Impact of Acute Exacerbation and Its Phenotypes on the Clinical Outcomes of Chronic Obstructive Pulmonary Disease in Hospitalized Patients: A Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
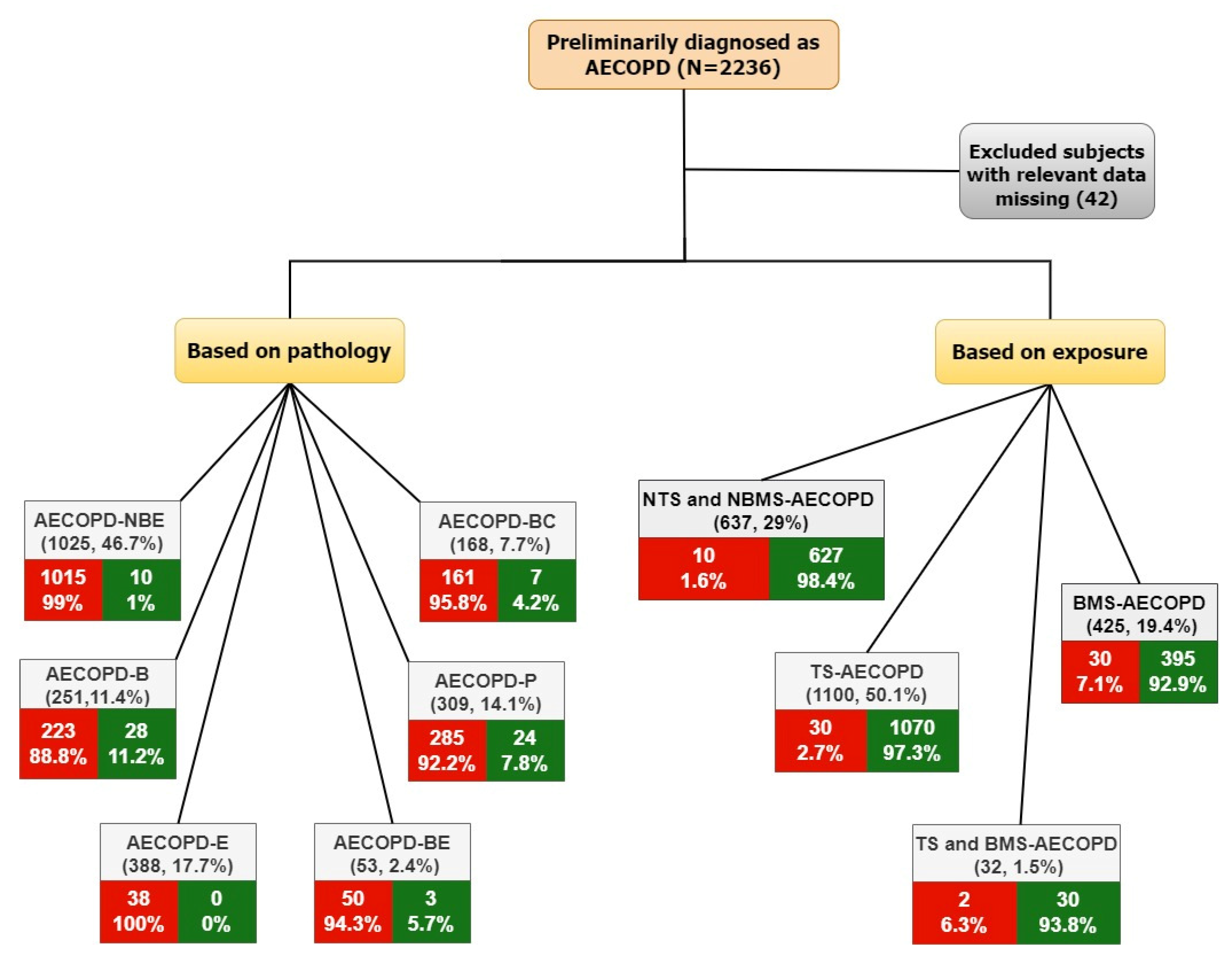
2.1. Cohort Description
2.2. Definitions
- AECOPD-Non-bacterial and non-eosinophilic (NBE) group, consisting of AECOPD subjects who had no bacterial infection and no peripheral blood eosinophilia;
- AECOPD-Bacterial (B) group, consisting of subjects who had bacterial AECOPD without eosinophilia;
- AECOPD-Eosinophilia (E) group, consisting of AECOPD subjects who had peripheral blood eosinophilia and no bacterial infections;
- AECOPD-Bacterial with eosinophilia (BE) group, consisting of subjects who had bacterial AECOPD with peripheral blood eosinophilia;
- AECOPD-Pneumonia (P) group, consisting of AECOPD subjects with pneumonia;
- AECOPD-Bronchiectasis (BC) group, consisting of AECOPD subjects with bronchiectasis.
- Non-tobacco smoke and non-biomass smoke (NTS and NBMS) AECOPD group, consisting of AECOPD subjects who were non-smokers with no biomass exposure;
- Tobacco smoke (TS) AECOPD group, consisting of AECOPD subjects who were smokers;
- Biomass smoke (BS) AECOPD group, consisting of AECOPD subjects who were exposed to biomass smoke;
- Tobacco smoke and biomass smoke (TS and BMS) AECOPD group, consisting of AECOPD subjects who were smokers with biomass exposure.
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pauwels, R.A.; Buist, A.S.; Calverley, P.M.A.; Jenkins, C.R.; Hurd, S.S. GOLD Scientific Committee Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease: NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop Summary. Am. J. Respir. Crit. Care Med. 2001, 163, 1256–1276. [Google Scholar] [CrossRef] [PubMed]
- GOLD Report 2022. 2022, pp. 1–165. Available online: https://goldcopd.org/2022-gold-reports-2/ (accessed on 1 November 2022).
- Varmaghani, M.; Dehghani, M.; Heidari, E.; Sharifi, F.; Saeedi Moghaddam, S.; Farzadfar, F. Global Prevalence of Chronic Obstructive Pulmonary Disease: Systematic Review and Meta-Analysis. East. Mediterr. Health J. 2019, 25, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Grigsby, M.; Siddharthan, T.; Chowdhury, M.; Siddiquee, A.; Rubinstein, A.; Sobrino, E.; Miranda, J.J.; Bernabe-Ortiz, A.; Alam, D.; Checkley, W. Socioeconomic Status and COPD among Low- and Middle-Income Countries. Int. J. Chron. Obstruct. Pulmon. Dis. 2016, 11, 2497–2507. [Google Scholar] [CrossRef]
- Buist, A.S.; McBurnie, M.A.; Vollmer, W.M.; Gillespie, S.; Burney, P.; Mannino, D.M.; Menezes, A.M.; Sullivan, S.D.; Lee, T.A.; Weiss, K.B.; et al. International Variation in the Prevalence of COPD (The BOLD Study): A Population-Based Prevalence Study. Lancet 2007, 370, 741–750. [Google Scholar] [CrossRef]
- World Health Organization. World Health Organization-Burden of COPD. Newsroom. 2022, p. 1. Available online: http://www.who.int/respiratory/copdburden/en/ (accessed on 1 November 2022).
