Chemical Composition and Chronic Toxicity of Disc-Cultured Antrodia cinnamomea Fruiting Bodies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mushroom Materials
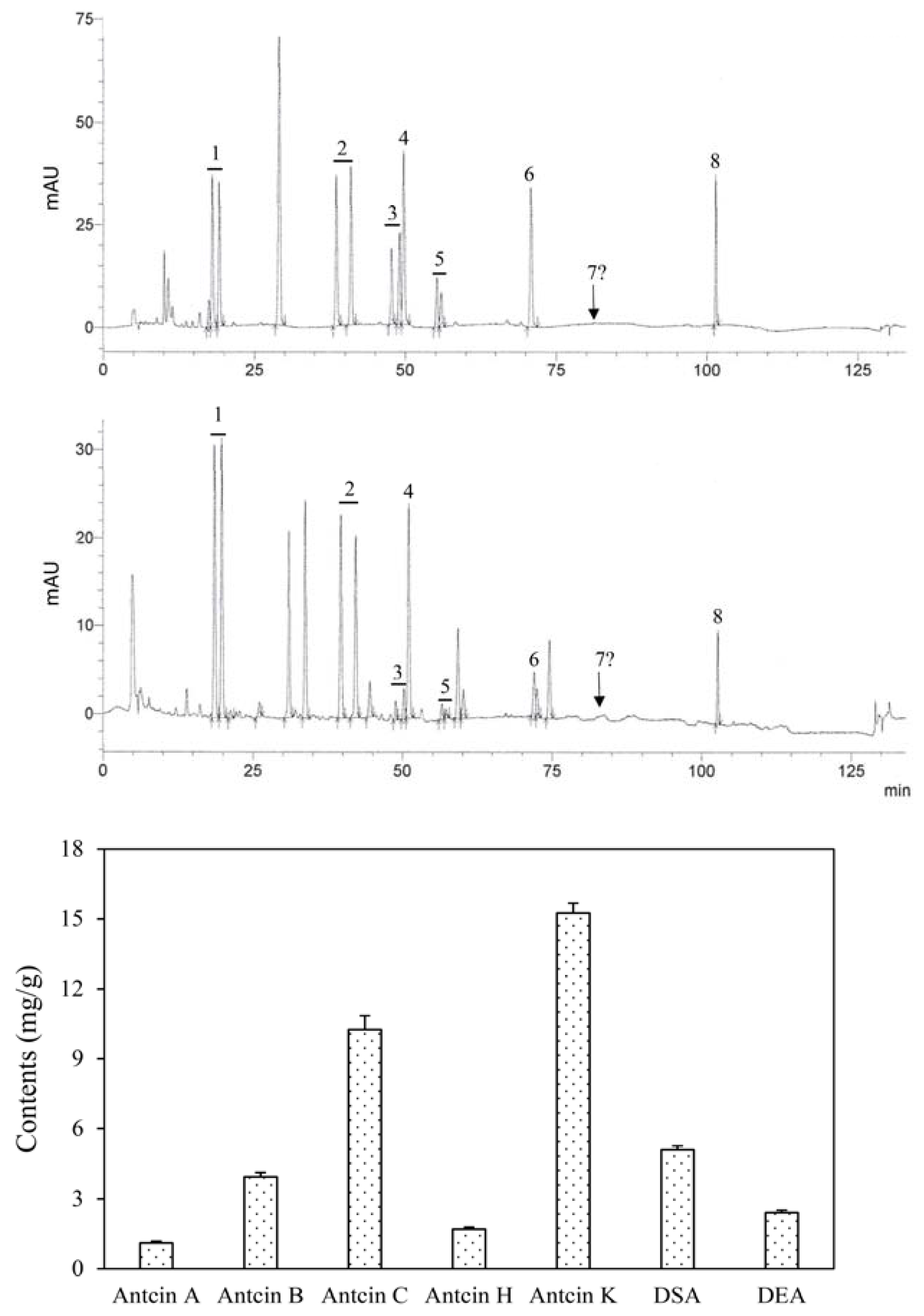
2.2. Determination of Index Compounds
2.3. Animals
2.4. Experimental Design
2.5. Hematological Measurements
2.6. Biochemical Measurements
2.7. Gross and Histological Examinations
2.8. Statistical Analysis
3. Results
3.1. Content of Bioactive Triterpenoids
3.2. Body Weight and Food Intake
3.3. Mortality, Clinical Symptoms, and Organ Weights
3.4. Hematological Parameters
3.5. Biochemical Parameters
3.6. Histopathological Observations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lu, M.C.; El-Shazly, M.; Wu, T.Y.; Du, Y.C.; Chang, T.T.; Chen, C.F.; Hsu, Y.M.; Lai, K.H.; Chiu, C.P.; Chang, F.R.; et al. Recent research and development of Antrodia cinnamomea. Pharmacol. Ther. 2013, 139, 124–156. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Y.; Li, B.; Wang, Z.; Qiao, X.; Ye, M. Terpenoids from the medicinal mushroom Antrodia camphorata: Chemistry and medicinal potential. Nat. Prod. Rep. 2021, 38, 83–102. [Google Scholar] [CrossRef]
- Lin, T.Y.; Chen, C.Y.; Chien, S.C.; Hsiao, W.W.; Chu, F.H.; Li, W.H.; Lin, C.C.; Shaw, J.F.; Wang, S.Y. Metabolite profiles for Antrodia cinnamomea fruiting bodies harvested at different culture ages and from different wood substrates. J. Agric. Food Chem. 2011, 59, 7626–7635. [Google Scholar] [CrossRef] [PubMed]
- Qiao, X.; Song, W.; Wang, Q.; Liu, K.D.; Zhang, Z.X.; Bo, T.; Li, R.Y.; Liang, L.N.; Tzeng, Y.M.; Guo, D.A.; et al. Comprehensive chemical analysis of triterpenoids and polysaccharides in the medicinal mushroom Antrodia cinnamomea. RSC Adv. 2015, 5, 47040–47052. [Google Scholar] [CrossRef]
- Yen, I.C.; Yao, C.W.; Kuo, M.T.; Chao, C.L.; Pai, C.Y.; Chang, W.L. Anti-cancer agents derived from solid-state fermented Antrodia camphorata mycelium. Fitoterapia 2015, 102, 115–119. [Google Scholar] [CrossRef]
