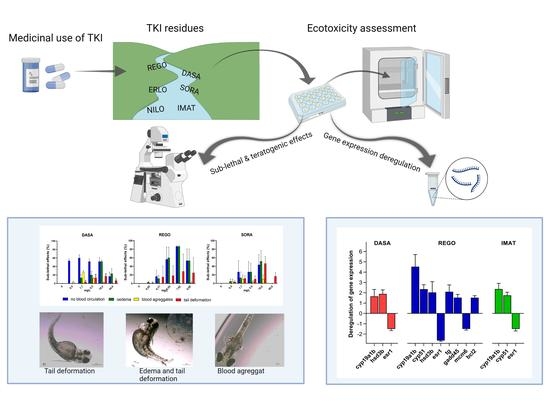
Lethal and Sub-Lethal Effects and Modulation of Gene Expression Induced by T Kinase Inhibitors in Zebrafish (Danio Rerio) Embryos
Abstract
:1. Introduction
2. Materials and Methods
2.1. Consumption of Tyrosine Kinase Inhibitors in Slovenia
2.2. Test Substances
2.3. Maintenance of Zebrafish
2.4. TKIs Solutions
2.5. Fish Embryo Toxicity Test (FET)
2.6. Gene Expression Analysis
2.7. Statistical Evaluations
3. Results and Discussion
3.1. Consumption of TKIs in Slovenia
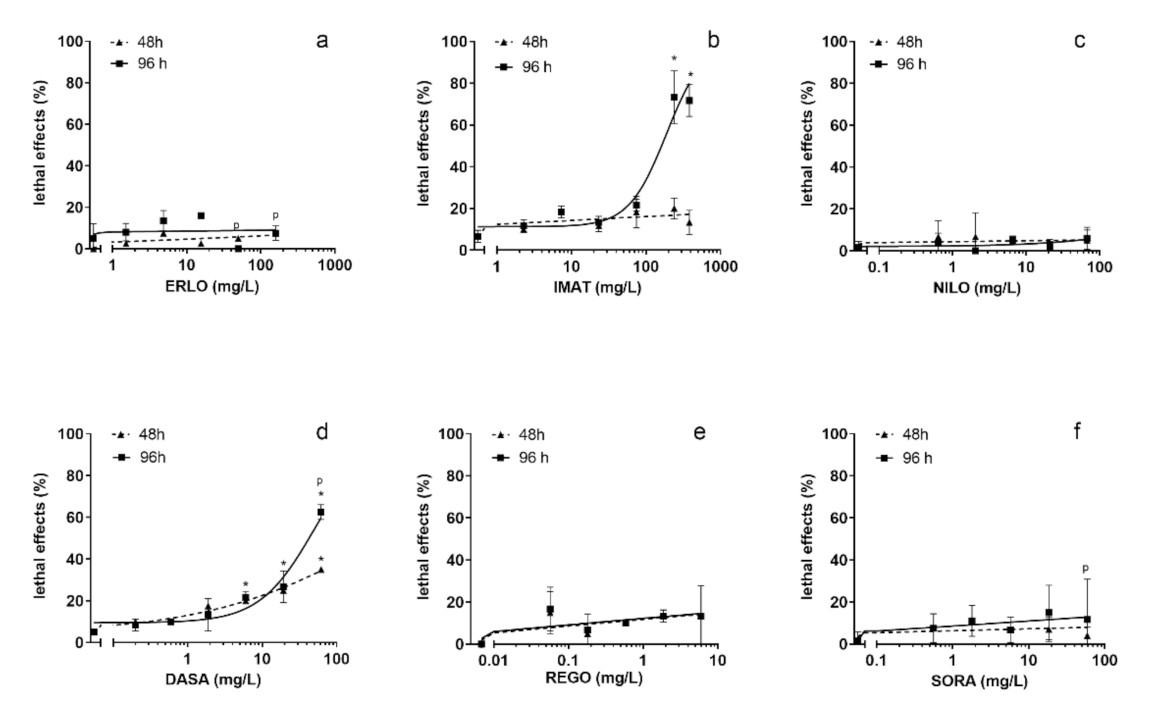
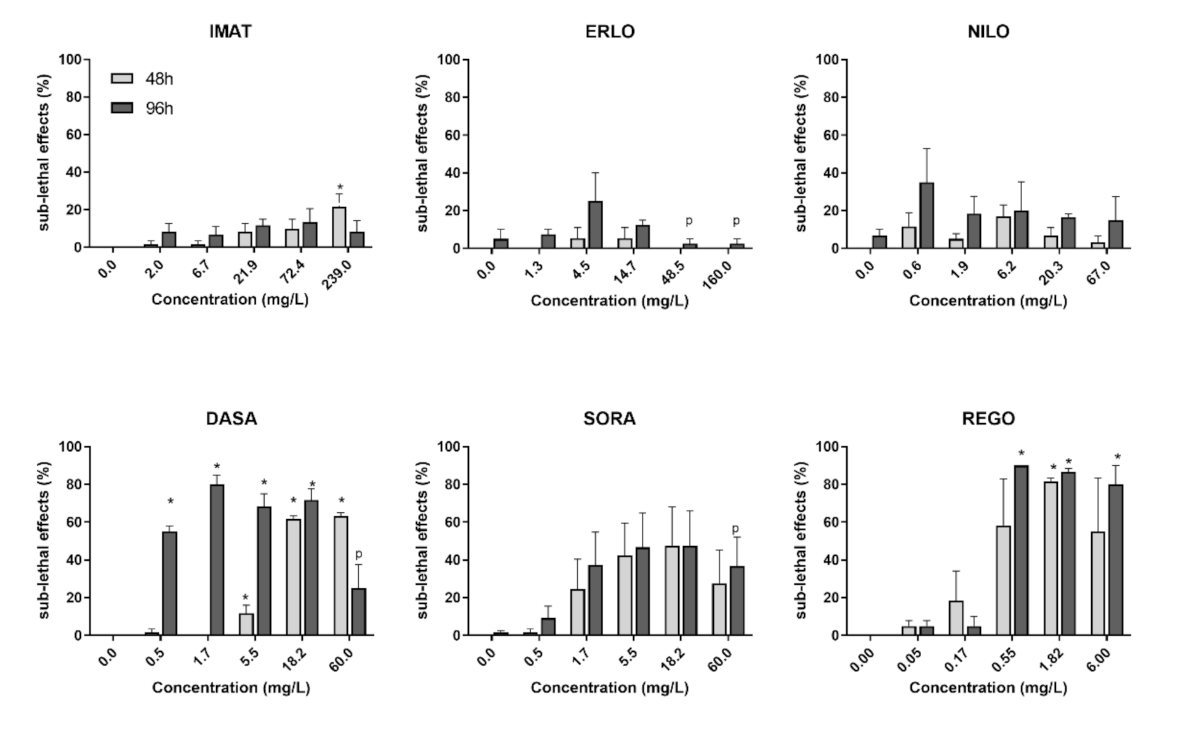
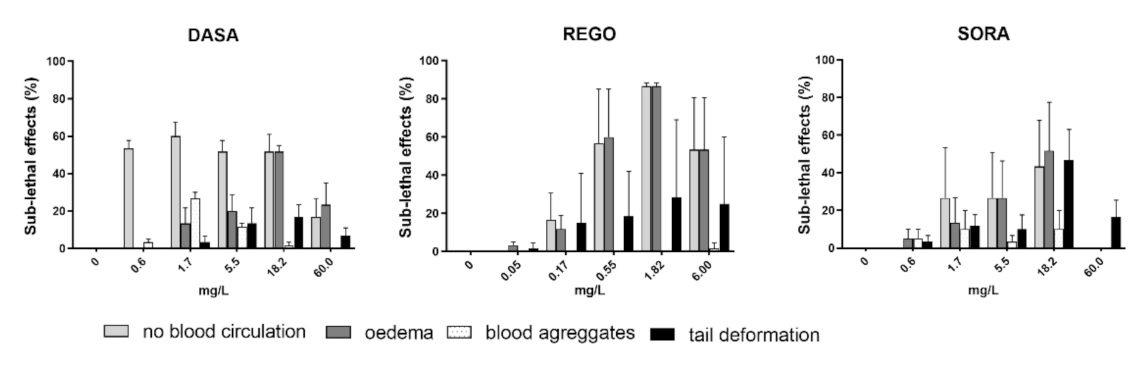
3.2. Fish Embryo Toxicity Test
3.3. Modulation of Gene Expression
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Besse, J.P.; Latour, J.F.; Garric, J. Anticancer drugs in surface waters: What can we say about the occurrence and environmental significance of cytotoxic, cytostatic and endocrine therapy drugs? Environ. Int. 2012, 39, 73–86. [Google Scholar] [CrossRef]
- Kümmerer, K.; Haiß, A.; Schuster, A.; Hein, A.; Ebert, I. Antineoplastic compounds in the environment—substances of special concern. Environ. Sci. Pollut. Res. 2016, 23, 14791–14804. [Google Scholar] [CrossRef] [PubMed]
- Kosjek, T.; Heath, E. Occurrence, fate and determination of cytostatic pharmaceuticals in the environment. TrAC Trends Anal. Chem. 2011, 30, 1065–1087. [Google Scholar] [CrossRef]
- Brezovšek, P.; Eleršek, T.; Filipič, M. Toxicities of four anti-neoplastic drugs and their binary mixtures tested on the green alga Pseudokirchneriella subcapitata and the cyanobacterium Synechococcus leopoliensis. Water Res. 2014, 52, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Parrella, A.; Lavorgna, M.; Criscuolo, E.; Russo, C.; Fiumano, V.; Isidori, M. Acute and chronic toxicity of six anticancer drugs on rotifers and crustaceans. Chemosphere 2014, 115, 59–66. [Google Scholar] [CrossRef]
- Parrella, A.; Lavorgna, M.; Criscuolo, E.; Russo, C.; Isidori, M. Eco-genotoxicity of six anticancer drugs using comet assay in daphnids. J. Hazard. Mater. 2015, 286, 573–580. [Google Scholar] [CrossRef] [Green Version]
- Česen, M.; Eleršek, T.; Novak, M.; Žegura, B.; Kosjek, T.; Filipič, M.; Heath, E. Ecotoxicity and genotoxicity of cyclophosphamide, ifosfamide, their metabolites/transformation products and their mixtures. Environ. Pollut. 2016, 210, 192–201. [Google Scholar] [CrossRef]