- World Health Organization. World Health Organization-The Top 10 Causes of Death. Newsroom. 2020, p. 1. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 1 November 2022).
- Viegi, G.; Maio, S.; Fasola, S.; Baldacci, S. Global Burden of Chronic Respiratory Diseases. J. Aerosol Med. Pulm. Drug Deliv. 2020, 33, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Soriano, J.B.; Kendrick, P.J.; Paulson, K.R.; Gupta, V.; Abrams, E.M.; Adedoyin, R.A.; Adhikari, T.B.; Advani, S.M.; Agrawal, A.; Ahmadian, E.; et al. Prevalence and Attributable Health Burden of Chronic Respiratory Diseases, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet Respir. Med. 2020, 8, 585–596. [Google Scholar] [CrossRef]
- Halpin, D.M.G.; Celli, B.R.; Criner, G.J.; Frith, P.; López Varela, M.V.; Salvi, S.; Vogelmeier, C.F.; Chen, R.; Mortimer, K.; Montes de Oca, M.; et al. The GOLD Summit on Chronic Obstructive Pulmonary Disease in Low- And Middle-Income Countries. Int. J. Tuberc. Lung Dis. 2019, 23, 1131–1141. [Google Scholar] [CrossRef] [PubMed]
- Salvi, S.; Kumar, G.A.; Dhaliwal, R.S.; Paulson, K.; Agrawal, A.; Koul, P.A.; Mahesh, P.A.; Nair, S.; Singh, V.; Aggarwal, A.N.; et al. The Burden of Chronic Respiratory Diseases and Their Heterogeneity across the States of India: The Global Burden of Disease Study 1990–2016. Lancet Glob. Health 2018, 6, 1363–1374. [Google Scholar] [CrossRef]
- Indian Council of Medical Research; Public Health Foundation of India; Institute for Health Metrics and Evaluation India. India: Health of the Nation’s States. The India State-Level Disease Burden Initiative: Disease Burden Trends in the States of India 1990 to 2016. 2017, pp. 1–214. Available online: https://www.healthdata.org/ (accessed on 1 November 2022).
- Papi, A. Pathophysiology of Exacerbations of Chronic Obstructive Pulmonary Disease. Proc. Am. Thorac. Soc. 2006, 3, 245–251. [Google Scholar] [CrossRef]
- 1Barberà, J.A.; Roca, J.; Ferrer, A.; Félez, M.A.; Díaz, O.; Roger, N.; Rodriguez-Roisin, R. Mechanisms of Worsening Gas Exchange during Acute Exacerbations of Chronic Obstructive Pulmonary Disease. Eur. Respir. J. 1997, 10, 1285–1291. [Google Scholar] [CrossRef]
- Vogelmeier, C.F.; Criner, G.J.; Martinez, F.J.; Anzueto, A.; Barnes, P.J.; Bourbeau, J.; Celli, B.R.; Chen, R.; Decramer, M.; Fabbri, L.M.; et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report: GOLD Executive Summary. Eur. Respir. J. 2017, 195, 557–582. [Google Scholar] [CrossRef]
- Seemungal, T.A.R.; Donaldson, G.C.; Paul, E.A.; Bestall, J.C.; Jeffries, D.J.; Wedzicha, J.A. Effect of Exacerbation on Quality of Life in Patients with Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 1998, 157 Pt 1, 1418–1422. [Google Scholar] [CrossRef]
- Miravitlles, M.; Ribera, A. Understanding the Impact of Symptoms on the Burden of COPD. Respir. Res. 2017, 18, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Han, M.K.; Agusti, A.; Calverley, P.M.; Celli, B.R.; Criner, G.; Curtis, J.L.; Fabbri, L.M.; Goldin, J.G.; Jones, P.W.; MacNee, W.; et al. Chronic Obstructive Pulmonary Disease Phenotypes: The Future of COPD. Am. J. Respir. Crit. Care Med. 2010, 182, 598–604. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Campos, J.L.; Agustí, A. Heterogeneity of Chronic Obstructive Pulmonary Disease Exacerbations: A Two-Axes Classification Proposal. Lancet Respir. Med. 2015, 3, 729–734. [Google Scholar] [CrossRef]
- Wu, C.-W.; Lan, C.-C.; Hsieh, P.-C.; Tzeng, I.-S.; Wu, Y.-K. Role of Peripheral Eosinophilia in Acute Exacerbation of Chronic Obstructive Pulmonary Disease. World J. Clin. Cases 2020, 8, 2727–2737. [Google Scholar] [CrossRef] [PubMed]
- Jabarkhil, A.; Moberg, M.; Janner, J.; Petersen, M.N.; Jensen, C.B.; Henrik Ängquist, L.; Vestbo, J.; Jess, T.; Porsbjerg, C. Elevated Blood Eosinophils in Acute COPD Exacerbations: Better Short- and Long-Term Prognosis. Eur. Clin. Respir. J. 2020, 7, 1–9. [Google Scholar] [CrossRef]
- Li, Q.; Larivée, P.; Courteau, J.; Couillard, S.; Poder, T.G.; Carrier, N.; Bélanger, M.; Vanasse, A. Greater Eosinophil Counts at First COPD Hospitalization Are Associated with More Readmissions and Fewer Deaths. Int. J. COPD 2019, 14, 331–341. [Google Scholar] [CrossRef]
- Ho, J.; He, W.; Chan, M.T.V.; Tse, G.; Liu, T.; Wong, S.H.; Leung, C.C.H.; Wong, W.T.; Tsang, S.; Zhang, L.; et al. Eosinophilia and Clinical Outcome of Chronic Obstructive Pulmonary Disease: A Meta-Analysis. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Salturk, C.; Karakurt, Z.; Takir, H.; Gungor, G.; Kargin, F.; Mocin, O.; Adiguzel, N.; Sari, R.; Celik, E.; Tuncay, E.; et al. Does Eosinophilic COPD Exacerbation Have a Better Patient Outcome than Non-Eosinophilic in the Intensive Care Unit? Int. J. Chron. Obstruct. Pulmon. Dis. 2015, 10, 1837–1846. [Google Scholar] [CrossRef]