- Chien, S.C.; Chen, M.L.; Kuo, H.T.; Tsai, Y.C.; Lin, B.F.; Kuo, Y.H. Anti-inflammatory activities of new succinic and maleic derivatives from the fruiting body of Antrodia camphorata. J. Agric. Food Chem. 2008, 56, 7017–7022. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, J.; Li, L.; Hu, W.; Qu, Y.; Ding, Y.; Meng, L.; Teng, L.; Wang, D. Hepatoprotective effects of Antrodia cinnamomea: The modulation of oxidative stress signaling in a mouse model of alcohol-induced acute liver injury. Oxid. Med. Cell Longev. 2017, 2017, 7841823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hseu, Y.C.; Chang, W.C.; Hseu, Y.T.; Lee, C.Y.; Yech, Y.J.; Chen, P.C.; Chen, J.Y.; Yang, H.L. Protection of oxidative damage by aqueous extract from Antrodia camphorata mycelia in normal human erythrocytes. Life Sci. 2002, 71, 469–482. [Google Scholar] [CrossRef]
- Chang, C.J.; Lu, C.C.; Lin, C.S.; Martel, J.; Ko, Y.F.; Ojcius, D.M.; Wu, T.R.; Tsai, Y.H.; Yeh, T.S.; Lu, J.J.; et al. Antrodia cinnamomea reduces obesity and modulates the gut microbiota in high-fat diet-fed mice. Int. J. Obes. 2018, 42, 231–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiao, X.; Wang, Q.; Ji, S.; Huang, Y.; Liu, K.D.; Zhang, Z.X.; Bo, T.; Tzeng, Y.M.; Guo, D.A.; Ye, M. Metabolites identification and multi-component pharmacokinetics of ergostane and lanostane triterpenoids in the anticancer mushroom Antrodia cinnamomea. J. Pharm. Biomed. Anal. 2015, 111, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Liu, Y.L.; Li, F.Y.; Chang, C.I.; Wang, S.Y.; Lee, K.Y.; Li, S.L.; Chen, Y.P.; Jinn, T.R.; Tzen, J.T. Antcin A, a steroid-like compound from Antrodia camphorata, exerts anti-inflammatory effect via mimicking glucocorticoids. Acta Pharmacol. Sin. 2011, 32, 904–911. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.C.; Rao, Y.K.; Whang-Peng, J.; Huang, C.Y.; Shyue, S.K.; Hsu, S.L.; Tzeng, Y.M. Antcin B and its ester derivative from Antrodia camphorata induce apoptosis in hepatocellular carcinoma cells involves enhancing oxidative stress coincident with activation of intrinsic and extrinsic apoptotic pathway. J. Agric. Food Chem. 2011, 59, 10943–10954. [Google Scholar] [CrossRef] [PubMed]