- Isidori, M.; Lavorgna, M.; Russo, C.; Kundi, M.; Žegura, B.; Novak, M.M.; Filipič, M.; Mišík, M.; Knasmueller, S.; de Alda, M.L.; et al. Chemical and toxicological characterisation of anticancer drugs in hospital and municipal wastewaters from Slovenia and Spain. Environ. Pollut. 2016, 219, 275–287. [Google Scholar] [CrossRef] [Green Version]
- Besse, J.-P.; Kausch-Barreto, C.; Garric, J. Exposure Assessment of Pharmaceuticals and Their Metabolites in the Aquatic Environment: Application to the French Situation and Preliminary Prioritization. Hum. Ecol. Risk Assess. Int. J. 2008, 14, 665–695. [Google Scholar] [CrossRef]
- Levitzki, A. Tyrosine Kinase Inhibitors: Views of Selectivity, Sensitivity, and Clinical Performance. Annu. Rev. Pharmacol. Toxicol. 2012, 53, 161–185. [Google Scholar] [CrossRef]
- Yamaoka, T.; Kusumoto, S.; Ando, K.; Ohba, M.; Ohmori, T. Receptor Tyrosine Kinase-Targeted Cancer Therapy. Int. J. Mol. Sci. 2018, 19, 3491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crisci, S.; Amitrano, F.; Saggese, M.; Muto, T.; Sarno, S.; Mele, S.; Vitale, P.; Ronga, G.; Berretta, M.; Di Francia, R. Overview of Current Targeted Anti-Cancer Drugs for Therapy in Onco-Hematology. Medicina 2019, 55, 414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.; Kurzrock, R. Toxicity of targeted therapy: Implications for response and impact of genetic polymorphisms. Cancer Treat. Rev. 2014, 40, 883–891. [Google Scholar] [CrossRef]
- Seiler, J.P. Pharmacodynamic activity of drugs and ecotoxicology—Can the two be connected? Toxicol. Lett. 2002, 131, 105–115. [Google Scholar] [CrossRef]
- Cohen, M.H.; Williams, G.; Johnson, J.R.; Duan, J.; Gobburu, J.; Rahman, A.; Benson, K.; Leighton, J.; Kim, S.K.; Wood, R.; et al. Approval Summary for Imatinib Mesylate Capsules in the Treatment of Chronic Myelogenous Leukemia Approval Summary for Imatinib Mesylate Capsules in the Treatment of Chronic Myelogenous Leukemia. Clin. Cancer Res. 2002, 8, 935–942. [Google Scholar]
- Booker, V.; Halsall, C.; Llewellyn, N.; Johnson, A.; Williams, R. Prioritising anticancer drugs for environmental monitoring and risk assessment purposes. Sci. Total Environ. 2014, 473-474, 159–170. [Google Scholar] [CrossRef] [Green Version]