- Bafadhel, M.; Greening, N.J.; Harvey-Dunstan, T.C.; Williams, J.E.A.; Morgan, M.D.; Brightling, C.E.; Hussain, S.F.; Pavord, I.D.; Singh, S.J.; Steiner, M.C. Blood Eosinophils and Outcomes in Severe Hospitalized Exacerbations of COPD. Chest 2016, 150, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Kandemir, Y.; Doğan, N.Ö.; Yaka, E.; Pekdemir, M.; Yılmaz, S. Clinical Characteristics of Neutrophilic, Eosinophilic and Mixed-Type Exacerbation Phenotypes of COPD. Am. J. Emerg. Med. 2021, 45, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Boixeda, R.; Almagro, P.; Diez-Manglano, J.; Cabrera, F.; Recio, J.; Martin-Garrido, I.; Soriano, J.B. Bacterial Flora in the Sputum and Comorbidity in Patients with Acute Exacerbations of COPD. Int. J. Chron. Obstruct. Pulmon. Dis. 2015, 10, 2581–2591. [Google Scholar] [CrossRef] [PubMed]
- Groenewegen, K.H.; Wouters, E.F.M. Bacterial Infections in Patients Requiring Admission for an Acute Exacerbation of COPD; a 1-Year Prospective Study. Respir. Med. 2003, 97, 770–777. [Google Scholar] [CrossRef]
- Fei, G.; Dai, M.-Y.; Qiao, J.-P.; Xu, Y.-H. Respiratory Infectious Phenotypes in Acute Exacerbation of COPD: An Aid to Length of Stay and COPD Assessment Test. Int. J. Chron. Obstruct. Pulmon. Dis. 2015, 10, 2257–2263. [Google Scholar] [CrossRef] [PubMed]
- Huerta, A.; Crisafulli, E.; Menéndez, R.; Martínez, R.; Soler, N.; Guerrero, M.; Montull, B.; Torres, A. Pneumonic and Nonpneumonic Exacerbations of COPD: Inflammatory Response and Clinical Characteristics. Chest 2013, 144, 1134–1142. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Fang, Q.; Li, Y. Independent Factors Associated with Pneumonia among Hospitalized Patients with Acute Exacerbations of Chronic Obstructive Pulmonary Disease. Medicine (Baltimore) 2018, 97, 1–6. [Google Scholar] [CrossRef]
- Dai, R.X.; Kong, Q.H.; Mao, B.; Xu, W.; Tao, R.J.; Wang, X.R.; Kong, Q.Y.; Xu, J.F. The Mortality Risk Factor of Community Acquired Pneumonia Patients with Chronic Obstructive Pulmonary Disease: A Retrospective Cohort Study. BMC Pulm. Med. 2018, 18, 1–10. [Google Scholar] [CrossRef]
- Jeong, S.W.; Lee, J.H.; Choi, K.J.; Hwangbo, Y.; Kim, Y.Y.; Lee, Y.J.; Yoon, W.K.; Kim, M.; Cha, S.I.; Park, J.Y.; et al. Comparisons of Clinical Characteristics and Outcomes in COPD Patients Hospitalized with Community-Acquired Pneumonia and Acute Exacerbation. Tuberc. Respir. Dis. 2010, 69, 31–38. [Google Scholar] [CrossRef][Green Version]
- Søgaard, M.; Madsen, M.; Løkke, A.; Hilberg, O.; Sørensen, H.T.; Thomsen, R.W. Incidence and Outcomes of Patients Hospitalized with COPD Exacerbation with and without Pneumonia. Int. J. Chron. Obstruct. Pulmon. Dis. 2016, 11, 455–465. [Google Scholar] [CrossRef]
- Lieberman, D.; Lieberman, D.; Gelfer, Y.; Varshavsky, R.; Dvoskin, B.; Leinonen, M.; Friedman, M.G. Pneumonic vs Nonpneumonic Acute Exacerbations of COPD. Chest 2002, 122, 1264–1270. [Google Scholar] [CrossRef]
- Sharafkhaneh, A.; Spiegelman, A.M.; Main, K.; Tavakoli-Tabasi, S.; Lan, C.; Musher, D. Mortality in Patients Admitted for Concurrent COPD Exacerbation and Pneumonia. COPD J. Chronic Obstr. Pulm. Dis. 2017, 14, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Shin, B.; Kim, S.H.; Yong, S.J.; Lee, W.Y.; Park, S.; Lee, S.J.; Lee, S.J.; Lee, M.K. Early Readmission and Mortality in Acute Exacerbation of Chronic Obstructive Pulmonary Disease with Community-Acquired Pneumonia. Chron. Respir. Dis. 2018, 16, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Restrepo, M.I.; Mortensen, E.M.; Pugh, J.A.; Anzueto, A. COPD Is Associated with Increased Mortality in Patients with Community-Acquired Pneumonia. Eur. Respir. J. 2006, 28, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Mao, B.; Lu, H.-W.; Li, M.-H.; Fan, L.-C.; Yang, J.-W.; Miao, X.-Y.; Xu, J.-F. The Existence of Bronchiectasis Predicts Worse Prognosis in Patients with COPD. Sci. Rep. 2015, 5, 10961. [Google Scholar] [CrossRef] [PubMed]
- Molino, A.; Vitale, C.; Valente, T.; Amato, M.D.; Mormile, M. Impact of Bronchiectasis on COPD Exacerbations. Int. J. Thorax 2018, 1, 1–7. [Google Scholar]
- Sánchez-Muñoz, G.; Lopez-de-Andrés, A.; Hernández-Barrera, V.; Jiménez-García, R.; Pedraza-Serrano, F.; Puente-Maestu, L.; de Miguel-Díez, J. Bronchiectasis in Patients Hospitalized with Acute Exacerbation of COPD in Spain: Influence on Mortality, Hospital Stay, and Hospital Costs (2006-2014) According to Gender. PLoS ONE 2019, 14, e0211222. [Google Scholar] [CrossRef]
- MacDonald, M.I.; Osadnik, C.R.; Bulfin, L.; Leahy, E.; Leong, P.; Shafuddin, E.; Hamza, K.; King, P.T.; Bardin, P.G. MULTI-PHACET: Multidimensional Clinical Phenotyping of Hospitalised Acute COPD Exacerbations. ERJ Open Res. 2021, 7, 00198–02021. [Google Scholar] [CrossRef]
