan, S.; Sun, G. Effects of L-Arginine on Physicochemical and Sensory Characteristics of Pork Sausage. Adv. J. Food Sci. Technol. 2014, 6, 660–667. [Google Scholar] [CrossRef]
- Guerrero-Beltran, J.A.; Swanson, B.G.; Barbosa-Canovas, G.V. Inhibition of polyphenoloxidase in mango puree with 4-hexylresorcinol, cysteine and ascorbic acid. LWT Food Sci. Technol. 2005, 38, 625–630. [Google Scholar] [CrossRef]
- Piste, P. Cysteine-masterantioxidant. Int. J. Pharm. Chem. Biol. Sci. 2013, 3, 143–149. [Google Scholar]
- Elias, R.J.; McClements, D.J.; Decker, E.A. Antioxidant activity of cysteine, tryptophan, and methionine residues in continuous phase β-Lactoglobulin in oil-in-water emulsions. J. Agric. Food Chem. 2005, 53, 10248–10253. [Google Scholar] [CrossRef]
- Cao, C.; Xie, J.; Hou, L.; Zhao, J.; Chen, F.; Xiao, Q.; Zhao, M.; Fan, M. Effect of glycine on reaction of cysteine-xylose: Insights on initial Maillard stage intermediates to develop meat flavor. Food Res. Int. 2017, 99, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Ghezzi, P.; Lemley, K.V.; Andrus, J.P.; De Rosa, S.C.; Holmgren, A.; Jones, D.; Jahoor, F.; Kopke, R.; Cotgreave, I.; Bottiglieri, T.; et al. Cysteine/Glutathione Deficiency: A Significant and Treatable Corollary of Disease. In The Therapeutic Use of N-Acetylcysteine (NAC) in Medicine; Adis: Singapore, 2019; pp. 349–386. [Google Scholar]
- Wu, Y.; Zhang, Y.; Wang, L.; Huyan, Y.; Li, H.; Pei, Z.; Tang, Y.; Sun, S.; Xu, Y. A simple ratiometric fluorescent sensor selectively compatible of different combinations of characteristic groups for identification of glutathione, cysteine and homocysteine. Sens. Actuators B Chem. 2020, 302, 127181. [Google Scholar] [CrossRef]
- Shin, H.S.; Rodgers, W.J.; Gomaa, A.; Strasburg, G.M.; Gray, J.I. Inhibition of heterocyclic aromatic amine formation in fried ground beef patties by garlic and selected garlic-related sulfur compounds. J. Food Sci. 2002, 65, 1766–1770. [Google Scholar] [CrossRef]
- Kiczorowska, B.; Samolinska, W.; AI-Yasiry, A.R.M.; Kowalczyk-Pecka, D. Effect of Boswellia serrata dietary supplementation on growth performance, gastrointestinal microflora, and morphology of broilers. Ann. Anim. Sci. 2016, 16, 835–849. [Google Scholar] [CrossRef]
- Kiczorowska, B.; Samolinska, W.; AI-Yasiry, A.; Zajac, M. Immunomodulant feed supplement Boswellia serrata to support broiler chickens’ health and dietary and technological meat quality. Poult. Sci. 2020, 99, 1052–1061. [Google Scholar] [CrossRef] [PubMed]
- Ceylan, E.; Fung, D.Y.C. Antimicrobial activity of spices. J. Rapid Methods Autom. Microbiol. 2004, 12, 1–55. [Google Scholar] [CrossRef]
- Oswell, N.J.; Thippareddi, H.; Pegg, R.B. Practical use of natural antioxidants in meat products in the U.S.: A review. Meat Sci. 2018, 145, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Cho, W.Y.; Lee, N.; Kim, D.H.; Lee, J.; Lee, H.J.; Seo, H.G.; Lee, C.H. Effects of Boswella serrata and whey protein powders on physicochemical properties of pork patties. Foods 2020, 9, 334. [Google Scholar] [CrossRef] [PubMed]
- Majcher, M.A.; Jelen, H.H. Effect of cysteine and cystine addition on sensory profile and potent odorants of extruded potato snacks. J. Agric. Food Chem. 2007, 55, 5754–5760. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis, 19th ed.; AOAC International: Gaithersburg, MD, USA, 2012. [Google Scholar]
