Organic Selenium as Antioxidant Additive in Mitigating Acrylamide in Coffee Beans Roasted via Conventional and Superheated Steam
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Selenium Pretreatment
2.3. Coffee Roasting
2.4. Determination of Acrylamide in Coffee
2.5. Determination of Selenium
2.6. Determination of Antioxidants Activity
2.6.1. FRAP Assay
2.6.2. DPPH Assay
2.7. Color Evaluation
2.8. Statistical and Data Analysis
3. Results and Discussion
3.1. Selenium Uptake by Green Coffee Beans and Retention in Se-Coffee Beans
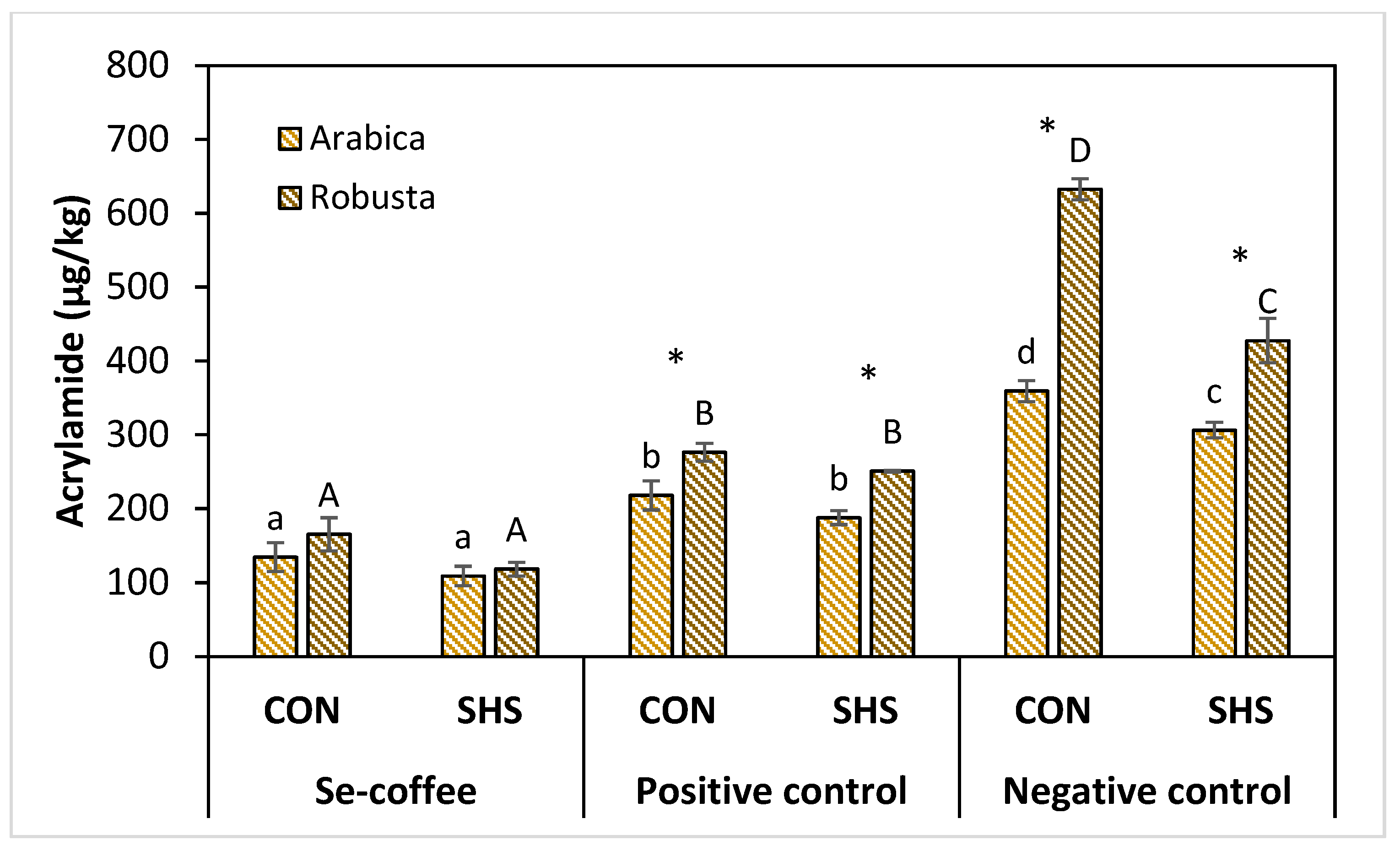
3.2. Effects of Selenium Pretreatment on Acrylamide Formation
3.3. Effects of Selenium Pretreatment on Antioxidant Activity of Green and Roasted Coffee Beans
3.3.1. Green Beans
3.3.2. Roasted Beans
3.4. Effects of Selenium Pretreatment on Color of Roasted coffee
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hu, G.L.; Wang, X.; Zhang, L.; Qiu, M.H. The sources and mechanisms of bioactive ingredients in coffee. Food Funct. 2019, 10, 3113–3126. [Google Scholar] [CrossRef] [PubMed]
- Higashi, Y. Coffee and endothelial function: A coffee paradox? Nutrients 2019, 11, 2104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gökcen, B.B.; Şanlier, N. Coffee consumption and disease correlations. Crit. Rev. Food Sci. Nutr. 2019, 59, 336–348. [Google Scholar] [CrossRef] [PubMed]
- Acidri, R.; Sawai, Y.; Sugimoto, Y.; Handa, T.; Sasagawa, D.; Masunaga, T.; Yamamoto, S.; Nishihara, E. Phytochemical profile and antioxidant capacity of coffee plant organs compared to green and roasted coffee beans. Antioxidants 2020, 9, 93. [Google Scholar] [CrossRef] [Green Version]