- Chiu, K.Y.; Chen, T.H.; Wen, C.L.; Lai, J.M.; Cheng, C.C.; Liu, H.C.; Hsu, S.L.; Tzeng, Y.M. Antcin-H isolated from Antrodia cinnamomea inhibits renal cancer cell invasion partly through inactivation of FAK-ERK-C/EBP-β/c-Fos-MMP-7 pathways. Evid. Based Complement. Alternat. Med. 2017, 2017, 5052870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gokila Vani, M.; Kumar, K.J.; Liao, J.W.; Chien, S.C.; Mau, J.L.; Chiang, S.S.; Lin, C.C.; Kuo, Y.H.; Wang, S.Y. Antcin C from Antrodia cinnamomea protects liver cells against free radical-induced oxidative stress and apoptosis in vitro and in vivo through Nrf2-dependent mechanism. Evid. Based Complement. Alternat. Med. 2013, 2013, 296082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huo, Y.; Win, S.; Than, T.A.; Yin, S.; Ye, M.; Hu, H.; Kaplowitz, N. Antcin H protects against acute liver injury through disruption of the interaction of c-Jun-N-terminal kinase with mitochondria. Antioxid. Redox Signal. 2017, 26, 207–220. [Google Scholar] [CrossRef]
- Kuo, Y.H.; Lin, C.H.; Shih, C.C.; Yang, C.S. Antcin K, a triterpenoid compound from Antrodia camphorata, displays antidiabetic and antihyperlipidemic effects via glucose transporter 4 and AMP-activated protein kinase phosphorylation in muscles. Evid. Based Complement. Alternat. Med. 2016, 2016, 4867092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuo, Y.H.; Lin, C.H.; Shih, C.C. Antidiabetic and antihyperlipidemic properties of a triterpenoid compound, dehydroeburicoic acid, from Antrodia camphorata in vitro and in streptozotocin-induced mice. J. Agric. Food Chem. 2015, 63, 10140–10151. [Google Scholar] [CrossRef]
- Lin, C.H.; Kuo, Y.H.; Shih, C.C. Eburicoic acid, a triterpenoid compound from Antrodia camphorata, displays antidiabetic and antihyperlipidemic effects in palmitate treated C2C12 myotubes and in high-fat diet-fed mice. Int. J. Mol. Sci. 2017, 18, 2314. [Google Scholar] [CrossRef] [Green Version]
- Yeh, C.T.; Huang, W.C.; Rao, Y.K.; Ye, M.; Lee, W.H.; Wang, L.S.; Tzeng, D.T.; Wu, C.H.; Shieh, Y.S.; Huang, C.Y.; et al. A sesquiterpene lactone antrocin from Antrodia camphorata negatively modulates JAK2/STAT3 signaling via microRNA let-7c and induces apoptosis in lung cancer cells. Carcinogenesis 2013, 34, 2918–2928. [Google Scholar] [CrossRef] [Green Version]
- CNS Number 16152; Fruiting Body of Niu-Chang-Chih (Ku). Chinese National Standards. The Ministry of Economic Affairs of Taiwan: Taipei, Taiwan, 2021; 15p.
