Pre-Fermentation Water Addition to High-Sugar Shiraz Must: Effects on Wine Composition and Sensory Properties
Abstract
:1. Introduction
2. Materials and Methods
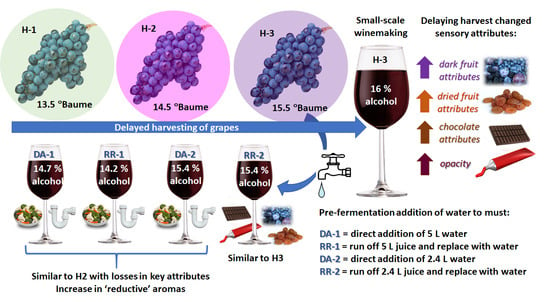
2.1. Grapes and Wines for the Ripening and Water Addition Trial
- DA-1: Direct addition of 5 L of water to the must
- DA-2: Direct addition of 2.4 L of water to the must
- RR-1: Juice substituted with 5 L of water
- RR-2: Juice substituted with 2.4 L of water
- RO-1: Removal of 5 L of juice without water addition
- RO-2: Removal of 2.4 L of juice without water addition
2.2. Wine Compositional Analysis
2.3. Sensory Analysis
2.4. Statistical Analysis of the Wine Composition and Sensory Results
3. Results and Discussion
3.1. Outcomes of the Harvest Date, Water Addition and Saigneé Treatments on Wine Composition
3.2. Outcomes of Grape Ripening, Water Addition and Saigneé on Wine Sensory Properties
3.3. Partial Least Squares Regression to Predict Wine Sensory Properties from the Wine Chemical Composition
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cameron, W.; Petrie, P.R.; Barlow, E.W.R.; Patrick, C.J.; Howell, K.; Fuentes, S. Advancement of grape maturity: Comparison between contrasting cultivars and regions. Aust. J. Grape Wine Res. 2020, 26, 53–67. [Google Scholar] [CrossRef]
- Sadras, V.O.; Petrie, P.R. Quantifying the onset, rate and duration of sugar accumulation in berries from commercial vineyards in contrasting climates of Australia. Aust. J. Grape Wine Res. 2011, 17, 190–198. [Google Scholar] [CrossRef]
- Schelezki, O.J.; Smith, P.A.; Hranilovic, A.; Bindon, K.A.; Jeffery, D.W. Comparison of consecutive harvests versus blending treatments to produce lower alcohol wines from Cabernet Sauvignon grapes: Impact on polysaccharide and tannin content and composition. Food Chem. 2018, 244, 50–59. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, M.G.; Coombe, B.G. Is weight loss in ripening grape berries cv. Shiraz caused by impeded phloem transport? Aust. J. Grape Wine Res. 1999, 5, 17–21. [Google Scholar] [CrossRef]
- De Orduna, R.M. Climate change associated effects on grape and wine quality and production. Food Res. Int. 2010, 43, 1844–1855. [Google Scholar] [CrossRef]
- Teng, B.; Petrie, P.R.; Smith, P.A.; Bindon, K.A. Comparison of water addition and early-harvest strategies to decrease alcohol concentration in Vitis vinifera cv. Shiraz wine: Impact on wine phenolics, tannin composition and colour properties. Aust. J. Grape Wine Res. 2020, 26, 158–171. [Google Scholar] [CrossRef]
- Schelezki, O.J.; Antalick, G.; Suklje, K.; Jeffery, D.W. Pre-fermentation approaches to producing lower alcohol wines from Cabernet Sauvignon and Shiraz: Implications for wine quality based on chemical and sensory analysis. Food Chem. 2020, 309, 125698. [Google Scholar] [CrossRef]