- Liang, N.; Kitts, D.D. Antioxidant property of coffee components: Assessment of methods that define mechanism of action. Molecules 2014, 19, 19180–19208. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Kitts, D.D. Confirmation that the Maillard reaction is the principle contributor to the antioxidant capacity of coffee brews. Food Res. Int. 2011, 44, 2418–2424. [Google Scholar] [CrossRef]
- Lee, B.H.; Nam, T.G.; Kim, S.Y.; Chun, O.K.; Kim, D.O. Estimated daily per capita intakes of phenolics and antioxidants from coffee in the Korean diet. Food Sci. Biotechnol. 2019, 28, 269–279. [Google Scholar] [CrossRef]
- Torres, T.; Farah, A. Coffee, maté, açaí and beans are the main contributors to the antioxidant capacity of Brazilian’s diet. Eur. J. Nutr. 2017, 56, 1523–1533. [Google Scholar] [CrossRef]
- Chindapan, N.; Soydok, S.; Devahastin, S. Roasting Kinetics and Chemical Composition Changes of Robusta Coffee Beans During Hot Air and Superheated Steam Roasting. J. Food Sci. 2019, 84, 292–302. [Google Scholar] [CrossRef]
- Opitz, S.; Smrke, S.; Goodman, B.; Keller, M.; Schenker, S.; Yeretzian, C. Antioxidant Generation during Coffee Roasting: A Comparison and Interpretation from Three Complementary Assays. Foods 2014, 3, 586–604. [Google Scholar] [CrossRef]
- IARC. Acrylamide. Monographs on the Evaluation of Carcinogenic Risks to Humans: Some Industrial Chemicals; IARC: Lyon, France, 1994; Volume 60. [Google Scholar]
- Europen Commission COMMISSION REGULATION (EU) 2017/2158 of 20 November 2017 establishing mitigation measures and benchmark levels for the reduction of the presence of acrylamide in food. Off. J. Eur. Union 2017, L 304, 24–44.