- Akwetey, W.Y.; Yamoah, G.A. Producing low-fat pork patties with solar-dried plantain (Musa Acuminate) flour. J. Anim. Sci. Adv. 2013, 3, 150–156. [Google Scholar] [CrossRef]
- Bourne, M.C. Texture profile analysis. Food Tech. 1978, 32, 62–66. [Google Scholar]
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. In Methods in Enzymology; Academic Press: New York, NY, USA, 1978; pp. 302–310. [Google Scholar]
- Conway, E.J. Determination of volatile amines. In Microdiffusion Analysis and Volumetric Error, 5th ed.; Crosby Lockwood: London, UK, 1962; pp. 195–200. [Google Scholar]
- Overland, M.; Borge, G.I.; Vogt, G.; Schoyen, H.F.; Skrede, A. Oxidative stability and sensory quality of meat from broiler chickens fed a bacterial meal produced on natural gas. Poult. Sci. 2011, 90, 201–210. [Google Scholar] [CrossRef]
- Warriss, P.D. The extraction of haem pigments from fresh meat. J. Food Technol. 1979, 14, 75–80. [Google Scholar] [CrossRef]
- Ha, J.H.; Lee, J.H.; Lee, J.J.; Choi, Y.I.; Lee, H.J. Effects of whey protein injection as a curing solution on chicken breast meat. Food Sci. Anim. Resour. 2019, 39, 494–502. [Google Scholar] [CrossRef]
- Cheng, Q.; Sun, D.W. Factors affecting the water holding capacity of red meat products: A review of recent research advances. Crit. Rev. Food Sci. 2008, 48, 137–159. [Google Scholar] [CrossRef] [PubMed]
- Vlahova-Vangelova, D.B.; Balev, D.K.; Dragoev, S.G.; Kirisheva, G.D. Improvement of technological and sensory properties of meat by whey marinating. Sci. Works Univ. Food Technol. 2016, 63, 7–13. [Google Scholar]
- Yuksel, Z.; Avci, E.; Erdem, Y.K. Characterization of binding interactions between green tea flavanoids and milk proteins. Food Sci. 2010, 121, 450–456. [Google Scholar] [CrossRef]
- Ning, C.; Bao, P.; Zhang, D.; Li, L.; Chen, L.; Fang, H.; Tang, Y.; Zhou, C. Reduction and coordination properties of L-Lysine/L-arginine/L-cysteine for the improvement of the color of cured sausage. Food Sci. 2020, 312, 126122. [Google Scholar] [CrossRef] [PubMed]
- Heywood, A.A.; Myers, D.J.; Bailey, T.B.; Johnson, L.A. Effect of value-enhanced texturized soy protein on the sensory and cooking properties of beef patties. J. Am. Oil Chem. Soc. 2002, 79, 703–707. [Google Scholar] [CrossRef]
- Stagsted, J.; Bendixen, E.; Andersen, H.J. Identification of specific oxidatively modified proteins in chicken muscles using a combined immunologic and proteomic approach. J. Agric. Food Chem. 2004, 52, 3967–3974. [Google Scholar] [CrossRef]
- Lund, M.N.; Lametsch, R.; Hviid, M.S.; Jensen, O.N.; Skibsted, L.H. High-oxygen packaging atmosphere influences protein oxidation and tenderness of porcine longissimus dorsi during chill storage. Meat Sci. 2007, 77, 295–303. [Google Scholar] [CrossRef]
- Ulu, H. Effect of wheat flour, whey protein concentrate and soya protein isolate on oxidative processes and textural properties of cooked meatballs. Food Chem. 2004, 87, 523–529. [Google Scholar] [CrossRef]
- Meynier, A.; Mottram, D.S. The effect of pH on the formation of volatile compounds in meat-related model systems. Food Chem. 1995, 52, 361–366. [Google Scholar] [CrossRef]
- Menis-Henrique, M.E.C.; Janzantti, N.S.; Monteiro, M.; Conti-Silva, A.C.C. Physical and sensory characteristics of cheese-flavored expanded snacks obtained using butyric acid and cysteine as aroma precursors: Effects of extrusion temperature and sunflower oil content. LWT Food Sci. Technol. 2020, 122, 109001. [Google Scholar] [CrossRef]