- Zhou, A.; Zhou, Z.; Zhao, Y.; Chen, P. The Recent Advances of Phenotypes in Acute Exacerbations of COPD. Int. J. Chron. Obstruct. Pulmon. Dis. 2017, 12, 1009–1018. [Google Scholar] [CrossRef]
- Salvi, S. Tobacco Smoking and Environmental Risk Factors for Chronic Obstructive Pulmonary Disease. Clin. Chest Med. 2014, 35, 17–27. [Google Scholar] [CrossRef]
- Ramírez-Venegas, A.; Montiel-Lopez, F.; Falfan-Valencia, R.; Pérez-Rubio, G.; Sansores, R.H. The “Slow Horse Racing Effect” on Lung Function in Adult Life in Chronic Obstructive Pulmonary Disease Associated to Biomass Exposure. Front. Med. 2021, 8, 700836. [Google Scholar] [CrossRef] [PubMed]
- Shetty, B.S.P.; D’Souza, G.; Padukudru Anand, M. Effect of Indoor Air Pollution on Chronic Obstructive Pulmonary Disease (COPD) Deaths in Southern Asia—A Systematic Review and Meta-Analysis. Toxics 2021, 9, 85. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Venegas, A.; Sansores, R.H.; Pérez-Padilla, R.; Regalado, J.; Velázquez, A.; Sánchez, C.; Mayar, M.E. Survival of Patients with Chronic Obstructive Pulmonary Disease Due to Biomass Smoke and Tobacco. Am. J. Respir. Crit. Care Med. 2006, 173, 393–397. [Google Scholar] [CrossRef]
- Camp, P.G.; Ramirez-Venegas, A.; Sansores, R.H.; Alva, L.F.; McDougall, J.E.; Sin, D.D.; Pare, P.D.; Muller, N.L.; Silva, C.I.S.; Rojas, C.E.; et al. COPD Phenotypes in Biomass Smoke- versus Tobacco Smoke-Exposed Mexican Women. Eur. Respir. J. 2014, 43, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Tabyshova, A.; Hurst, J.R.; Soriano, J.B.; Checkley, W.; Wan-Chun Huang, E.; Trofor, A.C.; Flores-Flores, O.; Alupo, P.; Gianella, G.; Ferdous, T.; et al. Gaps in COPD Guidelines of Low- and Middle-Income Countries. Chest 2021, 159, 575–584. [Google Scholar] [CrossRef]
- Crisafulli, E.; Barbeta, E.; Ielpo, A.; Torres, A. Management of Severe Acute Exacerbations of COPD: An Updated Narrative Review. Multidiscip. Respir. Med. 2018, 13, 36. [Google Scholar] [CrossRef] [PubMed]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A New Method of Classifying Prognostic Comorbidity in Longitudinal Studies: Development and Validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Brusselle, G.; Pavord, I.D.; Landis, S.; Pascoe, S.; Lettis, S.; Morjaria, N.; Barnes, N.; Hilton, E. Blood Eosinophil Levels as a Biomarker in COPD. Respir. Med. 2018, 138, 21–31. [Google Scholar] [CrossRef]
- Apte, K.; Salvi, S. Household Air Pollution and Its Effects on Health. F1000Research 2016, 5, 2593. [Google Scholar] [CrossRef]
- Smith, K.R. Biofuels, Air Pollution, and Health: A Global Review; Springer: Boston, MA, USA, 1987; ISBN 978-1-4612-8231-0. [Google Scholar]
- Smith, K.R.; Apte, M.G.; Yuqing, M.; Wongsekiarttirat, W.; Kulkarni, A. Air Pollution and the Energy Ladder in Asian Cities. Energy 1994, 19, 587–600. [Google Scholar] [CrossRef]
- Veeramachaneni, S.B.; Sethi, S. Pathogenesis of Bacterial Exacerbations of COPD. COPD J. Chronic Obstr. Pulm. Dis. 2006, 3, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, A.I.; Wedzicha, J.A. Definition, Causes, Pathogenesis, and Consequences of Chronic Obstructive Pulmonary Disease Exacerbations. Clin. Chest Med. 2020, 41, 421–438. [Google Scholar] [CrossRef] [PubMed]
- Berenson, C.S.; Kruzel, R.L.; Eberhardt, E.; Dolnick, R.; Minderman, H.; Wallace, P.K.; Sethi, S. Impaired Innate Immune Alveolar Macrophage Response and the Predilection for Copd Exacerbations. Thorax 2014, 69, 811–818. [Google Scholar] [CrossRef] [PubMed]
- King, P.T.; Sharma, R.; O’Sullivan, K.; Selemidis, S.; Lim, S.; Radhakrishna, N.; Lo, C.; Prasad, J.; Callaghan, J.; McLaughlin, P.; et al. Nontypeable Haemophilus Influenzae Induces Sustained Lung Oxidative Stress and Protease Expression. PLoS ONE 2015, 10, e0120371. [Google Scholar] [CrossRef] [PubMed]
- Sethi, S.; Murphy, T.F. Infection in the Pathogenesis and Course of Chronic Obstructive Pulmonary Disease. N. Engl. J. Med. 2008, 359, 2355–2365. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.T.; Campbell, E.J.; Hill, S.L.; Bayley, D.L.; Stockley, R.A. Association between Airway Bacterial Load and Markers of Airway Inflammation in Patients with Stable Chronic Bronchitis. Am. J. Med. 2000, 109, 288–295. [Google Scholar] [CrossRef]
- Sethi, S.; Evans, N.; Grant, B.J.B.; Murphy, T.F. New Strains of Bacteria and Exacerbations of Chronic Obstructive Pulmonary Disease. N. Engl. J. Med. 2002, 347, 465–471. [Google Scholar] [CrossRef]
- Soriano, J.B.; Visick, G.T.; Muellerova, H.; Payvandi, N.; Hansell, A.L. Patterns of Comorbidities in Newly Diagnosed COPD and Asthma in Primary Care. Chest 2005, 128, 2099–2107. [Google Scholar] [CrossRef]
- Müllerova, H.; Chigbo, C.; Hagan, G.W.; Woodhead, M.A.; Miravitlles, M.; Davis, K.J.; Wedzicha, J.A. The Natural History of Community-Acquired Pneumonia in COPD Patients: A Population Database Analysis. Respir. Med. 2012, 106, 1124–1133. [Google Scholar] [CrossRef]