- Jin, C.; Wu, X.; Zhang, Y. Relationship between antioxidants and acrylamide formation: A review. Food Res. Int. 2013, 51, 611–620. [Google Scholar] [CrossRef]
- Kocadagli, T.; Göncüoglu, N.; Hamzalioglu, A.; Gökmen, V. In depth study of acrylamide formation in coffee during roasting: Role of sucrose decomposition and lipid oxidation. Food Funct. 2012, 3, 970–975. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Zhang, Z.; Jiang, S.; Yu, M.; Huang, C.; Qiu, R.; Zou, Y.; Zhang, Q.; Ou, S.; Zhou, H.; et al. Chlorogenic acid increased acrylamide formation through promotion of HMF formation and 3-aminopropionamide deamination. J. Hazard. Mater. 2014, 268, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Gökmen, V.; Kocadaǧli, T.; Göncüoǧlu, N.; Mogol, B.A. Model studies on the role of 5-hydroxymethyl-2-furfural in acrylamide formation from asparagine. Food Chem. 2012, 132, 168–174. [Google Scholar] [CrossRef]
- Kápolna, E.; Gergely, V.; Dernovics, M.; Illés, A.; Fodor, P. Fate of selenium species in sesame seeds during simulated bakery process. J. Food Eng. 2007, 79, 494–501. [Google Scholar] [CrossRef]
- Institute of Medicine. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids; The National Academies Press: Washington, DC, USA; ISBN 9780309069496.
- Kieliszek, M.; Błazejak, S. Current knowledge on the importance of selenium in food for living organisms: A review. Molecules 2016, 21, 609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuršvietienė, L.; Mongirdienė, A.; Bernatonienė, J.; Šulinskienė, J.; Stanevičienė, I. Selenium anticancer properties and impact on cellular redox status. Antioxidants 2020, 9, 80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bodnar, M.; Szczyglowska, M.; Konieczka, P.; Namiesnik, J. Methods of Selenium Supplementation: Bioavailability and Determination of Selenium Compounds. Crit. Rev. Food Sci. Nutr. 2016, 56, 36–55. [Google Scholar] [CrossRef]
- Messaoudi, M.; Begaa, S.; Hamidatou, L.; Salhi, M. Determination of selenium in roasted beans coffee samples consumed in Algeria by radiochemical neutron activation analysis method. Radiochim. Acta 2018, 106, 141–146. [Google Scholar] [CrossRef]
- Meija, J.; Bryson, J.M.; Vonderheide, A.P.; Montes-Bayón, M.; Caruso, J.A. Studies of selenium-containing volatiles in roasted coffee. J. Agric. Food Chem. 2003, 51, 5116–5122. [Google Scholar] [CrossRef] [PubMed]
- Anese, M.; Nicoli, M.C.; Verardo, G.; Munari, M.; Mirolo, G.; Bortolomeazzi, R. Effect of vacuum roasting on acrylamide formation and reduction in coffee beans. Food Chem. 2014, 145, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Guenther, H.; Anklam, E.; Wenzl, T.; Stadler, R.H. Acrylamide in coffee: Review of progress in analysis, formation and level reduction. Food Addit. Contam. 2007, 24, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Narita, Y.; Inouye, K. Decrease in the acrylamide content in canned coffee by heat treatment with the addition of cysteine. J. Agric. Food Chem. 2014, 62, 12218–12222. [Google Scholar] [CrossRef] [PubMed]
- Lynglev, G.B.; Schoesler, S. Method for producing roasted coffee beans. U.S. Patent US20160295877A1, 13 October 2016. [Google Scholar]
