Effect of Heating Temperature on Ammonia Emission in the Mainstream Aerosols from Heated Tobacco Products
Abstract
:1. Introduction
pKa1 = 3.22 pKa2 = 8.11
2. Materials and Methods
2.1. Heated Tobacco Products
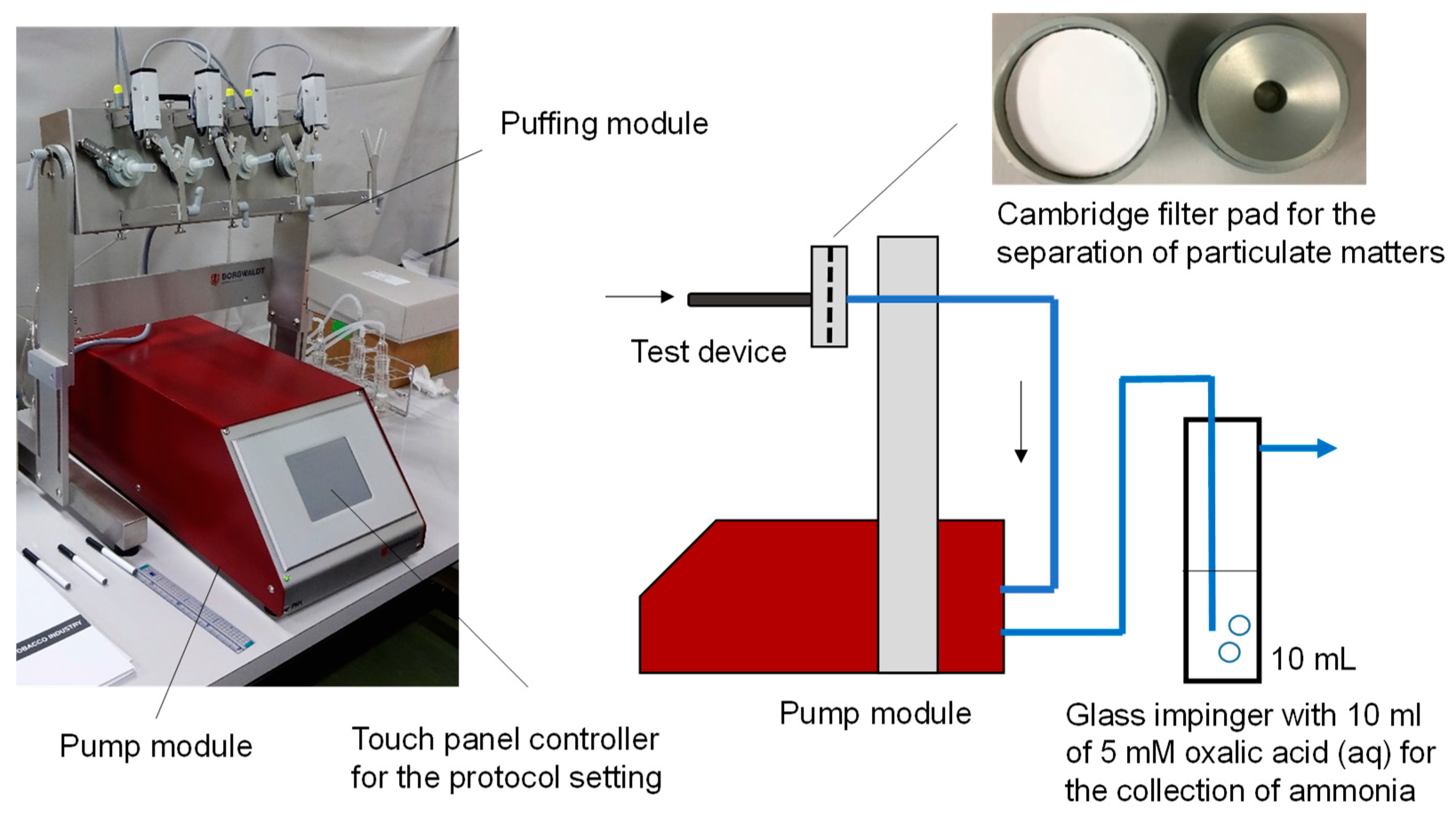
2.2. Experimental Setup
3. Results and Discussion
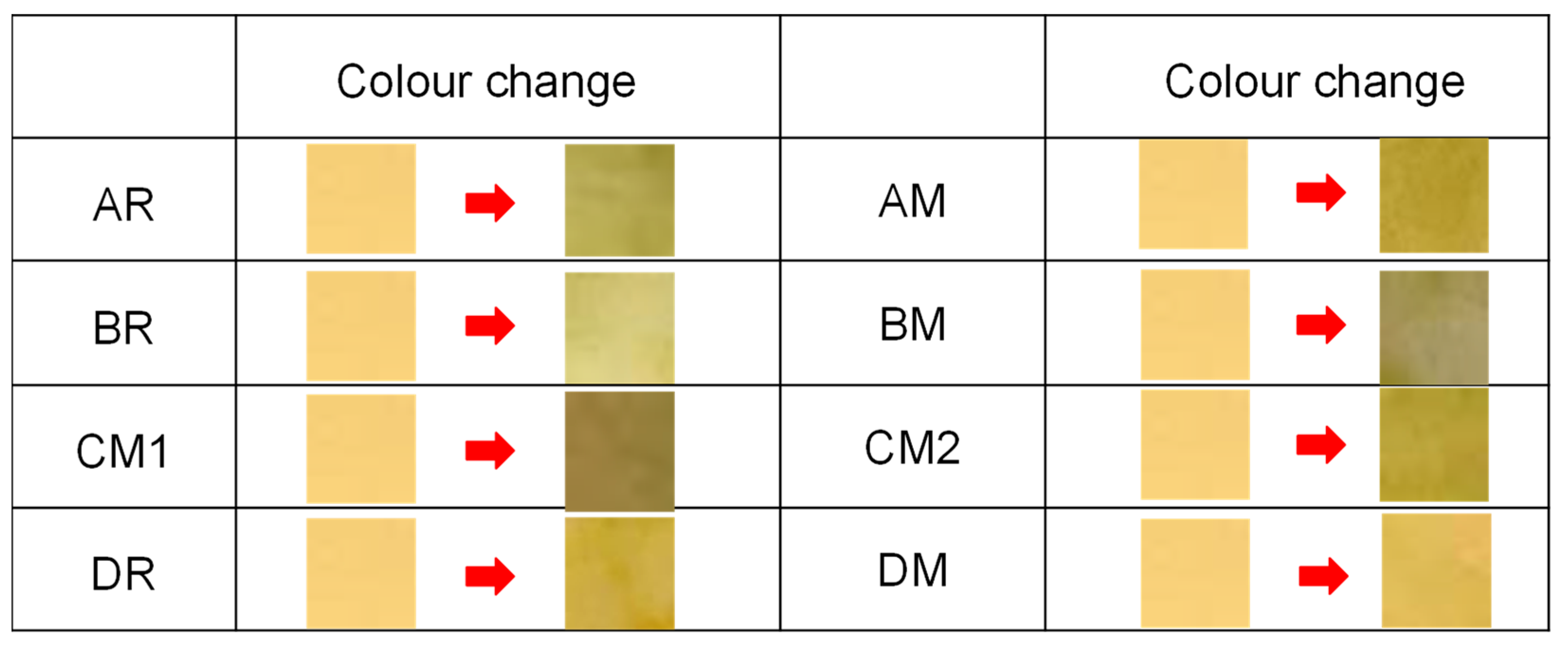
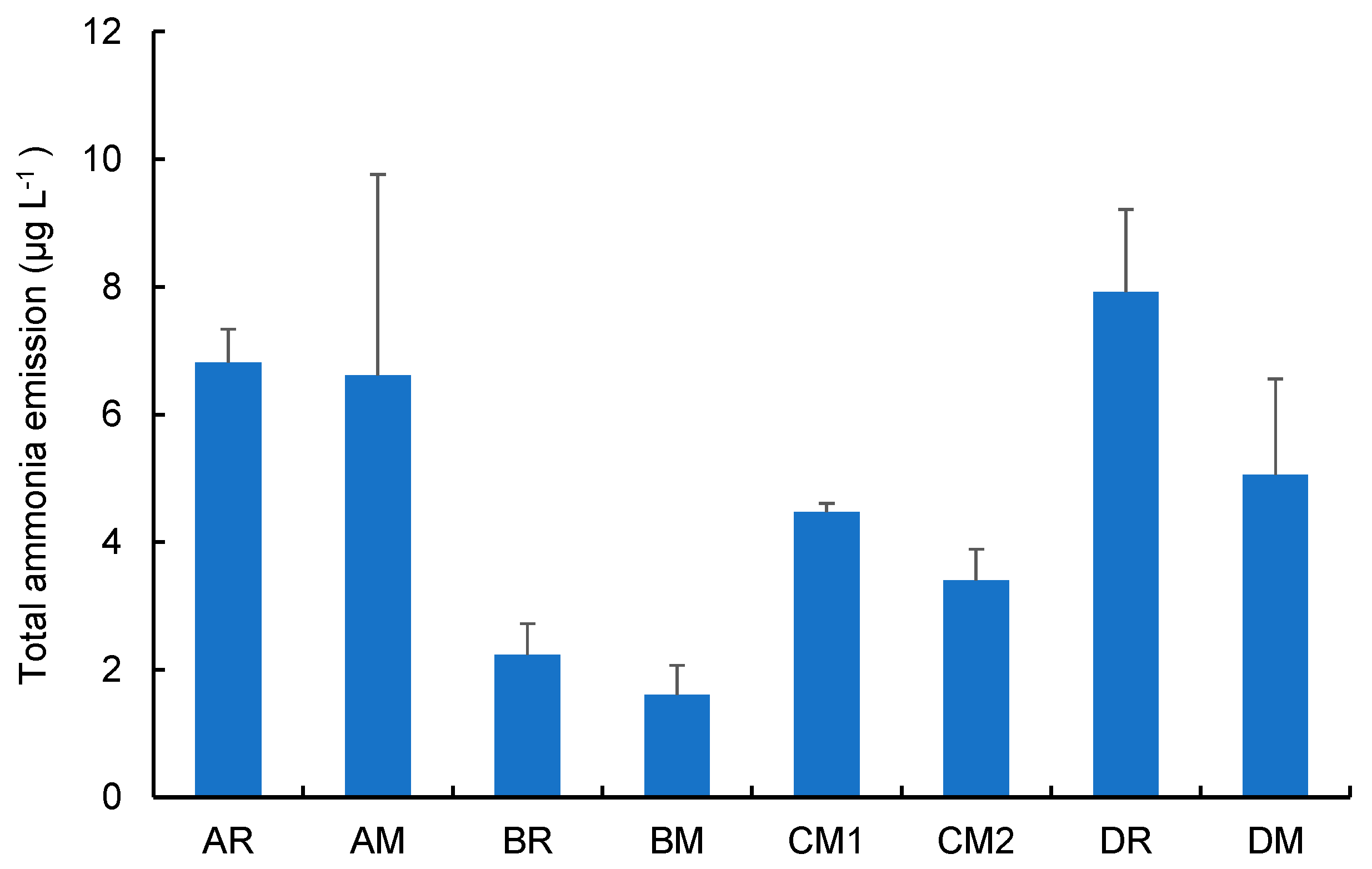
3.1. Aerosol pH and Total Ammonia Emission
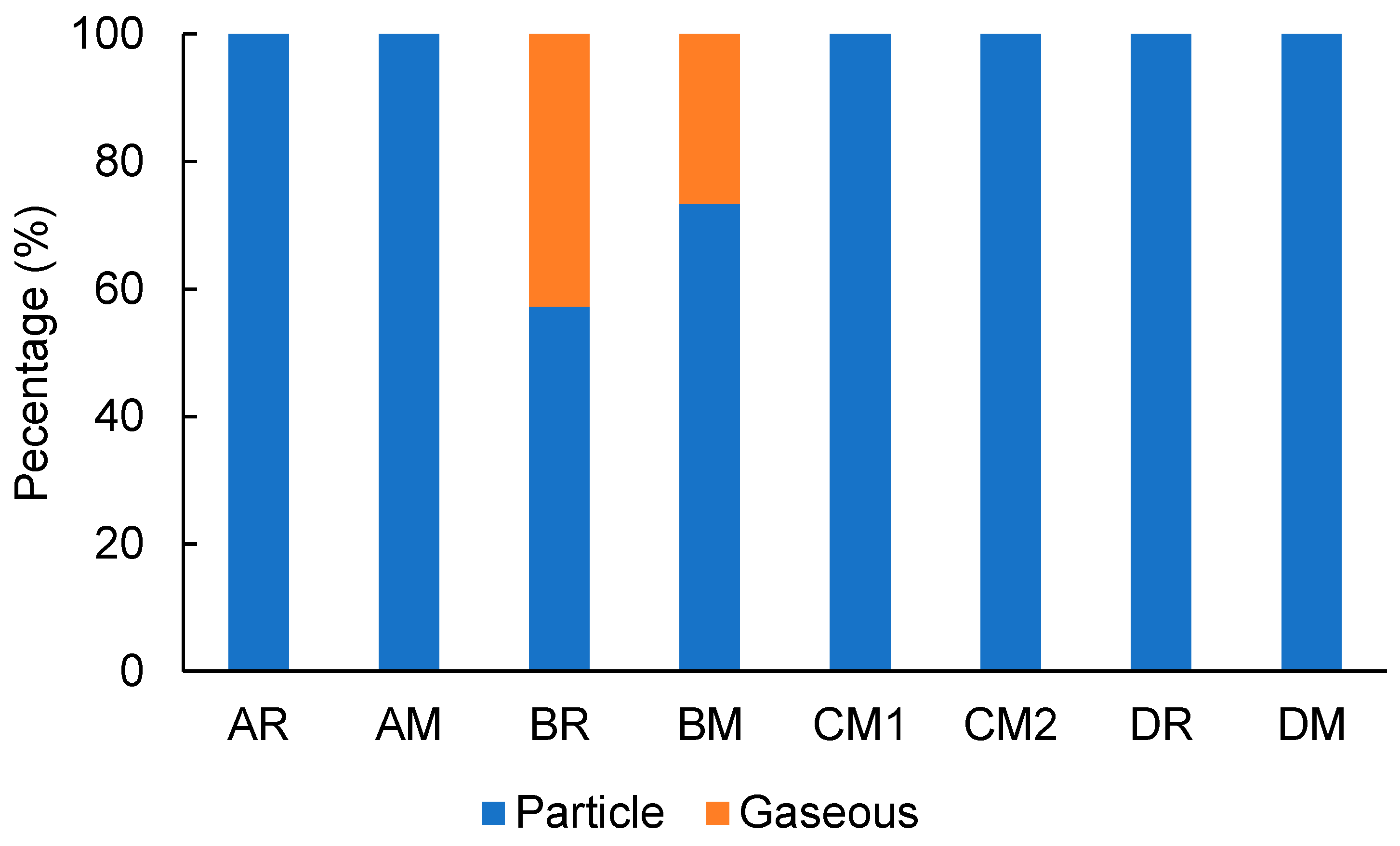
3.2. Gas–Particle Distribution of Ammonia
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dusautoir, R.; Zarcone, G.; Verriele, M.; Garçon, G.; Fronval, I.; Beauval, N.; Allorge, D.; Riffault, V.; Locoge, N.; Guidice, J.M.L.; et al. Comparison of the chemical composition of aerosols from heated tobacco products, electronic cigarettes and tobacco cigarettes and their toxic impacts on the human bronchial epithelial BEAS-2B cells. J. Hazard. Mater. 2021, 401, 123417. [Google Scholar] [CrossRef] [PubMed]