- OECD (Organization of Economic Co-operation and Development). Test No. 408: Repeated Dose 90-Day Oral Toxicity Study in Rodents; OECD Guidelines for the Testing of Chemicals, Section 4; OECD Publishing: Paris, France, 1998. [Google Scholar]
- Shackelford, C.; Long, G.; Wolf, J.; Okerberg, C.; Herbert, R. Qualitative and quantitative analysis of nonneoplastic lesions in toxicology studies. Toxicol. Pathol. 2002, 30, 93–96. [Google Scholar] [CrossRef]
- Senthil Kumar, K.J.; Gokila Vani, M.; Chen, C.Y.; Hsiao, W.W.; Li, J.; Lin, Z.X.; Chu, F.H.; Yen, G.C.; Wang, S.Y. A mechanistic and empirical review of antcins, a new class of phytosterols of formosan fungi origin. J. Food Drug. Anal. 2020, 28, 38–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.C.; Wang, S.E.; Wang, J.J.; Tsai, T.Y.; Lin, C.H.; Pan, T.M.; Lee, C.L. In vitro and in vivo comparisons of the effects of the fruiting body and mycelium of Antrodia camphorata against amyloid β-protein-induced neurotoxicity and memory impairment. Appl. Microbiol. Biotechnol. 2012, 94, 1505–1519. [Google Scholar] [CrossRef] [PubMed]
- Ezeja, M.I.; Anaga, A.O.; Asuzu, I.U. Acute and sub-chronic toxicity profile of methanol leaf extract of Gouania longipetala in rats. J. Ethnopharmacol. 2014, 151, 1155–1164. [Google Scholar] [CrossRef] [PubMed]
- Piao, Y.; Liu, Y.; Xie, X. Change trends of organ weight background data in sprague dawley rats at different ages. J. Toxicol. Pathol. 2013, 26, 29–34. [Google Scholar] [CrossRef] [Green Version]
- Contreras-Zentella, M.L.; Hernández-Muñoz, R. Is liver enzyme release really associated with cell necrosis induced by oxidant stress? Oxid. Med. Cell Longev. 2016, 2016, 3529149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Josef, O.; Ratner, M.; Shaw, M.; Bailey, W.; Schomaker, S. The current state of serum biomarkers of hepatotoxicity. Toxicology 2008, 245, 194–205. [Google Scholar]
- Nikolaou, M.; Parissis, J.; Yilmaz, M.B.; Seronde, M.F.; Kivikko, M.; Laribi, S.; Paugam-Burtz, C.; Cai, D.; Pohjanjousi, P.; Laterre, P.F.; et al. Liver function abnormalities, clinical profile, and outcome in acute decompensated heart failure. Eur. Heart J. 2013, 34, 742–749. [Google Scholar] [CrossRef]
- Agrawal, S.; Dhiman, R.K.; Limdi, J.K. Evaluation of abnormal liver function tests. Postgrad. Med. J. 2016, 92, 223–234. [Google Scholar] [CrossRef] [Green Version]
