Effect of Pulsed Electric Fields on the Growth and Acidification Kinetics of Lactobacillus delbrueckii Subsp. bulgaricus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strain and Preculture Conditions
2.2. Fermentation Conditions
2.2.1. Control Fermentation
2.2.2. PEF-Assisted Fermentation
2.3. Statistical Analysis
3. Results and Discussion
3.1. Effect of Recirculation Flow Rate on the Growth and Acidification Kinetics
3.2. The Impact of PEF Treatment on the Growth and Acidification Kinetics
3.2.1. The Impact of PEF Intensities
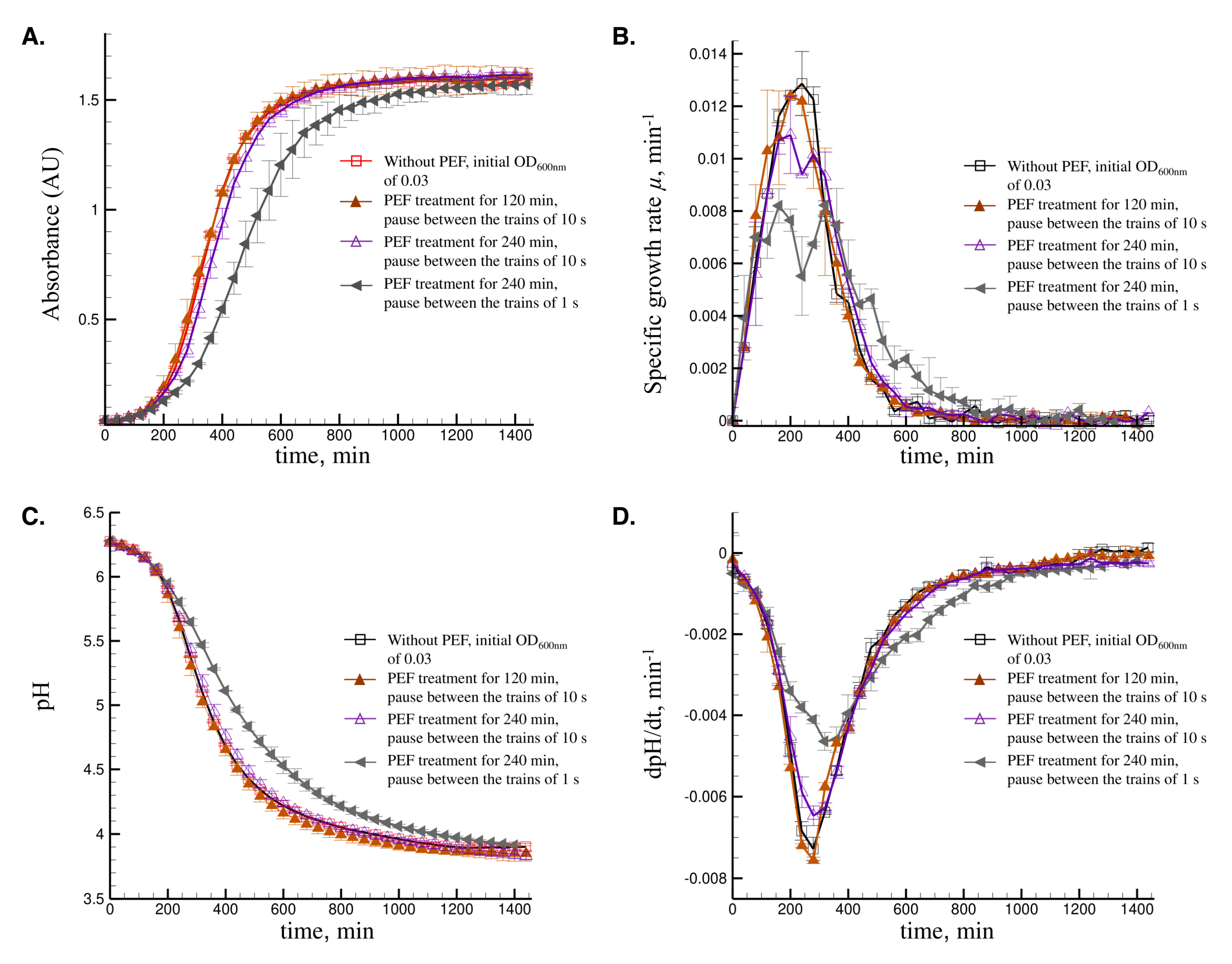
3.2.2. Effect of the Treatment Time and the Duration between the Trains
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lübeck, M.; Lübeck, P.S. Application of lactic acid bacteria in green biorefineries. FEMS Microbiol. Lett. 2019, 366. [Google Scholar] [CrossRef]
- Bintsis, T. Lactic acid bacteria as starter cultures: An update in their metabolism and genetics. AIMS Microbiol. 2018, 4, 665–684. [Google Scholar] [CrossRef] [PubMed]
- Reis, J.A.; Paula, A.T.; Casarotti, S.N.; Penna, A.L.B. Lactic acid bacteria antimicrobial compounds: Characteristics and applications. Food Eng. Rev. 2012, 4, 124–140. [Google Scholar] [CrossRef]
- Lorusso, A.; Coda, R.; Montemurro, M.; Rizzello, C.G. Use of selected lactic acid bacteria and quinoa flour for manufacturing novel yogurt-like beverages. Foods 2018, 7, 51. [Google Scholar] [CrossRef] [Green Version]
- Ayad, E.H.E.; Nashat, S.; El-Sadek, N.; Metwaly, H.; El-Soda, M. Selection of wild lactic acid bacteria isolated from traditional Egyptian dairy products according to production and technological criteria. Food Microbiol. 2004, 21, 715–725. [Google Scholar] [CrossRef]
- Venegas-Ortega, M.G.; Flores-Gallegos, A.C.; Aguilar, C.N.; Rodríguez-Herrera, R.; Martínez-Hernández, J.L.; Nevárez-Moorillón, G.V. Multi-functional potential of presumptive lactic acid bacteria isolated from Chihuahua Cheese. Foods 2020, 9, 276. [Google Scholar] [CrossRef] [Green Version]
- Hartley, D.L.; Denariaz, G. The role of lactic acid bacteria in yogurt fermentation. Int. J. Immunother. 1993, 9, 3–17. [Google Scholar]
- Najim, N.; Aryana, K.J. A mild pulsed electric field condition that improves acid tolerance, growth, and protease activity of Lactobacillus acidophilus LA-K and Lactobacillus delbrueckii subspecies bulgaricus LB-12. J. Dairy Sci. 2013, 96, 3424–3434. [Google Scholar] [CrossRef] [Green Version]