- Schelezki, O.J.; Deloire, A.; Jeffery, D.W. Substitution or dilution? Assessing Pre-fermentative water implementation to produce lower alcohol shiraz wines. Molecules 2020, 25, 2245. [Google Scholar] [CrossRef]
- Schelezki, O.J.; Suklje, K.; Boss, P.K.; Jeffery, D.W. Comparison of consecutive harvests versus blending treatments to produce lower alcohol wines from Cabernet Sauvignon grapes: Impact on wine volatile composition and sensory properties. Food Chem. 2018, 259, 196–206. [Google Scholar] [CrossRef]
- Suklje, K.; Zhang, X.Y.; Antalick, G.; Clark, A.C.; Deloire, A.; Schmidtke, L.M. Berry shriveling significantly alters shiraz (Vitis vinifera L.) grape and wine chemical composition. J. Agric. Food Chem. 2016, 64, 870–880. [Google Scholar] [CrossRef]
- Mercurio, M.D.; Dambergs, R.G.; Herderich, M.J.; Smith, P.A. High throughput analysis of red wine and grape phenolics-adaptation and validation of methyl cellulose precipitable tannin assay and modified somers color assay to a rapid 96 well plate format. J. Agric. Food Chem. 2007, 55, 4651–4657. [Google Scholar] [CrossRef] [PubMed]
- Kassara, S.; Kennedy, J.A. Relationship between red wine grade and phenolics. 2. tannin composition and size. J. Agric. Food Chem. 2011, 59, 8409–8412. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, J.A.; Taylor, A.W. Analysis of proanthocyanidins by high-performance gel permeation chromatography. J. Chromatogr. A 2003, 995, 99–107. [Google Scholar] [CrossRef]
- Bindon, K.A.; Kennedy, J.A. Ripening-induced changes in grape skin proanthocyanidins modify their interaction with cell walls. J. Agric. Food Chem. 2011, 59, 2696–2707. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, J.A.; Jones, G.P. Analysis of proanthocyanidin cleavage products following acid-catalysis in the presence of excess phloroglucinol. J. Agric. Food Chem. 2001, 49, 1740–1746. [Google Scholar] [CrossRef] [PubMed]
- Siebert, T.E.; Smyth, H.E.; Capone, D.L.; Neuwohner, C.; Pardon, K.H.; Skouroumounis, G.K.; Herderich, M.; Sefton, M.A.; Pollnitz, A.P. Stable isotope dilution analysis of wine fermentation products by HS-SPME-GC-MS. Anal. Bioanal. Chem. 2005, 381, 937–947. [Google Scholar] [CrossRef]
- Honda, S.; Akao, E.; Suzuki, S.; Okuda, M.; Kakehi, K.; Nakamura, J. High-performance liquid-chromatography of reducing carbohydrates as strongly ultraviolet-absorbing and electrochemically sensitive 1-phenyl-3-methyl-5-pyrazolone derivatives. Anal. Biochem. 1989, 180, 351–357. [Google Scholar] [CrossRef]
- Bindon, K.A.; Kassara, S.; Solomon, M.; Bartel, C.; Smith, P.A.; Barker, A.; Curtin, C. Commercial saccharomyces cerevisiae yeast strains significantly impact shiraz tannin and polysaccharide composition with implications for wine colour and astringency. Biomolecules 2019, 9, 466. [Google Scholar] [CrossRef] [Green Version]