- Kathak, R.R.; Sumon, A.H.; Molla, N.H.; Hasan, M.; Miah, R.; Tuba, H.R.; Habib, A.; Ali, N. The association between elevated lipid profile and liver enzymes: A study on Bangladeshi adults. Sci. Rep. 2022, 12, 1711. [Google Scholar] [CrossRef]
- Thapa, B.R.; Walia, A. Liver function tests and their interpretation. Indian J. Pediatr. 2007, 74, 663–671. [Google Scholar] [CrossRef]
- Guan, H.; Zheng, Y.; Zhou, X.; Xu, Y.; Fu, C.; Xiao, J.; Ye, Z. Efficacy of different urinary uric acid indicators in patients with chronic kidney disease. BMC Nephrol. 2020, 21, 290. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, P.J.; Liao, C.Y.; Kao, Y.H.; Chan, J.S.; Lin, Y.F.; Chuu, C.P.; Chen, J.S. Comparison of fractional excretion of electrolytes in patients at different stages of chronic kidney disease: A cross-sectional study. Medicine (Baltimore) 2020, 99, e18709. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.I.; Chen, C.C.; Lin, T.W.; Tsai, Y.T.; Nam, M.K. A 90-day subchronic toxicological assessment of Antrodia cinnamomea in Sprague-Dawley rats. Food Chem. Toxicol. 2011, 49, 429–433. [Google Scholar] [CrossRef]
- Chang, J.B.; Wu, M.F.; Lu, H.F.; Chou, J.; Au, M.K.; Liao, N.C.; Chang, C.H.; Huang, Y.P.; Wu, C.T.; Chung, J.G. Toxicological evaluation of Antrodia cinnamomea in BALB/c mice. In Vivo 2013, 27, 739–745. [Google Scholar] [PubMed]
- Lin, C.C.; Kumar, K.J.S.; Liao, J.W.; Kuo, Y.H.; Wang, S.Y. Genotoxic, teratotoxic and oral toxic assessments of Antrodia cinnamomea health food product (Leader Deluxe Antrodia cinnamomea®). Toxicol. Rep. 2015, 2, 1409–1417. [Google Scholar] [CrossRef]


| ACP (mg/kg) | ||||
|---|---|---|---|---|
| Control | 200 | 600 | 1000 | |
| Male rats | ||||
| Initial body weight (g) | 183.9 ± 9.1 | 185.8 ± 9.7 | 185.6 ± 6.8 | 187.4 ± 4.5 |
| Final body weight (g) | 511.0 ± 50.4 | 504.0 ± 23.2 | 510.0 ± 39.7 | 515.0 ± 35.0 |
| Total food intake (g) | 375.3 ± 16.9 | 374.1 ± 38.7 | 374.7 ± 33.9 | 373.1 ± 18.1 |
| Female rats | ||||
| Initial body weight (g) | 160.9 ± 4.4 | 160.4 ± 3.7 | 162.3 ± 4.6 | 161.1 ± 4.0 |
| Final body weight (g) | 285.0 ± 8.5 | 300.0 ± 18.9 | 306.0 ± 19.6 | 301.0 ± 23.3 |
| Total food intake (g) | 256.5 ± 20.0 | 258.8 ± 11.4 | 259.4 ± 25.1 | 260.6 ± 17.5 |
| ACP (mg/kg) | ||||
|---|---|---|---|---|
| Control | 200 | 600 | 1000 | |
| Male rats (Organ weights, g) | ||||
| Brain | 2.24 ± 0.08 | 2.21 ± 0.08 | 2.26 ± 0.10 | 2.25 ± 0.09 |
| Adrenal glands | 0.06 ± 0.01 | 0.06 ± 0.1 | 0.06 ± 0.01 | 0.06 ± 0.01 |