- Gutierrez, P.; Closa, D.; Piñer, R.; Bulbena, O.; Menéndez, R.; Torres, A. Macrophage Activation in Exacerbated COPD with and without Community-Acquired Pneumonia. Eur. Respir. J. 2010, 36, 285–291. [Google Scholar] [CrossRef]
- Chaturvedi, S.; Kumar, A.; Sapkota, S.; Rojas, S.; Carla Krissel Badiola, M.; Patel, P.; Upadhyaya, A.; Shah, H.; Karki, A. Impact of Sepsis on Outcomes of Hospitalizations Due to COPD. Chest 2021, 160, A1906. [Google Scholar] [CrossRef]
- Hasselbacher, D.A.; Mannino, D.M.; Berger, R. Patients with Chronic Obstructive Pulmonary Disease Are at Higher Risk of Sepsis. Chest 2005, 128, 378S. [Google Scholar] [CrossRef]
- Ernst, P.; Coulombe, J.; Brassard, P.; Suissa, S. The Risk of Sepsis with Inhaled and Oral Corticosteroids in Patients with COPD. COPD J. Chronic Obstr. Pulm. Dis. 2017, 14, 137–142. [Google Scholar] [CrossRef]
- Chen, C.-H.; Lai, C.-C.; Wang, Y.-H.; Wang, C.-Y.; Wang, H.-C.; Yu, C.-J.; Chen, L. Taiwan Clinical Trial Consortium for Respiratory Diseases (TCORE) The Impact of Sepsis on the Outcomes of COPD Patients: A Population-Based Cohort Study. J. Clin. Med. 2018, 7, 393. [Google Scholar] [CrossRef] [PubMed]
- Leigh, R.; Pizzichini, M.M.M.; Morris, M.M.; Maltais, F.; Hargreave, F.E.; Pizzichini, E. Stable COPD: Predicting Benefit from High-Dose Inhaled Corticosteroid Treatment. Eur. Respir. J. 2006, 27, 964–971. [Google Scholar] [CrossRef]
- Bafadhel, M.; McKenna, S.; Terry, S.; Mistry, V.; Reid, C.; Haldar, P.; McCormick, M.; Haldar, K.; Kebadze, T.; Duvoix, A.; et al. Acute Exacerbations of Chronic Obstructive Pulmonary Disease: Identification of Biologic Clusters and Their Biomarkers. Am. J. Respir. Crit. Care Med. 2011, 184, 662–671. [Google Scholar] [CrossRef]
- Pascoe, S.; Locantore, N.; Dransfield, M.T.; Barnes, N.C.; Pavord, I.D. Blood Eosinophil Counts, Exacerbations, and Response to the Addition of Inhaled Fluticasone Furoate to Vilanterol in Patients with Chronic Obstructive Pulmonary Disease: A Secondary Analysis of Data from Two Parallel Randomised Controlled Trials. Lancet Respir. Med. 2015, 3, 435–442. [Google Scholar] [CrossRef]
- Disantostefano, R.L.; Hinds, D.; Van Le, H.; Barnes, N.C. Relationship between Blood Eosinophils and Clinical Characteristics in a Cross-Sectional Study of a US Population-Based COPD Cohort. Respir. Med. 2016, 112, 88–96. [Google Scholar] [CrossRef]
- Holland, M.; Alkhalil, M.; Chandromouli, S.; Janjua, A.; Babores, M. Eosinopenia as a Marker of Mortality and Length of Stay in Patients Admitted with Exacerbations of Chronic Obstructive Pulmonary Disease. Respirology 2010, 15, 165–167. [Google Scholar] [CrossRef]
- Duman, D.; Aksoy, E.; Agca, M.C.; Kocak, N.D.; Ozmen, I.; Akturk, U.A.; Gungor, S.; Tepetam, F.M.; Eroglu, S.A.; Oztas, S.; et al. The Utility of Inflammatory Markers to Predict Readmissions and Mortality in COPD Cases with or without Eosinophilia. Int. J. COPD 2015, 11, 2469–2478. [Google Scholar] [CrossRef]
- Villalobos, R.; Magallanes, J.; David-Wang, A. P143 Blood Eosinophilia as Predictor for Patient Outcomes in COPD Exacerbations: A Systematic Review and Meta-Analysis. Thorax 2016, 71, 160. [Google Scholar] [CrossRef]
- Gonzalez-Barcala, F.J.; San-Jose, M.E.; Nieto-Fontarigo, J.J.; Calvo-Alvarez, U.; Carreira, J.M.; Garcia-Sanz, M.T.; Muñoz, X.; Perez-Lopez-Corona, M.P.; Gómez-Conde, M.J.; Casas-Fernández, A.; et al. Blood Eosinophils Could Be Useful as a Biomarker in Chronic Obstructive Pulmonary Disease Exacerbations. Int. J. Clin. Pract. 2019, 73, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Saad, A.B.; Khemakhem, R.; Mhamed, S.C.; Fahem, N.; Migaou, A.; Joobeur, S.; Rouatbi, N. Study of the Role of Blood Eosinophil Count in Patients with Severe Acute Exacerbation of Chronic Obstructive Pulmonary Disease Hospitalized in a Tunisian Center. Pan Afr. Med. J. 2019, 34, 138. [Google Scholar] [CrossRef]
- Lv, M.-Y.; Qiang, L.-X.; Li, Z.-H.; Jin, S.-D. The Lower the Eosinophils, the Stronger the Inflammatory Response? The Relationship of Different Levels of Eosinophils with the Degree of Inflammation in Acute Exacerbation Chronic Obstructive Pulmonary Disease (AECOPD). J. Thorac. Dis. 2021, 13, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Bélanger, M.; Couillard, S.; Courteau, J.; Larivée, P.; Poder, T.; Carrier, N.; Girard, K.; Vézina, F.-A.; Vanasse, A. Eosinophil Counts in First COPD Hospitalizations: A Comparison of Health Service Utilization. Int. J. Chron. Obstruct. Pulmon. Dis. 2018, 13, 3045–3054. [Google Scholar] [CrossRef]
- Kolsum, U.; Donaldson, G.C.; Singh, R.; Barker, B.L.; Gupta, V.; George, L.; Webb, A.J.; Thurston, S.; Brookes, A.J.; McHugh, T.D.; et al. Blood and Sputum Eosinophils in COPD; Relationship with Bacterial Load. Respir. Res. 2017, 18. [Google Scholar] [CrossRef]