- Delgado-Fernández, P.; Corzo, N.; Olano, A.; Hernández-Hernández, O.; Moreno, F.J. Effect of selected prebiotics on the growth of lactic acid bacteria and physicochemical properties of yoghurts. Int. Dairy J. 2019, 89, 77–85. [Google Scholar] [CrossRef] [Green Version]
- Costa, M.P.; Balthazar, C.F.; Franco, R.M.; Mársico, E.T.; Cruz, A.G.; Junior, C.C. Changes on expected taste perception of probiotic and conventional yogurts made from goat milk after rapidly repeated exposure. J. Dairy Sci. 2014, 97, 2610–2618. [Google Scholar] [CrossRef] [Green Version]
- Béal, C.; Marin, M.; Fontaine, E.; Fonseca, F.; Obert, J.-P. Production et conservation des ferments lactiques et probiotiques. In Bactéries Lactiques: De la Génétique aux Ferments; Corrieu, G., Luquet, F.-M., Eds.; Tec&Doc Lavoisier: Paris, France, 2008; pp. 661–785. [Google Scholar]
- Durso, L.; Hutkins, R. Starter cultures. In Encyclopedia of Food Sciences and Nutrition; Caballero, B., Ed.; Academic Press: Oxford, UK, 2003; pp. 5583–5593. ISBN 978-0-12-227055-0. [Google Scholar]
- Mota, M.J.; Lopes, R.P.; Koubaa, M.; Roohinejad, S.; Barba, F.J.; Delgadillo, I.; Saraiva, J.A. Fermentation at non-conventional conditions in food- and bio-sciences by the application of advanced processing technologies. Crit. Rev. Biotechnol. 2018, 38, 122–140. [Google Scholar] [CrossRef] [PubMed]
- Barba, F.J.; Koubaa, M.; Do Prado-Silva, L.; Orlien, V.; de Souza Sant’Ana, A. Mild processing applied to the inactivation of the main foodborne bacterial pathogens: A review. Trends Food Sci. Technol. 2017, 66, 20–35. [Google Scholar] [CrossRef]
- Koubaa, M.; Barba, F.J.; Grimi, N.; Mhemdi, H.; Koubaa, W.; Boussetta, N.; Vorobiev, E. Recovery of colorants from red prickly pear peels and pulps enhanced by pulsed electric field and ultrasound. Innov. Food Sci. Emerg. Technol. 2016, 37 Pt C, 336–344. [Google Scholar] [CrossRef]
- Parniakov, O.; Barba, F.J.; Grimi, N.; Marchal, L.; Jubeau, S.; Lebovka, N.; Vorobiev, E. Pulsed electric field assisted extraction of nutritionally valuable compounds from microalgae Nannochloropsis spp. using the binary mixture of organic solvents and water. Innov. Food Sci. Emerg. Technol. 2015, 27, 79–85. [Google Scholar] [CrossRef]