| Heart | 1.70 ± 0.17 | 1.64 ± 0.09 | 1.65 ± 0.09 | 1.61 ± 0.13 |
| Kidneys | 3.23 ± 0.35 | 3.07 ± 0.17 | 3.19 ± 0.32 | 3.16 ± 0.35 |
| Liver | 13.66 ± 1.93 | 13.01 ± 1.38 | 13.66 ± 1.70 | 13.24 ± 1.60 |
| Spleen | 0.69 ± 0.09 | 0.74 ± 0.10 | 0.70 ± 0.07 | 0.69 ± 0.08 |
| Testes | 3.53 ± 0.29 | 3.72 ± 0.38 | 3.47 ± 0.29 | 3.67 ± 0.33 |
| Thymus | 0.29 ± 0.06 | 0.28 ± 0.06 | 0.30 ± 0.04 | 0.34 ± 0.08 |
| Lung | 1.71 ± 0.24 | 1.74 ± 0.18 | 1.89 ± 0.17 | 1.76 ± 0.12 |
| Female rats (Organ weights, g) | ||||
| Brain | 2.07 ± 0.15 | 2.02 ± 0.15 | 2.09 ± 0.12 | 2.06 ± 0.13 |
| Adrenal glands | 0.07 ± 0.01 | 0.07 ± 0.01 | 0.07 ± 0.02 | 0.07 ± 0.02 |
| Heart | 0.97 ± 0.10 | 1.02 ± 0.11 | 1.11 ± 0.08 | 1.02 ± 0.07 |
| Kidneys | 1.82 ± 0.14 | 1.94 ± 0.24 | 1.89 ± 0.18 | 1.89 ± 0.21 |
| Liver | 8.93 ± 0.88 | 8.63 ± 0.63 | 9.03 ± 0.79 | 8.42 ± 0.13 |
| Spleen | 0.47 ± 0.04 | 0.47 ± 0.03 | 0.49 ± 0.05 | 0.48 ± 0.06 |
| Ovaries | 0.10 ± 0.02 | 0.13 ± 0.02 | 0.12 ± 0.03 | 0.11 ± 0.02 |
| Thymus | 0.34 ± 0.10 | 0.37 ± 0.14 | 0.34 ± 0.11 | 0.38 ± 0.14 |
| Lung | 1.26 ± 0.16 | 1.24 ± 0.13 | 1.30 ± 0.09 | 1.35 ± 0.09 |
| ACP (mg/kg) | ||||
|---|---|---|---|---|
| Control | 200 | 600 | 1000 | |
| Male rats | ||||
| WBC (103/µL) | 12.01 ± 2.69 | 11.81 ± 3.75 | 12.50 ± 1.80 | 12.91 ± 2.69 |
| RBC (106/µL) | 8.67 ± 0.34 | 8.71 ± 0.32 | 8.61 ± 0.23 | 8.52 ± 0.32 |
| RDW (%) | 15.75 ± 0.21 | 15.89 ± 0.17 | 15.69 ± 0.26 | 15.86 ± 0.22 |
| Hb (g/dL) | 16.28 ± 0.29 | 16.53 ± 0.29 | 16.40 ± 0.25 | 16.80 ± 0.51 |
| Hct (%) | 43.77 ± 3.23 | 45.50 ± 1.71 | 44.98 ± 1.97 | 44.77 ± 2.70 |
| MCV (fL) | 53.44 ± 1.81 | 53.51 ± 1.45 | 52.45 ± 1.94 | 54.14 ± 1.17 |
| MCH (pg) | 20.57 ± 0.51 | 20.87 ± 0.39 | 20.52 ± 0.52 | 20.07 ± 0.49 |
| MCHC (g/dL) | 33.57 ± 0.39 | 32.90 ± 0.41 | 33.11 ± 0.27 | 33.05 ± 0.36 |
| Plt (103/µL) | 873.7 ± 58.0 | 861.5 ± 70.6 | 873.9 ± 88.0 | 877.3 ± 73.0 |
| MPV (fL) | 7.65 ± 0.52 | 7.64 ± 0.40 | 7.82 ± 0.46 | 7.83 ± 0.45 |
| Lym (%) | 74.42 ± 3.81 | 75.93 ± 4.56 | 79.23 ± 3.48 | 74.68 ± 3.66 |
| Bas (%) | 0.14 ± 0.10 | 0.30 ± 0.16 | 0.41 ± 0.23 | 0.35 ± 0.12 |
| Mono (%) | 0.43 ± 0.11 | 0.44 ± 0.08 | 0.45 ± 0.11 | 0.58 ± 0.12 |
| Eos (%) | 0.13 ± 0.03 | 0.15 ± 0.04 | 0.11 ± 0.09 | 0.15 ± 0.04 |
| PT (sec) | 10.88 ± 0.65 | 10.70 ± 0.75 | 10.80 ± 0.70 | 11.42 ± 1.25 |
| APTT (sec) | 23.21 ± 0.14 | 23.48 ± 0.33 | 23.32 ± 0.38 | 23.26 ± 0.34 |
| Female rats | ||||
| WBC (103/µL) | 11.15 ± 3.27 | 10.90 ± 1.36 | 10.97 ± 1.16 | 11.09 ± 15.96 |