- Choi, J.; Oh, J.Y.; Lee, Y.S.; Hur, G.Y.; Lee, S.Y.; Shim, J.J.; Kang, K.H.; Min, K.H. The Association between Blood Eosinophil Percent and Bacterial Infection in Acute Exacerbation of Chronic Obstructive Pulmonary Disease. Int. J. COPD 2019, 14, 953–959. [Google Scholar] [CrossRef]
- Wang, F.; Yang, L.; Wang, M.; He, B.; Wei, P.F. Association between Blood Eosinophil Count and Bacterial Infection and Clinical Outcomes in Patients with Severe Exacerbations of Chronic Obstructive Pulmonary Disease. Chin. Med. J. (Engl.) 2022, 135, 462–464. [Google Scholar] [CrossRef]
- Hills, A.G.; Forsham, P.H.; Finch, C.A. Changes in Circulating Leukocytes Induced by the Administration of Pituitary Adrenocorticotrophic Hormone in Man. Blood 1948, 3, 755–768. [Google Scholar] [CrossRef]
- Bass, D.A. Behavior of Eosinophil Leukocytes in Acute Inflammation. II. Eosinophil Dynamics during Acute Inflammation. J. Clin. Investig. 1975, 56, 870–879. [Google Scholar] [CrossRef]
- Lv, J.; Bhatia, M.; Wang, X. Roles of Mitochondrial DNA in Energy Metabolism. In Mitochondrial DNA and Diseases; Sun, H., Wang, X., Eds.; Advances in Experimental Medicine and Biology; Springer: Singapore, 2017; Volume 1038, pp. 71–83. ISBN 978-981-10-6673-3. [Google Scholar]
- Yousefi, S.; Gold, J.A.; Andina, N.; Lee, J.J.; Kelly, A.M.; Kozlowski, E.; Schmid, I.; Straumann, A.; Reichenbach, J.; Gleich, G.J.; et al. Catapult-like Release of Mitochondrial DNA by Eosinophils Contributes to Antibacterial Defense. Nat. Med. 2008, 14, 949–953. [Google Scholar] [CrossRef] [PubMed]
- Kvarnhammar, A.M.; Cardell, L.O. Pattern-Recognition Receptors in Human Eosinophils. Immunology 2012, 136, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Lehrer, R.I.; Szklarek, D.; Barton, A.; Ganz, T.; Hamann, K.J.; Gleich, G.J. Antibacterial Properties of Eosinophil Major Basic Protein and Eosinophil Cationic Protein. J. Immunol. 1989, 142, 4428–4434. [Google Scholar] [PubMed]
- Giordano, L.; Gregory, A.D.; Pérez Verdaguer, M.; Ware, S.A.; Harvey, H.; DeVallance, E.; Brzoska, T.; Sundd, P.; Zhang, Y.; Sciurba, F.C.; et al. Extracellular Release of Mitochondrial DNA: Triggered by Cigarette Smoke and Detected in COPD. Cells 2022, 11, 369. [Google Scholar] [CrossRef] [PubMed]
- Szczesny, B.; Marcatti, M.; Ahmad, A.; Montalbano, M.; Brunyánszki, A.; Bibli, S.-I.; Papapetropoulos, A.; Szabo, C. Mitochondrial DNA Damage and Subsequent Activation of Z-DNA Binding Protein 1 Links Oxidative Stress to Inflammation in Epithelial Cells. Sci. Rep. 2018, 8, 914. [Google Scholar] [CrossRef] [PubMed]
- Avriel, A.; Rozenberg, D.; Raviv, Y.; Heimer, D.; Bar-Shai, A.; Gavish, R.; Sheynin, J.; Douvdevani, A. Prognostic Utility of Admission Cell-Free DNA Levels in Patients with Chronic Obstructive Pulmonary Disease Exacerbations. Int. J. Chron. Obstruct. Pulmon. Dis. 2016, 11, 3153–3161. [Google Scholar] [CrossRef]
- Yousefi, S.; Mihalache, C.; Kozlowski, E.; Schmid, I.; Simon, H.U. Viable Neutrophils Release Mitochondrial DNA to Form Neutrophil Extracellular Traps. Cell Death Differ. 2009, 16, 1438–1444. [Google Scholar] [CrossRef]
- Martinez-Garcia, M.A.; Miravitlles, M. Bronchiectasis in COPD Patients: More than a Comorbidity? Int. J. COPD 2017, 11, 1401–1411. [Google Scholar] [CrossRef]
- Patel, I.S.; Vlahos, I.; Wilkinson, T.M.A.; Lloyd-Owen, S.J.; Donaldson, G.C.; Wilks, M.; Reznek, R.H.; Wedzicha, J.A. Bronchiectasis, Exacerbation Indices, and Inflammation in Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2004, 170, 400–407. [Google Scholar] [CrossRef]
- Martínez-García, M.Á.; Soler-Catalunã, J.J.; Sanz, Y.D.; Serra, P.C.; Lerma, M.A.; Vicente, J.B.; Perpiñá-Tordera, M. Factors Associated with Bronchiectasis in Patients with COPD. Chest 2011, 140, 1130–1137. [Google Scholar] [CrossRef]
- Gatheral, T.; Kumar, N.; Sansom, B.; Lai, D.; Nair, A.; Vlahos, I.; Baker, E.H. COPD-Related Bronchiectasis; Independent Impact on Disease Course and Outcomes. COPD J. Chronic Obstr. Pulm. Dis. 2014, 11, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Harness-Brumley, C.L.; Elliott, A.C.; Rosenbluth, D.B.; Raghavan, D.; Jain, R. Gender Differences in Outcomes of Patients with Cystic Fibrosis. J. Womens Health 2014, 23, 1012–1020. [Google Scholar] [CrossRef]
- Rosenfeld, M.; Davis, R.; FitzSimmons, S.; Pepe, M.; Ramsey, B. Gender Gap in Cystic Fibrosis Mortality. Am. J. Epidemiol. 1997, 145, 794–803. [Google Scholar] [CrossRef]
- Raghavan, D.; Jain, R. Increasing Awareness of Sex Differences in Airway Diseases. Respirology 2016, 21, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Vidaillac, C.; Yong, V.F.L.; Jaggi, T.K.; Soh, M.M.; Chotirmall, S.H. Gender Differences in Bronchiectasis: A Real Issue? Breathe 2018, 14, 108–121. [Google Scholar] [CrossRef]