- Devahastin, S.; Mujumdar, A.S. Superheated Steam Drying of Foods and Biomaterials. Modern Drying Technol. 2014, 5, 57–84. [Google Scholar]
- Maki, Y.; Haruyama, T. Process for roasting coffee beans with steam. U.S. Patent US5681607A, 28 October 1997. [Google Scholar]
- Perkin, E. Acrylamide Analysis by Gas Chromatography. USA PerkinElmer Life Anal. Sci. 2004, 20, 5–7. [Google Scholar]
- Ku Madihah, K.Y.; Zaibunnisa, A.H.; Norashikin, S.; Rozita, O.; Misnawi, J. Optimization of roasting conditions for high-quality Arabica coffee. Int. Food Res. J. 2013, 20, 1623–1627. [Google Scholar]
- Choi, Y.; Kim, J.; Lee, H.S.; Kim, C.; Hwang, I.K.; Park, H.K.; Oh, C.H. Selenium content in representative Korean foods. J. Food Compos. Anal. 2009, 22, 117–122. [Google Scholar] [CrossRef]
- Moreira, D.P.; Monteiro, M.C.; Ribeiro-Alves, M.; Donangelo, C.M.; Trugo, L.C. Contribution of chlorogenic acids to the iron-reducing activity of coffee beverages. J. Agric. Food Chem. 2005, 53, 1399–1402. [Google Scholar] [CrossRef]
- Kwak, H.S.; Ji, S.; Jeong, Y. The effect of air flow in coffee roasting for antioxidant activity and total polyphenol content. Food Control 2017, 71, 210–216. [Google Scholar] [CrossRef]
- Virgen-Navarro, L.; Herrera-López, E.J.; Corona-González, R.I.; Arriola-Guevara, E.; Guatemala-Morales, G.M. Neuro-fuzzy model based on digital images for the monitoring of coffee bean color during roasting in a spouted bed. Expert Syst. Appl. 2016, 54, 162–169. [Google Scholar] [CrossRef]
- Buera, M.P.; Lozano, R.D.; Petriella, C. Definition of Color in the Non-enzymatic Browning Process. Die Farbe 1986, 33, 316–326. [Google Scholar]
- Ludwig, I.A.; Mena, P.; Calani, L.; Cid, C.; Del Rio, D.; Leand, M.E.; Crozier, A.; Lean, J. Variations in caffeine and chlorogenic acid contents of coffees: What are we drinking? Food Funct. 2014, 5, 1718–1726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.J.; De Bruyn, F.; Pothakos, V.; Contreras, G.F.; Cai, Z.; Moccand, C.; Weckx, S.; De Vuyst, L. Influence of Various Processing Parameters on the Microbial Community Dynamics, Metabolomic Profiles, and Cup Quality During Wet Coffee Processing. Front. Microbiol. 2019, 10, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Shadaksharaswamy, M.; Ramachandra, G. Changes in the oligosaccharides and the α-galactosidase content of coffee seeds during soaking and germination. Phytochemistry 1968, 7, 715–719. [Google Scholar] [CrossRef]
- Tsai, J.H.; Hiserodt, R.D.; Ho, C.T.; Hartman, T.G.; Rosen, R.T. Determination of Volatile organic Selenium Compounds from the Maillard Reaction in a Selenomethionine - Glucose Model System. J. Agric. Food Chem. 1998, 46, 2541–2545. [Google Scholar] [CrossRef]
- Wei, G.J.; Ho, C.T.; Huang, A.S. Determination of volatile compounds formed in a glucose-selenomethionine model system by gas chromatography-atomic emission detector and gas chromatography-mass spectrometry. Food Chem. 2009, 116, 774–778. [Google Scholar] [CrossRef]