- Al Daccache, M.; Koubaa, M.; Salameh, D.; Vorobiev, E.; Maroun, R.G.; Louka, N. Control of the sugar/ethanol conversion rate during moderate pulsed electric field-assisted fermentation of a Hanseniaspora sp. strain to produce low-alcohol cider. Innov. Food Sci. Emerg. Technol. 2020, 59, 102258. [Google Scholar] [CrossRef]
- Barba, F.J.; Parniakov, O.; Pereira, S.A.; Wiktor, A.; Grimi, N.; Boussetta, N.; Saraiva, J.A.; Raso, J.; Martin-Belloso, O.; Witrowa-Rajchert, D.; et al. Current applications and new opportunities for the use of pulsed electric fields in food science and industry. Food Res. Int. 2015, 77, 773–798. [Google Scholar] [CrossRef]
- Vaessen, E.M.J.; den Besten, H.M.W.; Patra, T.; van Mossevelde, N.T.M.; Boom, R.M.; Schutyser, M.A.I. Pulsed electric field for increasing intracellular trehalose content in Lactobacillus plantarum WCFS1. Innov. Food Sci. Emerg. Technol. 2018, 47, 256–261. [Google Scholar] [CrossRef]
- Koubaa, M.; Roselló-Soto, E.; Šic Žlabur, J.; Režek Jambrak, A.; Brnčić, M.; Grimi, N.; Boussetta, N.; Barba, F.J. Current and new insights in the sustainable and green recovery of nutritionally valuable compounds from Stevia rebaudiana Bertoni. J. Agric. Food Chem. 2015, 63, 6835–6846. [Google Scholar] [CrossRef]
- Ohba, T.; Uemura, K.; Nabetani, H. Moderate pulsed electric field treatment enhances exopolysaccharide production by Lactococcus lactis subspecies cremoris. Process Biochem. 2016, 51, 1120–1128. [Google Scholar] [CrossRef]
- Mattar, J.R.; Turk, M.F.; Nonus, M.; Lebovka, N.I.; El Zakhem, H.; Vorobiev, E.S. Cerevisiae fermentation activity after moderate pulsed electric field pre-treatments. Bioelectrochemistry 2015, 103, 92–97. [Google Scholar] [CrossRef]
- Al Daccache, M.; Koubaa, M.; Maroun, R.G.; Salameh, D.; Louka, N.; Vorobiev, E. Pulsed electric field-assisted fermentation of Hanseniaspora sp. yeast isolated from Lebanese apples. Food Res. Int. 2020, 129, 108840. [Google Scholar] [CrossRef] [PubMed]
- Ewe, J.-A.; Wan-Abdullah, W.-N.; Alias, A.K.; Liong, M.-T. Enhanced growth of lactobacilli and bioconversion of isoflavones in biotin-supplemented soymilk by electroporation. Int. J. Food Sci. Nutr. 2012, 63, 580–596. [Google Scholar] [CrossRef] [PubMed]