- Capistrano, S.; van Reyk, D.; Chen, H.; Oliver, B. Evidence of Biomass Smoke Exposure as a Causative Factor for the Development of COPD. Toxics 2017, 5, 36. [Google Scholar] [CrossRef] [PubMed]
- Ezzati, M.; Bailis, R.; Kammen, D.M.; Holloway, T.; Price, L.; Cifuentes, L.A.; Barnes, B.; Chaurey, A.; Dhanapala, K.N. Energy Management and Global Health. Annu. Rev. Environ. Resour. 2004, 29, 383–419. [Google Scholar] [CrossRef]
- Silverman, E.K.; Speizer, F.E. Risk Factors for the Development of Chronic Obstructive Pulmonary Disease. Med. Clin. N. Am. 1996, 80, 501–522. [Google Scholar] [CrossRef]
- Raherison, C.; Girodet, P.-O. Epidemiology of COPD. Eur. Respir. Rev. 2009, 18, 213–221. [Google Scholar] [CrossRef]
- Mannino, D.M.; Buist, A.S. Global Burden of COPD: Risk Factors, Prevalence, and Future Trends. Lancet 2007, 370, 765–773. [Google Scholar] [CrossRef]
- Laniado-Laborín, R. Smoking and Chronic Obstructive Pulmonary Disease (COPD). Parallel Epidemics of the 21 Century. Int. J. Environ. Res. Public. Health 2009, 6, 209–224. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Lu, M.; Sun, H.; Mao, Y. A Survival Analysis of Patients with an Acute Exacerbation of Chronic Obstructive Pulmonary Disease Discharged from the Respiratory Intensive Care Unit. Ann. Palliat. Med. 2020, 9, 1812–1820. [Google Scholar] [CrossRef] [PubMed]
- Gunen, H. Factors Affecting Survival of Hospitalised Patients with COPD. Eur. Respir. J. 2005, 26, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Xing, Z.; Long, H.; Huang, Y.; Zeng, P.; Janssens, J.-P.; Guo, Y. Predictors of Mortality in COPD Exacerbation Cases Presenting to the Respiratory Intensive Care Unit. Respir. Res. 2021, 22, 77. [Google Scholar] [CrossRef] [PubMed]
- Fruchter, O.; Yigla, M.; Kramer, M.R. D-Dimer as a Prognostic Biomarker for Mortality in Chronic Obstructive Pulmonary Disease Exacerbation. Am. J. Med. Sci. 2015, 349, 29–35. [Google Scholar] [CrossRef]
- Golpe, R.; Mengual-Macenlle, N.; Sanjuán-López, P.; Cano-Jiménez, E.; Castro-Añón, O.; Pérez-de-Llano, L.A. Prognostic Indices and Mortality Prediction in COPD Caused by Biomass Smoke Exposure. Lung 2015, 193, 497–503. [Google Scholar] [CrossRef]
- Bustamante-Fermosel, A.; De Miguel-Yanes, J.M.; Duffort-Falcó, M.; Muñoz, J. Mortality-Related Factors after Hospitalization for Acute Exacerbation of Chronic Obstructive Pulmonary Disease: The Burden of Clinical Features. Am. J. Emerg. Med. 2007, 25, 515–522. [Google Scholar] [CrossRef]
- Carey, M.A.; Card, J.W.; Voltz, J.W.; Arbes, S.J.; Germolec, D.R.; Korach, K.S.; Zeldin, D.C. It’s All about Sex: Gender, Lung Development and Lung Disease. Trends Endocrinol. Metab. 2007, 18, 308–313. [Google Scholar] [CrossRef]
- Chotirmall, S.H. The Microbiological Gender Gap in Cystic Fibrosis. J. Womens Health 2014, 23, 995–996. [Google Scholar] [CrossRef]
- Roggenbuck, M.; Anderson, D.; Barfod, K.K.; Feelisch, M.; Geldenhuys, S.; Sørensen, S.J.; Weeden, C.E.; Hart, P.H.; Gorman, S. Vitamin D and Allergic Airway Disease Shape the Murine Lung Microbiome in a Sex-Specific Manner. Respir. Res. 2016, 17. [Google Scholar] [CrossRef]
- Chamekh, M.; Deny, M.; Romano, M.; Lefèvre, N.; Corazza, F.; Duchateau, J.; Casimir, G. Differential Susceptibility to Infectious Respiratory Diseases between Males and Females Linked to Sex-Specific Innate Immune Inflammatory Response. Front. Immunol. 2017, 8, 1806. [Google Scholar] [CrossRef] [PubMed]
- Casimir, G.J.; Lefèvre, N.; Corazza, F.; Duchateau, J. Sex and Inflammation in Respiratory Diseases: A Clinical Viewpoint. Biol. Sex Differ. 2013, 4, 16. [Google Scholar] [CrossRef] [PubMed]
- Townsend, E.A.; Miller, V.M.; Prakash, Y.S. Sex Differences and Sex Steroids in Lung Health and Disease. Endocr. Rev. 2012, 33, 1–47. [Google Scholar] [CrossRef]
- Fletcher, C.; Peto, R. The Natural History of Chronic Airflow Obstruction. BMJ 1977, 1, 1645–1648. [Google Scholar] [CrossRef] [PubMed]
- Vaz Fragoso, C.A.; Gill, T.M. Respiratory Impairment and the Aging Lung: A Novel Paradigm for Assessing Pulmonary Function. J. Gerontol. A Biol. Sci. Med. Sci. 2012, 67A, 264–275. [Google Scholar] [CrossRef] [PubMed]
- Divo, M.J.; Celli, B.R.; Poblador-Plou, B.; Calderón-Larrañaga, A.; de-Torres, J.P.; Gimeno-Feliu, L.A.; Bertó, J.; Zulueta, J.J.; Casanova, C.; Pinto-Plata, V.M.; et al. Chronic Obstructive Pulmonary Disease (COPD) as a Disease of Early Aging: Evidence from the EpiChron Cohort. PLoS ONE 2018, 13, e0193143. [Google Scholar] [CrossRef] [PubMed]
- MacNee, W. Is Chronic Obstructive Pulmonary Disease an Accelerated Aging Disease? Ann. Am. Thorac. Soc. 2016, 13, S429–S437. [Google Scholar] [CrossRef]