- Jayawardana, B.C.; Liyanage, R.; Lalantha, N.; Iddamalgoda, S.; Weththasinghe, P. Antioxidant and antimicrobial activity of drumstick (Moringa oleifera) leaves in herbal chicken sausages. LWT Food Sci. Technol. 2015, 64, 1204–1208. [Google Scholar] [CrossRef]
- Park, S.Y.; Chin, K.B. Evaluation of pre-heating and extraction solvents in antioxidant and antimicrobial activities of garlic, and their application in fresh pork patties. Int. J. Food Sci. Technol. 2010, 45, 365–373. [Google Scholar] [CrossRef]
- Aktas, N.; Aksu, M.I.; Kaya, M. The effect of organic acid marination on tenderness, cooking loss and bound water content of beef. J. Muscle Foods 2003, 14, 181–194. [Google Scholar] [CrossRef]
- Lu, X.; Cornforth, D.P.; Carpenter, C.E.; Zhu, L.; Luo, X. Effect of oxygen concentration in modified atmosphere packaging on color changes of the M. longissimus thoraces et lumborum from dark cutting beef carcasses. Meat Sci. 2020, 161, 107999. [Google Scholar] [CrossRef] [PubMed]
- Ozer, O.; Saricoban, C.; Unal, K. The Effects of phytic acid, carnosine and butylated hydroxylanisole on some properties of mechanically deboned chicken patties during frozen storage. Selcuk J. Agric. Food Sci. 2018, 32, 502–509. [Google Scholar]
- McCarthy, T.L.; Kerry, J.P.; Kerry, J.F.; Lynch, P.B.; Buckley, D.J. Evaluation of the antioxidant potential of natural food/plant extracts as compared with synthetic antioxidants and vitamin E in raw and cooked pork patties. Meat Sci. 2001, 57, 45–52. [Google Scholar] [CrossRef]
- Joo, S.T.; Lee, J.I.; Ha, Y.L.; Park, G.B. Effects of dietary conjugated linoleic acid on fatty acid composition, lipid oxidation, color, and water-holding capacity of pork loin. J. Anim. Sci. 2002, 80, 108–112. [Google Scholar] [CrossRef]
- Faustman, C.; Sun, Q.; Mancini, R.; Suman, S.P. Myoglobin and lipid oxidation interactions: Mechanistic bases and control. Meat Sci. 2010, 86, 86–94. [Google Scholar] [CrossRef]
- Peng, X.; Kong, B. Antioxidant activity of whey protein hydrolysates on raw pork patties. Food Sci. 2010, 31, 14–18. [Google Scholar]
- Jung, S.; Choe, J.H.; Kim, B.; Yun, H.; Kruk, Z.A.; Jo, C. Effect of dietary mixture of gallic acid and linoleic acid on antioxidative potential and quality of breast meat from broilers. Meat Sci. 2010, 86, 520–526. [Google Scholar] [CrossRef]
- Kim, S.; Kubec, R.; Musah, R.A. Antibacterial and antifungal activity of sulfur-containing compounds from Petiveria alliacea L. J. Ethnopharmacol. 2006, 104, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Ahn, D.U.; Wolfe, F.H.; Sim, J.S. Prevention of lipid oxidation in pre-cooked turkey meat patties with hot packaging and antioxidant combinations. J. Food Sci. 1993, 58, 283–287. [Google Scholar] [CrossRef]
- Hawashin, M.D.; AI-Juhaimi, F.; Ahmed, I.A.M.; Ghafoor, K.; Babiker, E.E. Physicochemical, microbiological and sensory evaluation of beef patties incorporated with destoned olive cake powder. Meat Sci. 2016, 122, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Amirdivani, S.; Baba, A.S. Changes in yogurt fermentation characteristics, and antioxidant potential and in vitro inhibition of angiotensin-1 converting enzyme upon the inclusion of peppermint, dill and basil. LWT Food Sci. Technol. 2011, 44, 1458–1464. [Google Scholar] [CrossRef]