- Cristóvão, M.B.; Janssens, R.; Yadav, A.; Pandey, S.; Luis, P.; Van der Bruggen, B.; Dubey, K.K.; Mandal, M.K.; Crespo, J.G.; Pereira, V.J. Predicted concentrations of anticancer drugs in the aquatic environment: What should we monitor and where should we treat? J. Hazard. Mater. 2020, 392, 122330. [Google Scholar] [CrossRef]
- Pottier, C.; Fresnais, M.; Gilon, M.; Jérusalem, G.; Longuespée, R.; Sounni, N.E. Tyrosine kinase inhibitors in cancer: Breakthrough and challenges of targeted therapy. Cancers 2020, 12, 731. [Google Scholar] [CrossRef] [Green Version]
- Olalla, A.; Negreira, N.; de Alda, M.L.; Barceló, D.; Valcárcel, Y. A case study to identify priority cytostatic contaminants in hospital effluents. Chemosphere 2018, 190, 417–430. [Google Scholar] [CrossRef]
- Pichler, C.; Filipič, M.; Kundi, M.; Rainer, B.; Knasmueller, S.; Mišík, M. Assessment of genotoxicity and acute toxic effect of the imatinib mesylate in plant bioassays. Chemosphere 2014, 115, 54–58. [Google Scholar] [CrossRef]
- Parrella, A.; Kundi, M.; Lavorgna, M.; Criscuolo, E.; Russo, C.; Isidori, M. Toxicity of exposure to binary mixtures of four anti-neoplastic drugs in Daphnia magna and Ceriodaphnia dubia. Aquat. Toxicol. 2014, 157, 41–46. [Google Scholar] [CrossRef] [Green Version]
- Novak, M.; Baebler, Š.; Žegura, B.; Rotter, A.; Gajski, G.; Gerić, M.; Garaj-Vrhovac, V.; Bakos, K.; Csenki, Z.; Kovács, R.; et al. Deregulation of whole-transcriptome gene expression in zebrafish (Danio rerio) after chronic exposure to low doses of imatinib mesylate in a complete life cycle study. Chemosphere 2021, 263, 128097. [Google Scholar] [CrossRef]
- Novak, M.M.; Žegura, B.; Nunić, J.; Gajski, G.; Gerić, M.; Garaj-Vrhovac, V. Assessment of the genotoxicity of the tyrosine kinase inhibitor imatinib mesylate in cultured fish and human cells. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2017, 814, 14–21. [Google Scholar] [CrossRef]
- Novak, M.; Žegura, B.; Baebler, Š.; Štern, A.; Rotter, A.; Stare, K.; Filipič, M.; Novak, M.; Baebler, Š.; Štern, A.; et al. Influence of selected anti-cancer drugs on the induction of DNA double-strand breaks and changes in gene expression in human hepatoma HepG2 cells. Environ. Sci. Pollut. Res. 2016, 23, 14751–14761. [Google Scholar] [CrossRef]