- Zzaman, W.; Bhat, R.; Yang, T.A. Effect of superheated steam roasting on the phenolic antioxidant properties of cocoa beans. J. Food Process. Preserv. 2014, 38, 1932–1938. [Google Scholar] [CrossRef]
- Yodkaew, P.; Chindapan, N.; Devahastin, S. Influences of Superheated Steam Roasting and Water Activity Control as Oxidation Mitigation Methods on Physicochemical Properties, Lipid Oxidation, and Free Fatty Acids Compositions of Roasted Rice. J. Food Sci. 2017, 82, 69–79. [Google Scholar] [CrossRef]
- Lim, P.K.; Jinap, S.; Sanny, M.; Tan, C.P.; Khatib, A. The influence of deep frying using various vegetable oils on acrylamide formation in sweet potato (Ipomoea batatas L. Lam) chips. J. Food Sci. 2014, 79. [Google Scholar] [CrossRef]
- Capuano, E. Lipid Oxidation Promotes Acrylamide Formation in Fat-Rich Systems; Elsevier Inc.: Amsterdam, The Netherlands, 2016; ISBN 9780128028759. [Google Scholar]
- Zamora, R.; Hidalgo, F.J. Contribution of lipid oxidation products to acrylamide formation in model systems. J. Agric. Food Chem. 2008, 56, 6075–6080. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, F.J.; Delgado, R.M.; Zamora, R. Degradation of asparagine to acrylamide by carbonyl-amine reactions initiated by alkadienals. Food Chem. 2009, 116, 779–784. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef] [PubMed]
- Masek, A.; Latos-Brozio, M.; Kałuzna-Czaplińska, J.; Rosiak, A.; Chrzescijanska, E. Antioxidant properties of green coffee extract. Forests 2020, 11, 557. [Google Scholar] [CrossRef]
- Sentkowska, A.; Pyrzyńska, K. Investigation of antioxidant activity of selenium compounds and their mixtures with tea polyphenols. Mol. Biol. Rep. 2019, 46, 3019–3024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Bruyn, F.; Zhang, S.J.; Pothakos, V.; Torres, J.; Lambot, C.; Moroni, A.V.; Callanan, M.; Sybesma, W.; Weckx, S.; De Vuysta, L. Exploring the Impacts of Postharvest Processing on the Microbiota and Metabolite Profiles during Green Coffee Bean Production. Appl. Environ. Microbiol. 2017, 83, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Benzie, I.F.F.; Devaki, M. The ferric reducing/antioxidant power (FRAP) assay for non-enzymatic antioxidant capacity: Concepts, procedures, limitations and applications. In Measurement of Antioxidant Activity & Capacity; Apak, R., Capanoglu, E., Shahidi, F., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2018; pp. 77–106. ISBN 9781119135357. [Google Scholar]
- Vignoli, J.A.; Viegas, M.C.; Bassoli, D.G.; de Benassi, M.T. Roasting process affects differently the bioactive compounds and the antioxidant activity of arabica and robusta coffees. Food Res. Int. 2014, 61, 279–285. [Google Scholar] [CrossRef] [Green Version]
- Sacchetti, G.; Di Mattia, C.; Pittia, P.; Mastrocola, D. Effect of roasting degree, equivalent thermal effect and coffee type on the radical scavenging activity of coffee brews and their phenolic fraction. J. Food Eng. 2009, 90, 74–80. [Google Scholar] [CrossRef]
- Budryn, G.; Nebesny, E.; Oracz, J. Correlation between the stability of chlorogenic acids, antioxidant activity and acrylamide content in coffee beans roasted in different conditions. Int. J. Food Prop. 2015, 18, 290–302. [Google Scholar] [CrossRef]