- Orte, P.H.; Guitart, A.; Cacho, J. Amino acid determination in musts and wines by HPLC after derivatization with phenylisothiocyanate. Am. J. Enol. Vitic. 1997, 48, 229–235. [Google Scholar]
- Godden, P. Ask the AWRI: Saignee. Aust. N. Z. Grapegrow. Winemak. 2019, 655, 91–92. [Google Scholar]
- Bindon, K.; Varela, C.; Kennedy, J.; Holt, H.; Herderich, M. Relationships between harvest time and wine composition in Vitis vinifera L. cv. Cabernet Sauvignon 1. Grape and wine chemistry. Food Chem. 2013, 138, 1696–1705. [Google Scholar] [CrossRef] [PubMed]
- Duteurtre, B.; Bourgeois, C.; Chollot, B. Study of assimilation of proline by brewing yeasts. J. Inst. Brew. 1971, 77, 28–35. [Google Scholar] [CrossRef]
- Kaneko, S.; Kumazawa, K.; Masuda, H.; Henze, A.; Hofmann, T. Molecular and sensory studies on the umami taste of Japanese green tea. J. Agric. Food Chem. 2006, 54, 2688–2694. [Google Scholar] [CrossRef] [PubMed]
- Forde, C.G.; Cox, A.; Williams, E.R.; Boss, P.K. Associations between the sensory attributes and volatile composition of cabernet sauvignon wines and the volatile composition of the grapes used for their production. J. Agric. Food Chem. 2011, 59, 2573–2583. [Google Scholar] [CrossRef] [PubMed]
- Bindon, K.; Holt, H.; Williamson, P.O.; Varela, C.; Herderich, M.; Francis, I.L. Relationships between harvest time and wine composition in Vitis vinifera L. cv. Cabernet Sauvignon 2. Wine sensory properties and consumer preference. Food Chem. 2014, 154, 90–101. [Google Scholar] [CrossRef]
- Viana, F.; Gil, J.V.; Valles, S.; Manzanares, P. Increasing the levels of 2-phenylethyl acetate in wine through the use of a mixed culture of Hanseniaspora osmophila and Saccharomyces cerevisiae. Int. J. Food Microbiol. 2009, 135, 68–74. [Google Scholar] [CrossRef]
- Brennand, C.P.; Ha, J.K.; Lindsay, R.C. Aroma properties and thresholds of some branched-chain and other minor volatile fatty acids occurring in milkfat and meat lipids 1. J. Sens. Stud. 1989, 4, 105–120. [Google Scholar] [CrossRef]
- Martínez-Pérez, M.P.; Bautista-Ortín, A.B.; Pérez-Porras, P.; Jurado, R.; Gómez-Plaza, E. A new approach to the reduction of alcohol content in red wines: The use of high-power ultrasounds. Foods 2020, 9, 726. [Google Scholar] [CrossRef]
- Ricci, A.; Parpinello, G.P.; Banfi, B.A.; Olivi, F.; Versari, A. Preliminary study of the effects of pulsed electric field (PEF) treatments in wines obtained from early-harvested sangiovese grapes. Beverages 2020, 6, 34. [Google Scholar] [CrossRef]