- Gajski, G.; Gerić, M.; Domijan, A.M.; Golubović, I.; Garaj-Vrhovac, V. Evaluation of oxidative stress responses in human circulating blood cells after imatinib mesylate treatment—Implications to its mechanism of action. Saudi Pharm. J. 2019, 27, 1216–1221. [Google Scholar] [CrossRef] [PubMed]
- Parrella, A.; Lavorgna, M.; Criscuolo, E.; Russo, C.; Isidori, M.; Russo, C.; Parrella, A.; Criscuolo, E. Estrogenic activity and cytotoxicity of six anticancer drugs detected in water systems. Sci. Total Environ. 2014, 485, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Whomsley, R.; Brendler-Schwaab, S.; Griffin, E.; Jensen, J.; Moermond, C.; Scholz, B.; Nilssen, L.S.; Stemplewski, H.; Roennefahrt, I. Commentary on the draft revised guideline on the environmental risk assessment of medicinal products for human use. Environ. Sci. Eur. 2019, 31, 1–48. [Google Scholar] [CrossRef] [Green Version]
- Girardot, F.; Monnier, V.; Tricoire, H. Genome wide analysis of common and specific stress responses in adult drosophila melanogaster. BMC Genom. 2004, 5, 74. [Google Scholar] [CrossRef] [Green Version]
- Williams, T.D.; Mirbahai, L.; Chipman, J.K. The toxicological application of transcriptomics and epigenomics in zebrafish and other teleosts. Brief. Funct. Genom. 2014, 13, 157–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baebler, Š.; Svalina, M.; Petek, M.; Stare, K.; Rotter, A.; Pompe-Novak, M.; Gruden, K. quantGenius: Implementation of a decision support system for qPCR-based gene quantification. BMC Bioinform. 2017, 18, 276. [Google Scholar] [CrossRef]
- Hill, A.; Gotham, D.; Fortunak, J.; Meldrum, J.; Erbacher, I.; Martin, M.; Shoman, H.; Levi, J.; Powderly, W.G.; Bower, M. Target prices for mass production of tyrosine kinase inhibitors for global cancer treatment. BMJ Open 2016, 6, e009586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kovács, R.; Bakos, K.; Urbányi, B.; Kövesi, J.; Gazsi, G.; Csepeli, A.; Appl, Á.J.; Bencsik, D.; Csenki, Z.; Horváth, Á. Acute and sub-chronic toxicity of four cytostatic drugs in zebrafish. Environ. Sci. Pollut. Res. 2016, 23, 14718–14729. [Google Scholar] [CrossRef]