- Seratlić, S.; Bugarski, B.; Nedović, V.; Radulović, Z.; Wadsö, L.; Dejmek, P.; Galindo, F.G. Behavior of the surviving population of Lactobacillus plantarum 564 upon the application of pulsed electric fields. Innov. Food Sci. Emerg. Technol. 2013, 17, 93–98. [Google Scholar] [CrossRef]
- Corrieu, G.; Spinnler, H.E.; Picque, D.; Jomier, Y. Automated System to Follow up and Control the Acidification Activity of Lactic Acid Starters. U.S. Patent 8,804,456, 2 April 1988. [Google Scholar]
- Dalgaard, P.; Koutsoumanis, K. Comparison of maximum specific growth rates and lag times estimated from absorbance and viable count data by different mathematical models. J. Microbiol. Methods 2001, 43, 183–196. [Google Scholar] [CrossRef]
- Beal, C.; Louvet, P.; Corrieu, G. Influence of controlled pH and temperature on the growth and acidification of pure cultures of Streptococcus thermophilus 404 and Lactobacillus bulgaricus 398. Appl. Microbiol. Biotechnol. 1989, 32, 148–154. [Google Scholar] [CrossRef]
- El Darra, N.; Rajha, H.N.; Ducasse, M.-A.; Turk, M.F.; Grimi, N.; Maroun, R.G.; Louka, N.; Vorobiev, E. Effect of pulsed electric field treatment during cold maceration and alcoholic fermentation on major red wine qualitative and quantitative parameters. Food Chem. 2016, 213, 352–360. [Google Scholar] [CrossRef]
- Mercier, P.; Yerushalmi, L.; Rouleau, D.; Dochain, D. Kinetics of lactic acid fermentation on glucose and corn by Lactobacillus amylophilus. J. Chem. Technol. Biotechnol. 1992, 55, 111–121. [Google Scholar] [CrossRef]
- Gupta, S.; Abu-Ghannam, N.; Scannell, A.G. Growth and kinetics of Lactobacillus plantarum in the fermentation of edible Irish brown seaweeds. Food Bioprod. Process. 2011, 89, 346–355. [Google Scholar] [CrossRef] [Green Version]
- Kandler, O.; Weiss, N. Regular gram positive nonsporing rods. Bergey’s Man. Syst. Bacteriol. 1986, 2, 1208–1260. [Google Scholar]
- Marty-Teysset, C.; De La Torre, F.; Garel, J.-R. Increased production of hydrogen peroxide by Lactobacillus delbrueckii subsp. bulgaricus upon aeration: Involvement of an NADH oxidase in oxidative stress. Appl. Environ. Microbiol. 2000, 66, 262–267. [Google Scholar] [CrossRef] [Green Version]
- Murphy, M.G.; Condon, S. Comparison of aerobic and anaerobic growth of Lactobacillus plantarum in a glucose medium. Arch. Microbiol. 1984, 138, 49–53. [Google Scholar] [CrossRef]