- Cancelada, L.; Sleiman, M.; Tang, X.; Russell, M.L.; Montesinos, V.N.; Litter, M.I.; Gundel, L.A.; Destaillats, H. Heated tobacco products: Volatile emissions and their predicted impact on indoor air quality. Environ. Sci. Technol. 2019, 53, 7866–7876. [Google Scholar] [CrossRef]
- Uchiyama, S.; Noguchi, M.; Takagi, N.; Hayashida, H.; Inaba, Y.; Ogura, H.; Kunugita, N. Simple determination of gaseous and particulate compounds generated from heated tobacco products. Chem. Res. Toxicol. 2018, 31, 585–593. [Google Scholar] [CrossRef]
- Bekki, K.; Inaba, Y.; Uchiyama, S.; Kunugita, N. Comparison of chemicals in mainstream smoke in heat-not-burn tobacco and combustion cigarettes. J. UOEH 2017, 39, 201–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, S. Research trends on biological effects of heated tobacco product. Indoor Environ. 2021, 24, 109–116. (In Japanese) [Google Scholar] [CrossRef]
- Elias, J.; Dutra, L.M.; St Helen, G.; Ling, P.M. Revolution or redux? Assessing IQOS through a precursor 250 product. Tob. Control 2018, 27 (Suppl. S1), s102–s110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pankow, J.; Mader, B.T.; Lorne, M.I.; Luo, W.; Pavlick, A.; Liang, C. Conversion of nicotine in tobacco smoke to its volatile and available free-base form through the action of gaseous ammonia. Environ. Sci. Technol. 1997, 31, 2428–2433. [Google Scholar] [CrossRef]
- van Amsterdam, J.; Sleijffers, A.; van Spiegel, P.; Blom, R.; Witte, M.; van de Kassteele, J.; Blokland, M.; Steerenberg, P.; Opperhuizen, A. Effect of ammonia in cigarette tobacco on nicotine absorption in human smokers. Food and Chem. Toxicol. 2011, 49, 3025–3030. [Google Scholar] [CrossRef] [PubMed]
- Clayton, P.M.; Vas, C.A.; Bui, T.T.T.; Drake, A.F.; McAdam, K. Spectroscopic investigations into the acid–base properties of nicotine at different temperatures. Anal. Methods 2013, 5, 81–88. [Google Scholar] [CrossRef]