- Chimote, G.; Sreenivasan, J.; Pawar, N.; Subramanian, J.; Sivaramakrishnan, H.; Sharma, S. Comparison of effects of anti-angiogenic agents in the zebrafish efficacy-toxicity model for translational anti-angiogenic drug discovery. Drug Des. Dev. Ther. 2014, 8, 1107–1123. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.-S.; Huang, Y.-L.; Wang, Y.-R.S.; Hsiao, E.; Hsu, T.-A.; Shiao, H.-Y.; Jiaang, W.-T.; Sampurna, B.P.; Lin, K.-H.; Wu, M.-S.; et al. Identification of Novel Anti-Liver Cancer Small Molecules with Better Therapeutic Index Than Sorafenib via Zebrafish Drug Screening Platform. Cancers 2019, 11, 739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.Q.; Fan, R.Y.; Zhang, S.R.; Li, C.Y.; Shen, L.Z.; Wei, P.; He, Z.H.; He, M.F. A systematical comparison of anti-angiogenesis and anti-cancer efficacy of ramucirumab, apatinib, regorafenib and cabozantinib in zebrafish model. Life Sci. 2020, 247, 117402. [Google Scholar] [CrossRef]
- Kilarski, W.W.; Jura, N.; Gerwins, P. Inactivation of Src family kinases inhibits angiogenesis in vivo: Implications for a mechanism involving organization of the actin cytoskeleton. Exp. Cell Res. 2003, 291, 70–82. [Google Scholar] [CrossRef]
- Mouhayar, E.; Durand, J.-B.; Cortes, J. Cardiovascular toxicity of tyrosine kinase inhibitors. Expert Opin. Drug Saf. 2013, 12, 687–696. [Google Scholar] [CrossRef]
- Breccia, M.; Molica, M.; Alimena, G. How tyrosine kinase inhibitors impair metabolism and endocrine system function: A systematic updated review. Leuk. Res. 2014, 38, 1392–1398. [Google Scholar] [CrossRef]
- Lodish, M.B.; Stratakis, C.A. Endocrine side effects of broad-acting kinase inhibitors. Endocr. Relat. Cancer 2010, 17, R233–R244. [Google Scholar] [CrossRef] [Green Version]
- Torino, F.; Corsello, S.M.; Longo, R.; Barnabei, A.; Gasparini, G. Hypothyroidism related to tyrosine kinase inhibitors: An emerging toxic effect of targeted therapy. Nat. Rev. Clin. Oncol. 2009, 6, 219–228. [Google Scholar] [CrossRef] [Green Version]
- Lutterbeck, C.A.; Kern, D.I.; Machado, Ê.L.; Kümmerer, K. Evaluation of the toxic effects of four anti-cancer drugs in plant bioassays and its potency for screening in the context of waste water reuse for irrigation. Chemosphere 2015, 135, 403–410. [Google Scholar] [CrossRef]
- Chen, Y.; Tang, H.; Wang, L.; He, J.; Guo, Y.; Liu, Y.; Liu, X.; Lin, H. Fertility Enhancement but Premature Ovarian Failure in esr1-Deficient Female Zebrafish. Front. Endocrinol. 2018, 9, 567. [Google Scholar] [CrossRef]