| RBC (106/µL) | 8.06 ± 1.32 | 7.81 ± 0.52 | 7.99 ± 0.57 | 7.86 ± 0.48 |
| RDW (%) | 15.72 ± 0.23 | 15.86 ± 0.15 | 15.97 ± 0.24 | 15.90 ± 0.34 |
| Hb (g/dl) | 15.57 ± 0.69 | 14.36 ± 0.98 | 14.71 ± 0.95 | 14.67 ± 0.84 |
| Hct (%) | 45.05 ± 7.01 | 42.71 ± 2.66 | 44.02 ± 3.08 | 44.66 ± 2.71 |
| MCV (fl) | 56.00 ± 1.04 | 54.73 ± 1.63 | 55.11 ± 0.83 | 56.84 ± 1.24 |
| MCH (pg) | 20.05 ± 0.71 | 19.62 ± 0.55 | 20.53 ± 1.08 | 21.27 ± 0.95 |
| MCHC (g/dL) | 33.32 ± 0.50 | 33.62 ± 0.59 | 33.42 ± 0.59 | 32.85 ± 0.46 |
| Plt (103/µL) | 840.9 ± 64.8 | 838.6 ± 55.8 | 889.1 ± 81.8 | 857.6 ± 76.9 |
| MPV (fL) | 7.47 ± 2.35 | 7.80 ± 0.31 | 7.86 ± 0.39 | 7.72 ± 0.43 |
| Lym (%) | 80.89 ± 3.69 | 74.26 ± 6.47 | 79.67 ± 5.52 | 75.72 ± 8.24 |
| Bas (%) | 0.35 ± 0.14 | 0.34 ± 0.20 | 0.45 ± 0.13 | 0.49 ± 0.07 |
| Mono (%) | 0.52 ± 0.14 | 0.52 ± 0.16 | 0.51 ± 0.17 | 0.62 ± 0.15 |
| Eos (%) | 0.16 ± 0.09 | 0.14 ± 0.06 | 0.14 ± 0.02 | 0.20 ± 0.23 |
| PT (sec) | 10.84 ± 0.50 | 10.83 ± 0.75 | 10.53 ± 0.35 | 10.84 ± 0.86 |
| APTT (sec) | 23.59 ± 0.23 | 23.58 ± 0.23 | 25.22 ± 0.90 | 24.24 ± 1.18 |
| ACP (mg/kg) | ||||
|---|---|---|---|---|
| Control | 200 | 600 | 1000 | |
| Male rats | ||||
| AST (U/L) | 74.22 ± 5.12 | 74.50 ± 3.24 | 73.20 ± 6.78 | 72.50 ± 4.55 |
| ALT (U/L) | 30.80 ± 3.74 | 30.90 ± 4.70 | 29.30 ± 3.77 | 31.20 ± 4.69 |
| ALP (U/L) | 83.50 ± 6.75 | 86.10 ± 745 | 82.10 ± 9.62 | 82.60 ± 13.7 |
| TP (g/dL) | 6.30 ± 0.70 | 6.72 ± 0.39 | 7.03 ± 0.48 | 6.93 ± 0.30 |
| ALB (g/dL) | 3.40 ± 0.34 | 3.53 ± 0.19 | 3.64 ± 0.20 | 3.64 ± 0.13 |
| GLO (g/dL) | 2.09 ± 0.13 | 2.05 ± 0.19 | 1.97 ± 0.16 | 2.01 ± 0.12 |
| TBIL (mg/dL) | 0.05 ± 0.01 | 0.05 ± 0.01 | 0.04 ± 0.01 | 0.04 ± 0.01 |
| GGT (U/L) | 2.40 ± 1.26 | 2.30 ± 0.67 | 2.20 ± 0.63 | 2.60 ± 0.97 |
| BUN (mg/dL) | 16.15 ± 0.91 | 15.38 ± 0.97 | 15.11 ± 0.70 | 15.60 ± 0.35 |
| Cre (mg/dL) | 0.55 ± 0.11 | 0.58 ± 0.12 | 0.56 ± 0.07 | 0.53 ± 0.07 |
| TC (mg/dL) | 80.60 ± 5.44 | 78.90 ± 3.73 | 79.90 ± 4.43 | 80.10 ± 5.04 |
| TG (mg/dL) | 38.60 ± 4.03 | 38.80 ± 4.52 | 39.10 ± 8.29 | 40.00 ± 6.27 |
| GLU (mg/dL) | 108.6 ± 11.8 | 111.1 ± 9.62 | 124.2 ± 15.6 | 133.5 ± 9.01 |
| Na (mmol/L) | 147.2 ± 2.66 | 147.2 ± 1.32 | 147.3 ± 1.70 | 147.2 ± 1.55 |
| K (mmol/L) | 5.02 ± 0.72 | 5.10 ± 0.49 | 5.02 ± 0.36 | 5.21 ± 0.38 |
| Cl (mmol/L) | 102.1 ± 1.85 | 100.7 ± 1.57 | 101.6 ± 1.07 | 102.5 ± 0.97 |
| Ca (mg/dL) | 9.35 ± 0.41 | 9.81 ± 0.26 | 9.50 ± 0.49 | 9.50 ± 0.22 |