- Benowitz, N.L.; Hukkanen, J.; Jacob III, P. Nicotine Chemistry, Metabolism, Kinetics and Biomarkers. Handb. Exp. Pharmacol. 2009, 192, 29–60. [Google Scholar] [CrossRef]
- Stevenson, T.; Proctor, R.N. The secret and soul of Marlboro: Phillip Morris and the origins, spread, and denial of nicotine freebasing. Am. J. Public Health 2008, 98, 1184–1194. [Google Scholar] [CrossRef] [PubMed]
- Wayne, G.F.; Conolly, G.N.; Henningfield, J. Brand differences of free-base nicotine delivery in cigarette smoke: The view of the tobacco industry documents. Tob. Control. 2006, 15, 189–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nordlund, N.; Smith, M.; Maeder, S.; McGrath, T.; Schaller, J.P.; Pratte, P.; Picavet, P.; Peitsch, M. Scientific Substantiation of the Absence of Combustion and no Smoke Formation in the Electrically Heated Tobacco Product (EHTP); PMI Science: New York, NY, USA, 2019; Available online: https://www.pmiscience.com/library/publication/ (accessed on 22 August 2022).
- Sekine, Y.; Yamamoto, T. Physical and chemical property of mainstream aerosol generated from heated tobacco products. Indoor Environ. 2021, 24, 135–144. (In Japanese) [Google Scholar] [CrossRef]
- Agency for Toxic Substances and Disease Registry (ATSDR). ToxFAQs for Ammonia; Division of Toxicology, U.S. Department of Health and Human Services. Public Health Service: Atlanta, GA, USA, 2004. Available online: https://wwwn.cdc.gov/TSP/ToxFAQs/ToxFAQsDetails.aspx?faqid=10&toxid=2 (accessed on 22 August 2022).
- Agency for Toxic Substances and Disease Registry (ATSDR). Public Health Statement for Ammonia; U.S. Department of Health and Human Services. Public Health Service: Atlanta, GA, USA, 2004. Available online: https://wwwn.cdc.gov/TSP/PHS/PHS.aspx?phsid=9&toxid=2 (accessed on 22 August 2022).