- Trant, J.M.; Gavasso, S.; Ackers, J.; Chung, B.-C.; Place, A.R. Developmental expression of cytochrome P450 aromatase genes (CYP19a and CYP19b) in zebrafish fry (Danio rerio). J. Exp. Zool. 2001, 290, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Caballero-Gallardo, K.; Olivero-Verbel, J.; Freeman, J.L. Toxicogenomics to Evaluate Endocrine Disrupting Effects of Environmental Chemicals Using the Zebrafish Model. Curr. Genom. 2016, 17, 515–527. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.C.; Hu, S.; Ho, P.H.; Hsu, H.J.; Postlethwait, J.H.; Chung, B.C. Two zebrafish hsd3b genes are distinct in function, expression, and evolution. Endocrinology 2015, 156, 2854–2862. [Google Scholar] [CrossRef] [Green Version]
- Lepesheva, G.I.; Waterman, M.R. Sterol 14α-demethylase cytochrome P450 (CYP51), a P450 in all biological kingdoms. Biochim. Biophys. Acta 2007, 1770, 467–477. [Google Scholar] [CrossRef] [Green Version]
- Manchado, M.; Infante, C.; Asensio, E.; Planas, J.V.; Cañavate, J.P. Thyroid hormones down-regulate thyrotropin β subunit and thyroglobulin during metamorphosis in the flatfish Senegalese sole (Solea senegalensis Kaup). Gen. Comp. Endocrinol. 2008, 155, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Tian, X.; Fang, X.; Ji, F. Waterborne exposure to bisphenol F causes thyroid endocrine disruption in zebrafish larvae. Chemosphere 2016, 147, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Power, D.; Llewellyn, L.; Faustino, M.; Nowell, M.; Björnsson, B.T.; Einarsdottir, I.; Canario, A.V.; Sweeney, G. Thyroid hormones in growth and development of fish. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2001, 130, 447–459. [Google Scholar] [CrossRef]
- Campinho, M.A.; Saraiva, J.; Florindo, C.; Power, D.M. Maternal Thyroid Hormones are Essential for Neural Development in Zebrafish. Mol. Endocrinol. 2014, 28, 1136–1149. [Google Scholar] [CrossRef] [Green Version]
- Jin, S.; Antinore, M.J.; Lung, F.D.T.; Dong, X.; Zhao, H.; Fan, F.; Colchagie, A.B.; Blanck, P.; Roller, P.P.; Fornace, A.J.; et al. The GADD45 inhibition of Cdc2 kinase correlates with GADD45-mediated growth suppression. J. Biol. Chem. 2000, 275, 16602–16608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.W.; Zhan, Q.; Coursen, J.D.; Khan, M.A.; Kontny, H.U.; Yu, L.; Hollander, M.C.; O’Connor, P.M.; Fornace, A.J.; Harris, C.C. GADD45 induction of a G2/M cell cycle checkpoint. Proc. Natl. Acad. Sci. USA 1999, 96, 3706–3711. [Google Scholar] [CrossRef] [Green Version]