- Cheng, J.; Chen, X.; Zhao, S.; Zhang, Y. Antioxidant-capacity-based models for the prediction of acrylamide reduction by flavonoids. Food Chem. 2015, 168, 90–99. [Google Scholar] [CrossRef]
- Hamzalıoğlu, A.; Gökmen, V. 5-Hydroxymethylfurfural accumulation plays a critical role on acrylamide formation in coffee during roasting as confirmed by multiresponse kinetic modelling. Food Chem. 2020, 318, 126467. [Google Scholar] [CrossRef] [PubMed]
- Summa, C.A.; de la Calle, B.; Brohee, M.; Stadler, R.H.; Anklam, E. Impact of the roasting degree of coffee on the in vitro radical scavenging capacity and content of acrylamide. LWT Food Sci. Technol. 2007, 40, 1849–1854. [Google Scholar] [CrossRef]




| Pretreatments | Arabica Coffee | Robusta Coffee | ||
|---|---|---|---|---|
| FRAP 1 (mmol Fe2+ eq/g) | DPPH EC50 1 (mg/mL) | FRAP 1 (mmol Fe2+ eq/g) | DPPH EC50 1 (mg/mL) | |
| Se-Coffee | 0.58 ± 0.02 a | 3.19 ± 0.02 b | 1.00 ± 0.03 b | 3.20 ± 0.02 b |
| Positive Control | 0.74 ± 0.01 a | 3.38 ± 0.02 c | 0.84 ± 0.02 a | 3.15 ± 0.05 b |
| Negative Control | 1.86 ± 0.07 b | 1.93 ± 0.01 a | 2.07 ± 0.02 c | 1.92 ± 0.03 a |
| Arabica Coffee | Robusta Coffee | |||
|---|---|---|---|---|
| FRAP 1 (mmol Fe2+ eq/g) | DPPH EC50 1 (mg/mL) | FRAP 1 (mmol Fe2+ eq/g) | DPPH EC50 1 (mg/mL) | |
| Se-coffee | ||||
| CON | 1.42 ± 0.01 Ba | 2.59 ± 0.03 Aa | 1.45 ± 0.05 Ba | 2.33 ± 0.08 Aa |
| SHS | 1.49 ± 0.01 Bb | 2.57 ± 0.03 Aa | 1.71 ±0.03 Bb | 2.30 ± 0.05 Aa |
| Positive control | ||||
| CON | 1.23 ± 0.03 Aa | 3.03 ± 0.02 Ca | 1.36 ± 0.04 Aa | 2.96 ± 0.05 Ca |
| SHS | 1.40 ± 0.04 Ab | 3.18 ± 0.04 Bb | 1.45 ±0.07 Ab | 2.93 ± 0.01 Ba |
| Negative control | ||||
| CON | 1.37 ± 0.02 Ba | 2.80 ± 0.04 Bb | 1.39 ± 0.01 Aa | 2.69 ± 0.04 Bb |
| SHS | 1.46 ± 0.02 Bb | 2.64 ± 0.03 Aa | 1.65 ±0.03 Bb | 2.45 ± 0.04 Aa |
| Two-way ANOVA analysis (F-value) | ||||
| Pretreatment | 34.8 *** | 295.5 *** | 15.8 *** | 215.5 *** |
| Roasting | 59.4 *** | NS | 62.4 *** | 8.6 *** |
| Pretreatment × Roasting | 4.6 *** | 24.9 *** | 4.8 *** | 14.2 *** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alafeef, A.K.; Ariffin, F.; Zulkurnain, M. Organic Selenium as Antioxidant Additive in Mitigating Acrylamide in Coffee Beans Roasted via Conventional and Superheated Steam. Foods 2020, 9, 1197. https://doi.org/10.3390/foods9091197
Alafeef AK, Ariffin F, Zulkurnain M. Organic Selenium as Antioxidant Additive in Mitigating Acrylamide in Coffee Beans Roasted via Conventional and Superheated Steam. Foods. 2020; 9(9):1197. https://doi.org/10.3390/foods9091197
Chicago/Turabian StyleAlafeef, Ahmad K., Fazilah Ariffin, and Musfirah Zulkurnain. 2020. "Organic Selenium as Antioxidant Additive in Mitigating Acrylamide in Coffee Beans Roasted via Conventional and Superheated Steam" Foods 9, no. 9: 1197. https://doi.org/10.3390/foods9091197
APA StyleAlafeef, A. K., Ariffin, F., & Zulkurnain, M. (2020). Organic Selenium as Antioxidant Additive in Mitigating Acrylamide in Coffee Beans Roasted via Conventional and Superheated Steam. Foods, 9(9), 1197. https://doi.org/10.3390/foods9091197