- Sacchetti, G.; Mattia, C.D.; Pittia, P.; Martino, G. Application of a radical scavenging activity test to measure the total antioxidant activity of poultry meat. Meat Sci. 2008, 80, 1081–1085. [Google Scholar] [CrossRef]
- Ahmed, L.; Martin-Diana, A.B.; Rico, D.; Barry-Ryan, C. The antioxidant properties of whey permeate treated fresh-cut tomatoes. Food Chem. 2011, 124, 1451–1457. [Google Scholar] [CrossRef][Green Version]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]

| Ingredient | Treatment 1 | ||||
|---|---|---|---|---|---|
| CON | W | C | WC | WBC | |
| Pork fillet | 75 | 75 | 75 | 75 | 75 |
| Back fat | 25 | 25 | 25 | 25 | 25 |
| Salt | 1 | 1 | 1 | 1 | 1 |
| Cysteine | - | - | 0.25 | 0.25 | 0.25 |
| Whey | - | 2 | - | 2 | 2 |
| B. serrata | - | - | - | - | 1 |
| Total | 101 | 103 | 101.25 | 103.25 | 104.25 |
| Treatment 1 | Moisture | Fat | Protein | Ash |
|---|---|---|---|---|
| CON | 54.27 a | 25.23 | 17.89 c | 1.82 b |
| W | 53.22 b | 24.70 | 19.03 a | 1.90 a |
| C | 54.45 a | 25.18 | 18.13 bc | 1.81 b |
| WC | 53.39 b | 24.54 | 18.47 b | 1.87 ab |
| WBC | 53.04 b | 24.15 | 18.50 ab | 1.90 a |
| SEM 2 | 0.26 | 0.58 | 0.16 | 0.02 |
| p-value | *** | 0.365 | *** | ** |
| Treatment 1 | Springiness (mm) | Cohesiveness | Chewiness (kg) | Gumminess (kg) | Hardness (kg) | WHC3 | Cooking loss |
|---|---|---|---|---|---|---|---|
| CON | 0.72 a | 0.25 | 3.79 a | 4.98 a | 19.61 b | 45.04 c | 41.73 a |
| W | 0.68 ab | 0.22 | 3.30 b | 4.60 b | 20.92 a | 47.60 b | 39.34 b |
| C | 0.59 d | 0.21 | 1.82 d | 3.16 d | 13.84 d | 47.58 b | 38.97 b |
| WC | 0.65 bc | 0.22 | 2.67 c | 4.15 c | 18.08 c | 48.61 ab | 38.17 bc |
| WBC | 0.63 cd | 0.23 | 2.59 c | 4.05 c | 17.99 c | 49.01 a | 37.60 c |
| SEM 2 | 0.02 | 0.01 | 0.09 | 0.11 | 0.43 | 0.39 | 0.43 |
| p-value | *** | 0.078 | *** | *** | *** | *** | *** |
| Treatment 1 | Color | Flavor | Tenderness | Juiciness | Taste | Overall Acceptability |
|---|---|---|---|---|---|---|
| CON | 5.83 | 6.25 | 6.08 | 5.00 | 5.25 | 5.50 |
| W | 5.83 | 6.33 | 5.50 | 5.83 | 6.08 | 6.17 |
| C | 6.25 | 6.83 | 7.50 | 5.92 | 7.17 | 6.58 |
| WC | 6.17 | 6.92 | 6.75 | 6.25 | 7.42 | 6.75 |
| WBC | 6.08 | 7.25 | 7.25 | 6.42 | 6.83 | 7.08 |
| SEM 2 | 0.30 | 0.29 | 0.33 | 0.24 | 0.34 | 0.30 |
| p-value | 0.527 | ** | *** | *** | *** | *** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, F.; Cho, W.-Y.; Seo, H.G.; Jeon, B.-T.; Kim, J.-H.; Kim, Y.H.B.; Wang, Y.; Lee, C.-H. Effect of L-cysteine, Boswellia serrata, and Whey Protein on the Antioxidant and Physicochemical Properties of Pork Patties. Foods 2020, 9, 993. https://doi.org/10.3390/foods9080993
Yang F, Cho W-Y, Seo HG, Jeon B-T, Kim J-H, Kim YHB, Wang Y, Lee C-H. Effect of L-cysteine, Boswellia serrata, and Whey Protein on the Antioxidant and Physicochemical Properties of Pork Patties. Foods. 2020; 9(8):993. https://doi.org/10.3390/foods9080993
Chicago/Turabian StyleYang, Fengqi, Won-Young Cho, Han Geuk Seo, Byong-Tae Jeon, Ji-Han Kim, Yuan H. Brad Kim, Yanmei Wang, and Chi-Ho Lee. 2020. "Effect of L-cysteine, Boswellia serrata, and Whey Protein on the Antioxidant and Physicochemical Properties of Pork Patties" Foods 9, no. 8: 993. https://doi.org/10.3390/foods9080993
APA StyleYang, F., Cho, W.-Y., Seo, H. G., Jeon, B.-T., Kim, J.-H., Kim, Y. H. B., Wang, Y., & Lee, C.-H. (2020). Effect of L-cysteine, Boswellia serrata, and Whey Protein on the Antioxidant and Physicochemical Properties of Pork Patties. Foods, 9(8), 993. https://doi.org/10.3390/foods9080993