- Zhan, Q. Gadd45a, a p53- and BRCA1-regulated stress protein, in cellular response to DNA damage. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2005, 569, 133–143. [Google Scholar] [CrossRef]
- Drissi, R.; Chauvin, A.; McKenna, A.; Lévesque, D.; Blais-Brochu, S.; Jean, D.; Boisvert, F.M. Destabilization of the MiniChromosome Maintenance (MCM) complex modulates the cellular response to DNA double strand breaks. Cell Cycle 2018, 17, 2593–2609. [Google Scholar] [CrossRef] [Green Version]
- Youle, R.J.; Strasser, A. The BCL-2 protein family: Opposing activities that mediate cell death. Nat. Rev. Mol. Cell Biol. 2008, 9, 47–59. [Google Scholar] [CrossRef]
- Hsu, F.T.; Sun, C.C.; Wu, C.H.; Lee, Y.J.; Chiang, C.H.; Wang, W.S. Regorafenib Induces Apoptosis and Inhibits Metastatic Potential of Human Bladder Carcinoma Cells. Anticancer. Res. 2017, 37, 4919–4926. [Google Scholar] [CrossRef] [Green Version]
- Tsai, J.-J.; Pan, P.-J.; Hsu, F.-T. Regorafenib induces extrinsic and intrinsic apoptosis through inhibition of ERK/NF-κB activation in hepatocellular carcinoma cells. Oncol. Rep. 2017, 37, 1036–1044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elmore, S. Apoptosis: A Review of Programmed Cell Death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]


| Compound | Kinase Target |
|---|---|
| Imatinib mesylate | BCR-ABL, PDGFR, c-KIT |
| Erlotinib | EGFR |
| Nilotinib | BCR-ABL, PDGFR, DDR1 |
| Sorafenib | VEGFR2, PDGFR, KIT, FLT3, BRAF |
| Dasatinib | BCR-ABL, SRC family (SRC, LCK, YES, FYN), c-KIT, EPHA2, PDGFRβ |
| Regorafenib | VEGFR, PDGFR, BRAF, RAF-1, KIT, Ret, TIE-2, FGFR |
| Gene | REGO | DASA | IMAT | |||
|---|---|---|---|---|---|---|
| Symbol | 0.2 mg/L | 0.6 mg/L | 0.2 mg/L | 0.6 mg/L | 2.3 mg/L | 210 mg/L |
| tg | 1.67 ± 0.18* | 2.09 ± 0.67 * | 1.26 ± 0.37 | 0.74 ± 0.18 | 0.72 ± 0.01 * | 1.27 ± 0.33 |
| tshb | 0.99 ± 0.12 | 0.92 ± 0.13 | 1.02 ± 0.13 | 0.98 ± 0.18 | 0.95 ± 0.07 | 1.16 ± 0.19 |
| thrb | 0.99 ± 0.12 | 0.85 ± 0.14 | 0.91 ± 0.12 | 0.88 ± 0.08 | 0.94 ± 0.09 | 0.98 ± 0.16 |
| thraa | 1.05 ± 0.08 | 0.98 ± 0.12 | 1.00 ± 0.07 | 0.91 ± 0.12 | 0.86 ± 0.14 | 0.92 ± 0.08 |
| dio2 | 0.95 ± 0.13 | 0.69 ± 0.15 * | 1.05 ± 0.23 | 1.11 ± 0.06 | 0.94 ± 0.19 | 0.74 ± 0.18 |