- Maza, M.A.; Martínez, J.M.; Cebrián, G.; Sánchez-Gimeno, A.C.; Camargo, A.; Álvarez, I.; Raso, J. Evolution of polyphenolic compounds and sensory properties of wines obtained from grenache grapes treated by pulsed electric fields during aging in bottles and in Oak Barrels. Foods 2020, 9, 542. [Google Scholar] [CrossRef]
- Kang, W.; Bindon, K.A.; Wang, X.; Muhlack, R.A.; Smith, P.A.; Niimi, J.; Bastian, S.E. Chemical and sensory impacts of accentuated cut edges (ACE) grape must polyphenol extraction technique on shiraz wines. Foods 2020, 9, 1027. [Google Scholar] [CrossRef] [PubMed]


| Phenolic Measure | Unit | H-1 ‡ | DA-1 | RR-1 | RO-1 | H-2 | DA-2 | RR-2 | RO-2 | H-3 | ANOVA p Value |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total phenolics | (A.U.) | 24.43 ± 0.52 f | 32.74 ± 0.63 d | 32.95 ± 0.46 d | 38.79 ± 1.13 a | 28.38 ± 1.05 e | 34.62 ± 0.89 c | 33.91 ± 0.58 cd | 36.84 ± 0.91 b | 36.75 ± 0.52 b | <0.0001 |
| Colour measures | |||||||||||
| Colour density | (A.U.) | 7.64 ± 0.14 e | 10.58 ± 0.29 c | 10.73 ± 0.17 c | 12.64 ± 0.49 a | 9.47 ± 0.21 d | 11.03 ± 0.29 c | 10.85 ± 0.19 c | 11.82 ± 0.40 b | 11.75 ± 0.23 b | <0.0001 |
| Hue | Ratio | 0.54 ± 0.01 e | 0.57 ± 0.01 d | 0.56 ± 0.00 de | 0.66 ± 0.02 a | 0.54 ± 0.01 e | 0.59 ± 0.00 c | 0.59 ± 0.00 c | 0.64 ± 0.00 b | 0.64 ± 0.00 b | <0.0001 |
| Total Anthocyanin | (mg/L) | 340 ± 10.66 d | 442 ± 16.85 b | 452 ± 5.42 ab | 438 ± 27.84 b | 411 ± 10.62 c | 472 ± 10.71 a | 453 ± 9.1 ab | 439 ± 14.04 b | 438 ± 8.94 b | <0.0001 |
| Non-bleachable pigment | (A.U.) | 1.35 ± 0.12 e | 1.99 ± 0.09 c | 2.01 ± 0.08 c | 3.14 ± 0.26 a | 1.58 ± 0.05 d | 2.04 ± 0.10 c | 2.10 ± 0.03 c | 2.75 ± 0.07 b | 2.62 ± 0.05 b | <0.0001 |
| Polymeric pigment | (mg/L) | 10.9 ± 0.63 g | 18.4 ± 0.56 e | 19.6 ± 0.29 e | 36.2 ± 1.96 a | 15.5 ± 0.18 f | 20.1 ± 1.15 de | 22.1 ± 0.35 cd | 31.9 ± 0.48 b | 24.5 ± 0.1 c | <0.0001 |
| Tannin concentration and composition | |||||||||||
| Tannin | (mg/L) | 232 ± 20.01 e | 539 ± 27.95 b | 513 ± 2.29B c | 82 ± 73.22 a | 369 ± 29.37 d | 545 ± 44.99 b | 548 ± 12.53 b | 742 ± 73.40 a | 723 ± 7.29 a | <0.0001 |
| Molecular mass (subunit) § | (g/mol) | 1144 ± 32 f | 1372 ± 32 d | 1351 ± 19 d | 2026 ± 65 a | 1183 ± 5 ef | 1333 ± 44 de | 1441 ± 14 cd | 1770 ± 15 b | 1541 ± 22 c | <0.0001 |
| Molecular mass (50% GPC) § | (g/mol) | 1444 ± 50 abc | 1457 ± 25 abc | 1430 ± 17 bc | 1537 ± 71 a | 1473 ± 25 abc | 1423 ± 35 c | 1453 ± 25 abc | 1477 ± 15 abc | 1531 ± 9 ab | <0.01 |
| mDP †† | no units | 3.79 ± 0.12 f | 4.52 ± 0.11 d | 4.43 ± 0.07 d | 6.64 ± 0.20 a | 3.90 ± 0.03 ef | 4.38 ± 0.14 de | 4.72 ± 0.04 cd | 5.81 ± 0.06 b | 5.05 ± 0.07 c | <0.0001 |
| Mass conversion‡‡ | (%) | 13.68 ± 0.64 d | 26.01 ± 0.60 a | 27.11 ± 0.97 a | 20.48 ± 1.40 bc | 17.57 ± 0.67 cd | 24.35 ± 0.87 ab | 24.69 ± 0.10 a | 17.92 ± 0.77 c | 17.50 ± 0.57 cd | <0.0001 |