- Fu, W.; Mathews, A.P. Lactic acid production from lactose by Lactobacillus plantarum: Kinetic model and effects of pH, substrate, and oxygen. Biochem. Eng. J. 1999, 3, 163–170. [Google Scholar] [CrossRef]
- Tango, M.S.A.; Ghaly, A.E. Amelioration of lactic acid production from cheese whey using micro-aeration. Biomass Bioenergy 1999, 17, 221–238. [Google Scholar] [CrossRef]
- Jeanson, S.; Hilgert, N.; Coquillard, M.-O.; Seukpanya, C.; Faiveley, M.; Neveu, P.; Abraham, C.; Georgescu, V.; Fourcassié, P.; Beuvier, E. Milk acidification by Lactocccus lactis is improved by decreasing the level of dissolved oxygen rather than decreasing redox potential in the milk prior to inoculation. Int. J. Food Microbiol. 2009, 131, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Ewe, J.-A.; Wan-Abdullah, W.-N.; Alias, A.K.; Liong, M.-T. Bioconversion of isoflavones and the probiotic properties of the electroporated parent and subsequent three subcultures of Lactobacillus fermentum BT 8219 in biotin-soymilk. J. Microbiol. Biotechnol. 2012, 22, 947–959. [Google Scholar] [CrossRef] [Green Version]


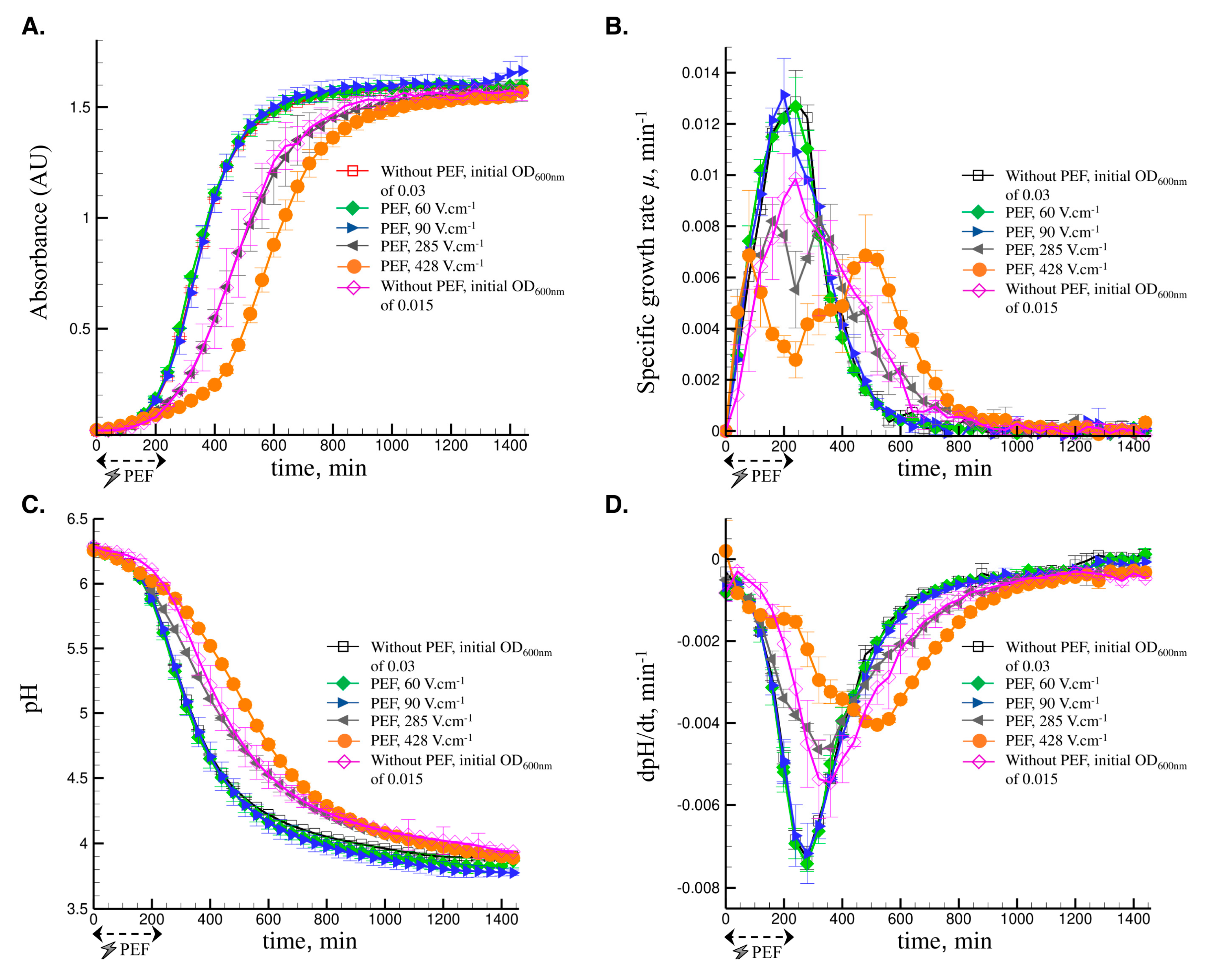
| µmax (min−1) | dpH/dt (min−1) | ||||
|---|---|---|---|---|---|
| Initial OD600nm | Treatment Intensity (V cm−1) | Average | SD | Average | SD |
| 0.015 | 0 | 0.0099 | 0.0001 | −0.0055 | 0.0008 |
| 0.03 | 0 | 0.0129 | 0.0012 | −0.0073 | 0.0003 |
| 0.03 | 60 | 0.0127 | 0.0011 | −0.0074 | 0.0002 |
| 0.03 | 90 | 0.0131 | 0.0014 | −0.0072 | 0.0007 |
| 0.03 | 285 | 0.0082; 160 min | 0.0007 | −0.0046 | 0.0002 |
| 0.0082; 320 min | 0.0006 | ||||
| 0.03 | 428 | 0.0069; 80 min | 0.0025 | −0.0040 | 0.0000 |
| 0.0069; 480 min | 0.0016 | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, K.; Koubaa, M.; Bals, O.; Vorobiev, E. Effect of Pulsed Electric Fields on the Growth and Acidification Kinetics of Lactobacillus delbrueckii Subsp. bulgaricus. Foods 2020, 9, 1146. https://doi.org/10.3390/foods9091146
Peng K, Koubaa M, Bals O, Vorobiev E. Effect of Pulsed Electric Fields on the Growth and Acidification Kinetics of Lactobacillus delbrueckii Subsp. bulgaricus. Foods. 2020; 9(9):1146. https://doi.org/10.3390/foods9091146
Chicago/Turabian StylePeng, Kaidi, Mohamed Koubaa, Olivier Bals, and Eugène Vorobiev. 2020. "Effect of Pulsed Electric Fields on the Growth and Acidification Kinetics of Lactobacillus delbrueckii Subsp. bulgaricus" Foods 9, no. 9: 1146. https://doi.org/10.3390/foods9091146