- Schaller, J.P.; Keller, D.; Poget, L.; Pratte, P.; Kaelin, E.; McHugh, D.; Cudazzo, G.; Smart, D.; Tricker, A.R.; Gautier, L.; et al. Evaluation of the Tobacco Heating System 2.2. Part 2: Chemical composition, genotoxicity, cytotoxicity, and physical properties of the aerosol. Regul. Toxicol. Pharmacol. 2016, 81 (Suppl. S2), S27–S47. [Google Scholar] [CrossRef] [Green Version]
- Adamson, J.; Jaunky, T.; Thorne, D.; Gaça, M.D. Characterisation of the borgwaldt LM4E system for in vitro exposures to undiluted aerosols from next generation tobacco and nicotine products (NGPs). Food Chem. Toxicol. 2018, 113, 337–344. [Google Scholar] [CrossRef]
- Cooperation Centre for Scientific Research Relative to Tobacco (CORESTA). Recommended Method No.81—Routine Analytical Machine for e-Cigarette Aerosol Generation and Collection—Definitions and Standard Conditions; CORESTA: Paris, France, 2015. [Google Scholar]
- Health Canada. Method T-115—Determination of “Tar”, Nicotine and Carbon Monoxide in Mainstream Tobacco Smoke; Health Canada: Ottawa, ON, Canada, 1999.
- Sohara, K.; Sekine, Y.; Yamamoto, T.; Sato, S.; Nakai, S.; Yanagisawa, Y. Influence of puffing protocol on carbonyl compounds emitted from electronic cigarette and heat-not-burn tobacco. Indoor Environ. 2020, 23, 89–97. [Google Scholar] [CrossRef]
- Beauval, N.; Verrièle, M.; Garat, A.; Fronval, I.; Dusautoir, R.; Anthérieu, S.; Garçon, G.; Lo-Guidice, J.M.; Allorge, D.; Locoge, N. Influence of puffing conditions on the carbonyl composition of e-cigarette aerosols. Int. J. Hyg. Environ. Health 2019, 222, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Sleiman, M.; Logue, J.M.; Montesinos, V.N.; Russell, M.L.; Litter, M.I.; Gundel, L.A.; Destaillats, H. Emissions from Electronic Cigarettes: Key Parameters Affecting the Release of Harmful Chemicals. Environ. Sci. Technol. 2016, 50, 9644–9651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biegen, J.R.; Czanderna, A.W. Analysis of thermal processes: The exponential integral. J. Therm Anal. 1972, 4, 39–45. [Google Scholar] [CrossRef]
- Tonokura, K.; Hayashi, R. Analysis of Tobacco Smoke Formation from Heat-Not-Burn Cigarettes and Electronic Cigarettes, Smoking Research Foundation Annual Research Report 2020; Smoking Research Foundation: Tokyo, Japan, 2021; pp. 839–844. (In Japanese) [Google Scholar]

| Device | Heating Temperature (°C) | Flavour Type | Operation Mode | Abbreviation |
|---|---|---|---|---|
| A | 350 | Regular | default | AR |
| Menthol | AM | |||
| B | 40 | Regular | default | BR |
| Menthol | BM | |||
| C | 200 | Menthol fresh | rapid heating | CM1 |
| default | CM2 | |||
| D | 350 | Regular | default | DR |
| Menthol | DM |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamamoto, T.; Sekine, Y.; Sohara, K.; Nakai, S.; Yanagisawa, Y. Effect of Heating Temperature on Ammonia Emission in the Mainstream Aerosols from Heated Tobacco Products. Toxics 2022, 10, 592. https://doi.org/10.3390/toxics10100592
Yamamoto T, Sekine Y, Sohara K, Nakai S, Yanagisawa Y. Effect of Heating Temperature on Ammonia Emission in the Mainstream Aerosols from Heated Tobacco Products. Toxics. 2022; 10(10):592. https://doi.org/10.3390/toxics10100592
Chicago/Turabian StyleYamamoto, Takumi, Yoshika Sekine, Koki Sohara, Satoshi Nakai, and Yukio Yanagisawa. 2022. "Effect of Heating Temperature on Ammonia Emission in the Mainstream Aerosols from Heated Tobacco Products" Toxics 10, no. 10: 592. https://doi.org/10.3390/toxics10100592