| Galloylation §§ | (%) | 5.80 ± 0.90 ab | 5.65 ± 0.34 ab | 5.88 ± 0.08 ab | 4.88 ± 0.13 ab | 6.94 ± 0.67 a | 6.11 ± 0.20 ab | 5.49 ± 0.07 ab | 4.51 ± 0,25 b | 6.25 ± 0.34 ab | <0.05 |
| Epigallocatechin (ext.) §§ | (mol %) | 18.34 ± 1.69 d | 30.74 ± 0.93 c | 34.22 ± 0.49 bc | 44.88 ± 2.0 a | 17.00 ± 1.23 d | 30.47 ± 2.46 c | 40.96 ± 1.29 ab | 46.10 ± 1.49 a | 33.08 ± 1.69 c | <0.0001 |
| Catechin (ext.) §§ | (mol %) | 4.16 ± 0.23 a | 3.43 ± 0.09 abc | 3.48 ± 0.15 abc | 2.12 ± 0.12 e | 3.55 ± 0.18 abc | 3.59 ± 0.17 ab | 3.14 ± 0.01 bcd | 2.42 ± 0.23 de | 2.78 ± 0.12 cde | <0.0001 |
| Epicatechin (ext.) §§ | (mol %) | 47.31 ± 1.03 a | 39.23 ± 0.77 b | 35.17 ± 0.90 bc | 34.06 ± 1.70 bc | 49.23 ± 1.30 a | 38.69 ± 1.76 b | 30.80 ± 1.05 c | 30.91 ± 1.94 c | 39.55 ± 1.44 b | <0.0001 |
| Epicatechin-gallate (ext.) §§ | (mol %) | 3.75 ± 0.64 | 4.43 ± 0.29 | 4.57 ± 0.08 | 3.86 ± 0.02 | 4.57 ± 0.28 | 4.36 ± 0.15 | 3.92 ± 0.07 | 3.37 ± 0.25 | 4.78 ± 0.26 | ns |
| Catechin (ter.) §§ | (mol %) | 16.48 ± 0.24 a | 13.07 ± 0.25 b | 12.92 ± 0.33 b | 8.63 ± 0.49 c | 16.74 ± 0.29 a | 13.19 ± 0.39 b | 12.13 ± 0.17 b | 10.05 ± 0.20 c | 11.80 ± 0.06 b | <0.0001 |
| Epicatechin (ter.) §§ | (mol %) | 7.90 ± 0.61 ab | 7.89 ± 0.28 ab | 8.33 ± 0.14 a | 5.43 ± 0.24 c | 6.55 ± 0.03 bc | 7.95 ± 0.39 ab | 7.47 ± 0.03 ab | 6.02 ± 0.04 c | 6.54 ± 0.21 ab | <0.0001 |
| Epicatechin-gallate (ter.) §§ | (mol %) | 2.05 ± 0.32 ab | 1.21 ± 0.05 bc | 1.31 ± 0.04 bc | 1.02 ± 0.12 c | 2.37 ± 0.43 a | 1.75 ± 0.11 abc | 1.58 ± 0.01 abc | 1.14 ± 0.01 bc | 1.47 ± 0.11 abc | <0.001 |
| Polysaccharide Component | H-1 | DA-1 | RR-1 | RO-1 | H-2 | DA-2 | RR-2 | RO-2 | H-3 | ANOVA p Value |
|---|---|---|---|---|---|---|---|---|---|---|
| Total polysaccharide | 655 ± 33 abc | 673 ± 2 abc | 674 ± 5 abc | 809 ± 17 a | 629 ± 25 c | 638 ± 69 bc | 763 ± 19 abc | 802 ± 1.5 ab | 771 ± 35 abc | <0.01 |
| Mannose ‡ | 141.18 ± 12 ab | 143.29 ± 1 ab | 130.9 ± 1 ab | 172.35 ± 5 a | 123.77 ± 6 b | 138.05 ± 16 ab | 155.43 ± 5 ab | 173.67 ± 3 a | 164.43 ± 5 ab | <0.01 |
| Rhamnose | 46.86 ± 3 bc | 48.54 ± 1 bc | 51.72 ± 0.3 abc | 61.42 ± 1 a | 42.85 ± 2 c | 48.77 ± 3 bc | 55.63 ± 2 ab | 58.92 ± 1 a | 55.69 ± 2 ab | <0.0001 |
| Glucuronic acid | 8.98 ± 1 d | 12.11 ± 1 bcd | 10.87 ± 1 bcd | 15.22 ± 1 a | 10.55 ± 1 cd | 12.15 ± 1 abcd | 13.53 ± 0.3 abc | 14.15 ± 1 ab | 14.87 ± 0.3 a | <0.0001 |
| Galacturonic acid | 196.94 ± 3 a | 167.43 ± 8 ab | 191.8 ± 1 ab | 179.13 ± 4 ab | 175.73 ± 5 ab | 143.30 ± 21 b | 198.64 ± 1 a | 186.95 ± 2 ab | 182.72 ± 2 ab | <0.05 |
| Glucose | 33.70 ± 2 c | 43.58 ± 1 bc | 41.73 ± 2 bc | 67.96 ± 1 a | 33.60 ± 2 c | 44.46 ± 5 bc | 53.71 ± 5 ab | 60.71 ± 3 a | 58.10 ± 2 ab | <0.0001 |