| P (mg/dL) | 7.89 ± 0.60 | 7.80 ± 1.33 | 7.79 ± 0.45 | 7.83 ± 0.43 |
| Female rats | ||||
| AST (U/L) | 72.90 ± 7.13 | 72.80 ± 4.39 | 74.10 ± 4.72 | 72.40 ± 5.10 |
| ALT (U/L) | 31.40 ± 3.69 | 29.40 ± 4.03 | 31.60 ± 4.35 | 30.10 ± 4.01 |
| ALP (U/L) | 76.90 ± 12.68 | 76.40 ± 10.74 | 76.10 ± 8.39 | 75.00 ± 11.30 |
| TP (g/dL) | 6.70 ± 0.65 | 7.17 ± 0.46 | 7.32 ± 0.40 | 6.96 ± 0.50 |
| ALB (g/dL) | 3.77 ± 0.34 | 3.89 ± 0.31 | 4.11 ± 0.24 | 3.91 ± 0.32 |
| GLO (g/dL) | 2.00 ± 0.08 | 2.01 ± 0.11 | 2.02 ± 0.13 | 1.98 ± 0.20 |
| TBIL (mg/dL) | 0.03 ± 0.01 | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.03 ± 0.01 |
| GGT (U/L) | 2.30 ± 0.67 | 2.50 ± 0.97 | 2.15 ± 0.57 | 2.40 ± 1.07 |
| BUN (mg/dL) | 15.43 ± 0.40 | 15.19 ± 0.72 | 14.19 ± 0.46 | 14.38 ± 0.35 |
| Cre (mg/dL) | 0.50 ± 0.07 | 0.48 ± 0.14 | 0.49 ± 0.08 | 0.48 ± 0.06 |
| TC (mg/dL) | 79.20 ± 4.24 | 80.40 ± 6.35 | 79.70 ± 4.45 | 80.00 ± 4.83 |
| TG (mg/dL) | 37.70 ± 3.31 | 38.60 ± 3.84 | 39.00 ± 2.11 | 37.70 ± 4.11 |
| GLU (mg/dL) | 113.5 ± 15.7 | 119.0 ± 14.0 | 125.2 ± 15.5 | 131.5 ± 6.10 |
| Na (mmol/L) | 143.9 ± 1.10 | 145.4 ± 1.07 | 144.7 ± 1.06 | 145.6 ± 0.70 |
| K (mmol/L) | 5.19 ± 0.53 | 4.82 ± 0.45 | 4.43 ± 0.27 | 4.95 ± 0.41 |
| Cl (mmol/L) | 102.7 ± 1.25 | 102.4 ± 1.90 | 102.0 ± 1.33 | 103.3 ± 1.95 |
| Ca (mg/dL) | 9.75 ± 0.40 | 10.07 ± 0.30 | 10.08 ± 0.34 | 9.93 ± 0.36 |
| P (mg/dL) | 7.60 ± 0.73 | 7.90 ± 0.96 | 7.87 ± 0.47 | 7.98 ± 0.43 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.-C.; Wu, T.-Y.; Hsu, T.-H.; Lai, M.-N.; Wu, Y.-C.; Ng, L.-T. Chemical Composition and Chronic Toxicity of Disc-Cultured Antrodia cinnamomea Fruiting Bodies. Toxics 2022, 10, 587. https://doi.org/10.3390/toxics10100587
Liu S-C, Wu T-Y, Hsu T-H, Lai M-N, Wu Y-C, Ng L-T. Chemical Composition and Chronic Toxicity of Disc-Cultured Antrodia cinnamomea Fruiting Bodies. Toxics. 2022; 10(10):587. https://doi.org/10.3390/toxics10100587
Chicago/Turabian StyleLiu, Shou-Chou, Tung-Ying Wu, Tai-Hao Hsu, Ming-Nan Lai, Yang-Chang Wu, and Lean-Teik Ng. 2022. "Chemical Composition and Chronic Toxicity of Disc-Cultured Antrodia cinnamomea Fruiting Bodies" Toxics 10, no. 10: 587. https://doi.org/10.3390/toxics10100587
APA StyleLiu, S.-C., Wu, T.-Y., Hsu, T.-H., Lai, M.-N., Wu, Y.-C., & Ng, L.-T. (2022). Chemical Composition and Chronic Toxicity of Disc-Cultured Antrodia cinnamomea Fruiting Bodies. Toxics, 10(10), 587. https://doi.org/10.3390/toxics10100587