| cyp51 | 1.14 ± 0.36 | 2.35 ± 0.43 * | 1.07 ± 0.39 | 1.13 ± 0.18 | 1.17 ± 0.05 | 1.75 ± 0.30 * |
| cyp19a1b | 4.43 ± 1.06 * | 4.35 ± 1.17 * | 0.96 ± 0.30 | 1.65 ± 0.67 | 1.78 ± 0.73 | 2.36 ± 0.55 * |
| hsd3b | 2.21 ± 0.67 * | 2.04 ± 1.04 * | 1.89 ± 0.39 * | 0.66 ± 0.03 * | 0.58 ± 0.13 * | 1.21 ± 0.35 |
| hsd17b3 | 1.06 ± 0.39 | 1.15 ± 0.21 | 1.07 ± 0.43 | 0.89± 0.08 | 1.31 ± 0.65 | 1.32 ± 0.20 |
| esr2b | 1.09 ± 0.03 | 0.89 ± 0.15 | 1.07 ± 0.43 | 0.98 ± 0.08 | 1.07 ± 0.29 | 1.16 ± 0.30 |
| esr1 | 0.52 ± 0.02 * | 0.38 ± 0.07 * | 0.68 ± 0.10 | 0.63 ± 0.16 * | 0.66 ± 0.15 | 0.65 ± 0.20 |
| apoeb | 0.86 ± 0.34 | 1.13 ± 0.52 | 1.13 ± 0.41 | 0.82 ± 0.16 | 0.94 ± 0.06 | 0.96 ± 0.30 |
| gadd45 | 1.53 ± 0.31 * | 0.95 ± 0.37 | 1.14 ± 0.42 | 1.10 ± 0.52 | 0.86 ± 0.05 | 1.01 ± 0.10 |
| mcm6 | 0.86 ± 0.14 | 0.64 ± 0.11 * | 0.96 ± 0.11 | 0.89 ± 0.07 | 0.78 ± 0.17 | 0.78 ± 0.05 |
| dnajb9 | 0.89 ± 0.11 | 0.74 ± 0.02 | 0.97 ± 0.03 | 0.84 ± 0.06 | 1.05 ± 0.15 | 1.03 ± 0.10 |
| bcl2 | 0.95 ± 0.18 | 1.52 ± 0.20 * | 1.02 ± 0.03 | 0.72 ± 0.05 * | 0.95 ± 0.14 | 0.90 ± 0.17 |
| pax2a | 1.19 ± 0.39 | 1.09 ± 0.37 | 1.03 ± 0.45 | 0.73 ± 0.001 | 0.95 ± 0.29 | 1.13 ± 0.20 |
| slc35 | 0.87 ± 0.20 | 0.69 ± 0.13 | 1.05 ± 0.05 | 0.86 ± 0.08 | 0.77 ± 0.18 | 0.67 ± 0.10 |
| nkx1 | 1.01 ± 0.07 | 0.73 ± 0.05 | 1.00 ± 0.11 | 0.96 ± 0.15 | 0.80 ± 0.17 | 0.95 ± 0.32 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elersek, T.; Novak, M.; Mlinar, M.; Virant, I.; Bahor, N.; Leben, K.; Žegura, B.; Filipič, M. Lethal and Sub-Lethal Effects and Modulation of Gene Expression Induced by T Kinase Inhibitors in Zebrafish (Danio Rerio) Embryos. Toxics 2022, 10, 4. https://doi.org/10.3390/toxics10010004
Elersek T, Novak M, Mlinar M, Virant I, Bahor N, Leben K, Žegura B, Filipič M. Lethal and Sub-Lethal Effects and Modulation of Gene Expression Induced by T Kinase Inhibitors in Zebrafish (Danio Rerio) Embryos. Toxics. 2022; 10(1):4. https://doi.org/10.3390/toxics10010004
Chicago/Turabian StyleElersek, Tina, Matjaž Novak, Mateja Mlinar, Igor Virant, Nika Bahor, Karin Leben, Bojana Žegura, and Metka Filipič. 2022. "Lethal and Sub-Lethal Effects and Modulation of Gene Expression Induced by T Kinase Inhibitors in Zebrafish (Danio Rerio) Embryos" Toxics 10, no. 1: 4. https://doi.org/10.3390/toxics10010004