| Galactose | 115.18 ± 9 | 120.59 ± 1 | 113.9 ± 1 | 145.39 ± 4 | 118.52 ± 6 | 117.15 ± 12 | 133.35 ± 5 | 142.19 ± 1 | 138.80 ± 6 | <0.05 |
| Xylose | 8.41 ± 1 c | 10.95 ± 0.2 bc | 10.6 ± 0.1 bc | 17.27 ± 0.4 a | 8.10 ± 1 c | 11.52 ± 1 bc | 14.54 ± 1 ab | 16.65 ± 0.3 a | 16.01 ± 1 a | <0.0001 |
| Arabinose | 103.50 ± 8 c | 127.02 ± 3 abc | 122.9 ± 2 abc | 150.52 ± 2 a | 115.75 ± 4 bc | 122.78 ± 11 abc | 137.79 ± 5 ab | 148.66 ± 2 a | 140.69 ± 7 ab | <0.001 |
| Fermentation-Derived Compound | Unit | H-1 | DA-1 | RR-1 | RO-1 | H-2 | DA-2 | RR-1 | RO-1 | H-3 | ANOVA p Value |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetate esters | |||||||||||
| Ethyl acetate | mg/L | 16.23 ± 0.33 c | 19.87 ± 1.9 abc | 19.2 ± 0.27 abc | 23.31 ± 1.90 a | 17.72 ± 0.24 bc | 21.58 ± 0.51 ab | 20.6 ± 0.68 abc | 23.28 ± 0.15 a | 23.01 ± 0.63 a | <0.001 |
| Hexyl acetate | µg/L | 10.56 ± 0.62 bc | 10.19 ± 0.63 bc | 12.37 ± 0.57 b | 6.67 ± 1.11 d | 17.63 ± 0.41 a | 10.12 ± 0.44 bc | 10.98 ± 0.14 bc | 6.58 ± 0.26 d | 8.40 ± 1.16 cd | <0.0001 |
| 3-Methylbutyl acetate | mg/L | 0.61 ± 0.0 abc | 0.71 ± 0.02 ab | 0.69 ± 0.02 ab | 0.42 ± 0.09 c | 0.71 ± 0.02 ab | 0.75 ± 0.04 a | 0.79 ± 0.03 a | 0.51 ± 0.05 bc | 0.60 ± 0.06 abc | <0.001 |
| 2-Methylbutyl acetate | µg/L | 58 ± 4.42 bc | 82 ± 7.43 abc | 77 ± 2.90 abc | 54 ± 8.69 c | 76 ± 3.24 abc | 90 ± 7.65 a | 84 ± 4.68 ab | 61 ± 4.85 abc | 69 ± 6.80 abc | <0.01 |
| 2-Phenylethyl acetate | µg/L | 76 ± 2.68 b | 72 ± 4.19 bc | 71 ± 3.83 bc | 52 ± 4.32 c | 103 ± 4.06 a | 72 ± 4.12 bc | 77 ± 0.89 b | 61 ± 2.86 bc | 62 ± 7.66 bc | <0.0001 |
| Ethyl esters | |||||||||||
| Ethyl butanoate | µg/L | 103 ± 2.64 | 121 ± 4.41 | 127 ± 3.55 | 109 ± 15.9 | 122 ± 3.91 | 121 ± 11.85 | 137 ± 6.81 | 121 ± 7.87 | 117 ± 11.51 | ns |
| Ethyl propanoate | µg/L | 51.40 ± 2.52 ab | 50.77 ± 1.04 ab | 45.98 ± 0.64 b | 50.44 ± 1.90 ab | 54.71 ± 3.06 a | 46.99 ± 0.67 ab | 49.02 ± 0.42 ab | 53.15 ± 1.56 ab | 47.21 ± 2.01 ab | <0.05 |
| Ethyl hexanoate | µg/L | 136 ± 0.04 cde | 150 ± 5.52 bcd | 159 ± 2.15 ab | 123 ± 6.37 e | 177 ± 5.42 a | 157 ± 0.08 abc | 159 ± 5.23 ab | 135 ± 1.01 de | 139 ± 6.81 bcde | <0.0001 |
| Ethyl octanoate | µg/L | 109 ± 1.0 cd | 138 ± 2.23 a | 137 ± 0.6 ab | 105 ± 2.96 d | 136 ± 7.82 ab | 137 ± 1.77 ab | 128 ± 2.23 abc | 117 ± 2.35 bcd | 131 ± 8.12 ab | <0.0001 |
| Ethyl decanoate | µg/L | 19.22 ± 1.88 b | 24.16 ± 0.45 ab | 23.41 ± 0.49 ab | 19.49 ± 0.58 ab | 20.87 ± 2.02 ab | 23.52 ± 0.82 ab | 20.49 ± 0.50 ab | 18.59 ± 0.15 b | 25.17 ± 1.79 a | <0.01 |
| Ethyl-3-methylbutanoate | µg/L | 27.01 ± 2.17 b | 34.15 ± 1.0 ab | 34.28 ± 1.26 ab | 27.16 ± 0.51 b | 39.86 ± 4.01 a | 30.30 ± 0.84 b | 28.91 ± 0.66 b | 31.88 ± 0.64 ab | 28.37 ± 0.79 b | <0.001 |
| Ethyl-2-methylpropanoate | µg/L | 62.73 ± 3.93 | 61.81 ± 3.62 | 61.61 ± 0.95 | 53.47 ± 4.77 | 60.53 ± 3.14 | 56.17 ± 2.90 | 55.52 ± 1.88 | 59.10 ± 6.53 | 59.76 ± 2.48 | ns |
| Higher alcohols | |||||||||||
| Butanol | mg/L | 1.32 ± 0.06 c | 1.27 ± 0.07 c | 1.59 ± 0.07 bc | 1.98 ± 0.08 ab | 1.90 ± 0.05 ab | 1.20 ± 0.18 c | 1.84 ± 0.14 ab | 2.25 ± 0.04 a | 1.58 ± 0.14 bc | <0.0001 |
| 2-Methylbutanol | mg/L | 107.93 ± 1.70 | 120.47 ± 4.09 | 120.88 ± 4.78 | 106.02 ± 3.35 | 128.36 ± 4.23 | 117.84 ± 9.33 | 118.19 ± 3.36 | 118.05 ± 8.51 | 108.93 ± 2.52 | ns |
| 3-Methylbutanol | mg/L | 233 ± 4.71 | 265 ± 15.04 | 253 ± 6.46 | 237 ± 9.87 | 267 ± 4.64 | 263 ± 19.06 | 257 ± 5.52 | 266 ± 17.75 | 239 ± 8.67 | ns |
| Hexanol | mg/L | 4.22 ± 0.29 b | 4.01 ± 0.24 b | 4.34 ± 0.20 b | 4.53 ± 0.33 b | 7.09 ± 0.49 a | 4.25 ± 0.26 b | 4.46 ± 0.10 b | 4.74 ± 0.29 b | 4.60 ± 0.23 b | <0.0001 |
| 2-Phenylethanol | mg/L | 50.59 ± 3.83 b | 58.35 ± 4.1 ab | 56.60 ± 2.81 ab | 52.62 ± 2.31 ab | 63.44 ± 1.54 ab | 54.67 ± 1.33 ab | 54.77 ± 1.56 ab | 64.90 ± 2.97 a | 57.75 ± 3.6 ab | <0.05 |
| 2-Methylpropanol | mg/L | 40.30 ± 0.78 | 38.63 ± 4.18 | 38.15 ± 3.10 | 37.62 ± 2.64 | 36.33 ± 3.59 | 40.48 ± 1.91 | 40.02 ± 1.95 | 41.67 ± 2.56 | 39.24 ± 2.12 | ns |
| Volatile acids | |||||||||||
| Butanoic acid | mg/L | 1.02 ± 0.16 | 1.04 ± 0.01 | 1.08 ± 0.08 | 1.19 ± 0.13 | 1.10 ± 0.02 | 1.16 ± 0.05 | 1.08 ± 0.06 | 1.11 ± 0.07 | 0.97 ± 0.03 | ns |
| Acetic acid | mg/L | 273 ± 53.9 | 227 ± 38.4 | 272 ± 45.0 | 356.35 ± 35.9 | 214.60 ± 11.5 | 237.18 ± 13.0 | 281.54 ± 39.0 | 276.38 ± 3.19 | 283.34 ± 20.2 | ns |
| Propanoic acid | mg/L | 9.17 ± 0.75 | 11.38 ± 1.61 | 12.46 ± 1.14 | 12.56 ± 1.37 | 11.16 ± 0.70 | 12.69 ± 2.77 | 11.86 ± 0.91 | 13.02 ± 0.83 | 12.64 ± 1.42 | ns |
| Hexanoic acid | mg/L | 1.89 ± 0.22 | 1.96 ± 0.1 | 2.46 ± 0.42 | 1.68 ± 0.1 | 2.48 ± 0.12 | 1.80 ± 0.02 | 1.78 ± 0.04 | 1.78 ± 0.09 | 1.82 ± 0.22 | ns |
| Octanoic acid | mg/L | 1.07 ± 0.01 abc | 1.19 ± 0.01 ab | 1.28 ± 0.03 a | 0.84 ± 0.03 c | 1.14 ± 0.08 ab | 1.16 ± 0.06 ab | 1.06 ± 0.06 abc | 0.88 ± 0.02 c | 1.01 ± 0.09 bc | <0.001 |
| Decanoic acid | µg/L | 154 ± 12.3 abc | 189 ± 6.89 ab | 200 ± 7.76 a | 151 ± 11.61 bc | 160 ± 9.27 abc | 188 ± 7.17 ab | 158 ± 7.24 abc | 138 ± 10.44 c | 176 ± 10.3 abc | <0.05 |
| 2-Methylbutanoic acid | mg/L | 1.04 ± 0.02 | 1.24 ± 0.06 | 1.16 ± 0.03 | 1.10 ± 0.06 | 1.05 ± 0.02 | 1.12 ± 0.03 | 1.17 ± 0.05 | 1.21 ± 0.08 | 1.03 ± 0.06 | ns |
| 3-Methylbutanoic acid | mg/L | 1.20 ± 0.02 | 1.47 ± 0.11 | 1.39 ± 0.05 | 1.32 ± 0.08 | 1.37 ± 0.04 | 1.37 ± 0.09 | 1.47 ± 0.04 | 1.44 ± 0.03 | 1.31 ± 0.09 | ns |
| Sensory Attribute | Data Selected | PLS Model Parameters ‡ | ||||
|---|---|---|---|---|---|---|
| Factor No | R2cal | R2val | RMSEcal | RMSEval | ||
| Opacity | all variables | 1 | 0.82 | 0.58 | 0.34 | 0.58 |
| sig var § | 3 | 0.96 | 0.58 | 0.15 | 0.58 | |
| Brown colour | all variables | 1 | 0.92 | 0.86 | 0.10 | 0.14 |
| sig var | 3 | 0.98 | 0.94 | 0.05 | 0.09 | |
| Red fruit aroma | all variables | 1 | 0.61 | 0.49 | 0.16 | 0.21 |
| sig var | 3 | 0.57 | 0.38 | 0.17 | 0.23 | |
| Dark fruit aroma | all variables | 1 | 0.72 | 0.50 | 0.20 | 0.30 |
| sig var | 3 | 0.96 | 0.58 | 0.07 | 0.28 | |
| Dried fruit aroma | all variables | 1 | 0.83 | 0.73 | 0.13 | 0.18 |
| sig var | 3 | 0.87 | 0.73 | 0.13 | 0.20 | |
| Spice aroma | all variables | 1 | - | - | - | - |
| sig var | 3 | 0.79 | 0.64 | 0.10 | 0.14 | |
| Earthy aroma | all variables | 1 | 0.79 | 0.67 | 0.10 | 0.13 |
| sig var | 3 | 0.78 | 0.62 | 0.09 | 0.14 | |
| Pepper aroma | all variables | 1 | 0.70 | 0.53 | 0.07 | 0.09 |
| sig var | 3 | 0.70 | 0.36 | 0.07 | 0.11 | |
| Pungent aroma | all variables | 1 | 0.79 | 0.65 | 0.11 | 0.15 |
| sig var | 3 | 0.87 | 0.62 | 0.08 | 0.16 | |
| Sweetness | all variables | 1 | 0.81 | 0.71 | 0.10 | 0.14 |
| sig var | 3 | 0.96 | 0.85 | 0.05 | 0.10 | |
| Viscosity | all variables | 1 | 0.69 | 0.43 | 0.16 | 0.24 |
| sig var | 3 | 0.89 | 0.35 | 0.09 | 0.25 | |
| Dark fruit flavour | all variables | 1 | 0.73 | 0.43 | 0.23 | 0.38 |
| sig var | 3 | 0.90 | 0.40 | 0.14 | 0.39 | |
| Dried fruit flavour | all variables | 1 | 0.95 | 0.88 | 0.09 | 0.15 |
| sig var | 3 | 0.95 | 0.90 | 0.09 | 0.14 | |
| Chocolate flavour | all variables | 1 | 0.61 | 0.40 | 0.19 | 0.26 |
| sig var | 3 | 0.79 | 0.38 | 0.14 | 0.26 | |
| Earthy flavour | all variables | 1 | 0.74 | 0.53 | 0.14 | 0.21 |
| sig var | 3 | 0.75 | 0.29 | 0.14 | 0.26 | |
| Spice flavour | all variables | 1 | 0.63 | 0.51 | 0.16 | 0.21 |
| sig var | 3 | 0.94 | 0.77 | 0.07 | 0.14 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teng, B.; Petrie, P.R.; Espinase Nandorfy, D.; Smith, P.; Bindon, K. Pre-Fermentation Water Addition to High-Sugar Shiraz Must: Effects on Wine Composition and Sensory Properties. Foods 2020, 9, 1193. https://doi.org/10.3390/foods9091193
Teng B, Petrie PR, Espinase Nandorfy D, Smith P, Bindon K. Pre-Fermentation Water Addition to High-Sugar Shiraz Must: Effects on Wine Composition and Sensory Properties. Foods. 2020; 9(9):1193. https://doi.org/10.3390/foods9091193
Chicago/Turabian StyleTeng, Bo, Paul R. Petrie, Damian Espinase Nandorfy, Paul Smith, and Keren Bindon. 2020. "Pre-Fermentation Water Addition to High-Sugar Shiraz Must: Effects on Wine Composition and Sensory Properties" Foods 9, no. 9: 1193. https://doi.org/10.3390/foods9091193
APA StyleTeng, B., Petrie, P. R., Espinase Nandorfy, D., Smith, P., & Bindon, K. (2020). Pre-Fermentation Water Addition to High-Sugar Shiraz Must: Effects on Wine Composition and Sensory Properties. Foods, 9(9), 1193. https://doi.org/10.3390/foods9091193