| Total (N = 2194) | AECOPD-NBE (N = 1025) | AECOPD-B (N = 251) | AECOPD-E (N = 388) | AECOPD-BE (N = 53) | AECOPD-P(N = 309) | AECOPD-BC (N = 168) | p-Value | ||
|---|---|---|---|---|---|---|---|---|---|
| Age in years | Median (IQR) | 67.0 (60.0 to 74.0) | 67.0 (60.0 to 74.0) | 66.0 (60.0 to 74.5) | 66.0 (59.0 to 74.0) | 70.0 (59.0 to 75.0) | 67.0 (60 to 75.0) | 65.0 (56 to 71.0) | 0.016 * |
| Gender | Male (n, %) | 1649 (75.2) | 805 (78.5) | 189 (75.3) | 279 (71.9) | 40 (75.5) | 230 (74.4) | 106 (63.1) | <0.001 † |
| Female (n, %) | 545 (24.8) | 220 (21.5) | 62 (24.7) | 109 (28.1) | 13 (24.5) | 79 (25.6) | 62 (36.9) | ||
| LOS in days | Median (IQR) | 5.0 (3.0 to 8.0) | 5.0 (3.0 to 7.0) | 6.0 (4.0 to 9.5) | 4.0 (3.0 to 6.0) | 6.0 (4.0 to 9.0) | 6.0 (4.0 to 10.0) | 5.0 (4.0 to 8.0) | <0.001 * |
| Admission to Ward/ICU | Ward (n, %) | 1794 (81.8) | 926 (90.3) | 123 (49.0) | 380 (97.9) | 37 (69.8) | 199 (64.4) | 129 (76.8) | <0.001 † |
| ICU (n, %) | 400 (18.2) | 99 (9.7) | 128 (51.0) | 8 (2.1) | 16 (30.2) | 110 (35.6) | 39 (23.2) | ||
| Hospital outcome | Alive (n, %) | 2122 (96.7) | 1015 (99) | 223 (88.8) | 388 (100) | 50 (94.3) | 285 (92.2) | 161 (95.8) | <0.001 † |
| Dead (n, %) | 72 (3.3) | 10 (1.0) | 28 (11.2) | 0 (0) | 3 (5.7) | 24 (7.8) | 7 (4.2) | ||
| Bacterial infection | Yes (n, %) | 432 (19.7) | 0 (0) | 251 (100) | 0 (0) | 53 (100) | 85 (27.5) | 43 (25.6) | <0.001 † |
| Alcohol consumption | Yes (n, %) | 310 (14.1) | 170 (16.6) | 21 (8.4) | 52 (13.4) | 2 (3.8) | 50 (16.2) | 15 (8.9) | 0.001 † |
| NTS and NBMS-AECOPD | Yes (n, %) | 637 (29.0) | 295 (28.8) | 75 (29.9) | 128 (33.0) | 17 (32.1) | 76 (24.6) | 46 (27.4) | <0.001 † |
| TS-AECOPD | Yes (n, %) | 1100 (50.1) | 560 (54.6) | 114 (45.4) | 179 (46.1) | 21 (39.6) | 165 (53.4) | 61 (36.3) | |
| BMS-AECOPD | Yes (n, %) | 425 (19.4) | 164 (16.0) | 54 (21.5) | 77 (19.8) | 13 (24.5) | 66 (21.4) | 51 (30.4) | |
| TS and BMS-AECOPD | Yes (n, %) | 32 (1.5) | 6 (0.6) | 8 (3.2) | 4 (1.0) | 2 (3.8) | 2 (0.6) | 10 (6.0) | |
| CCI | Median (IQR) | 4.0 (3.0 to 5.0) | 4.0 (3.0 to 5.0) | 4.0 (3.0 to 5.0) | 3.0 (2.0 to 4.0) | 4.0 (3.0 to 5.0) | 4.0 (3.0 to 5.0) | 3.0 (2.0 to 4.0) | <0.001 * |
| Comorbidities | |||||||||
| Diabetes mellitus | Yes (n, %) | 522 (23.8) | 249 (24.3) | 60 (23.9) | 88 (22.7) | 14 (26.4) | 77 (24.9) | 34 (20.2) | 0.850 † |
| Heart diseases | Yes (n, %) | 327 (14.9) | 162 (15.8) | 37 (14.7) | 53 (13.7) | 9 (17.0) | 45 (14.6) | 21 (12.5) | 0.834 † |
| Renal diseases | Yes (n, %) | 186 (8.5) | 86 (8.4) | 26 (10.4) | 20 (5.2) | 5 (9.4) | 41 (13.3) | 8 (4.8) | 0.002 † |
| Liver diseases | Yes (n, %) | 56 (2.6) | 29 (2.8) | 13 (5.2) | 5 (1.3) | 1 (1.9) | 7 (2.3) | 1 (0.6) | 0.028 † |
| Cor pulmonale | Yes (n, %) | 452 (20.6) | 217 (21.2) | 62 (24.7) | 57 (14.7) | 5 (9.4) | 63 (20.4) | 48 (28.6) | <0.001 † |
| Hypertension | Yes (n, %) | 758 (34.5) | 362 (35.3) | 88 (35.1) | 140 (36.1) | 23 (43.4) | 100 (32.4) | 45 (26.8) | 0.176 † |
| Obesity | Yes (n, %) | 129 (5.9) | 61 (6.0) | 14 (5.6) | 33 (8.5) | 7 (13.2) | 12 (3.9) | 2 (1.2) | 0.002 † |
| OSA | Yes (n, %) | 137 (6.2) | 64 (6.2) | 12 (4.8) | 37 (9.5) | 7 (13.2) | 16 (5.2) | 1 (0.6) | <0.001 † |
| PAH | Yes (n, %) | 383 (17.5) | 163 (15.9) | 49 (19.5) | 65 (16.8) | 17 (32.1) | 46 (14.9) | 43 (25.6) | 0.001 † |
| Sepsis | Yes (n, %) | 94 (4.3) | 19 (1.9) | 15 (6.0) | 1 (0.3) | 0 (0) | 53 (17.2) | 6 (3.6) | <0.001 † |
| T1RF | Yes (n, %) | 80 (3.6) | 35 (3.4) | 12 (4.8) | 13 (3.4) | 1 (1.9) | 15 (4.9) | 4 (2.4) | 0.595 † |
| T2RF | Yes (n, %) | 371 (16.9) | 166 (16.2) | 61 (24.3) | 30 (7.7) | 14 (26.4) | 69 (22.3) | 31 (18.5) | <0.001 † |
| Lymphocytes % | Median (IQR) | 13.5 (7.3 to 21) | 12.6 (6.7 to 19.3) | 10.0 (5.1 to 16.5) | 20.4 (14.2 to 27.5) | 18.1 (11.0 to 25.7) | 9.6 (5.05 to 16.4) | 14.5 (8.15 to 19.8) | <0.001 * |
| Eosinophils % | Median (IQR) | 1.05 (0.2 to 3.3) | 0.5 (0.1 to 1.3) | 0.2 (0.0 to 1.2) | 4.90 (3.9 to 6.73) | 4.40 (3.8 to 5.50) | 0.5 (0.1 to 1.85) | 0.9 (0.2 to 3.0) | <0.001 * |
| Monocytes % | Median (IQR) | 4.5 (3.1 to 6.0) | 4.3 (3.0 to 5.9) | 4.3 (2.8 to 5.8) | 5.0 (3.9 to 6.2) | 4.35 (3.10 to 5.93) | 3.95 (2.4 to 5.45) | 4.7 (2.85 to 6.0) | <0.001 * |
| Basophils % | Median (IQR) | 0.3 (0.2 to 0.5) | 0.3 (0.2 to 0.5) | 0.3 (0.2 to 0.5) | 0.4 (0.3 to 0.7) | 0.4 (0.2 to 0.6) | 0.3 (0.2 to 0.5) | 0.3 (0.2 to 0.5) | <0.001 * |
| RBC count (million/cumm) | Median (IQR) | 4.63 (4.16 to 5.15) | 4.68 (4.21 to 5.2) | 4.66 (4.28 to 5.21) | 4.59 (4.15 to 5.06) | 4.70 (4.35 to 5.05) | 4.46 (4.0 to 4.97) | 4.58 (4.09 to 5.23) | 0.002 * |
| Platelet count (lakh cells/cumm) | Median (IQR) | 2.49 (1.94 to 3.17) | 2.51 (1.97 to 3.27) | 2.46 (1.83 to 3.19) | 2.5 (2.08 to 3.03) | 2.47 (2.03 to 2.84) | 2.43 (1.69 to 3.11) | 2.54 (1.97 to 3.29) | 0.483 * |
| NLR | Median (IQR) | 5.9 (3.3 to 12.0) | 6.55 (3.8 to 13.3) | 8.50 (4.6 to 17.6) | 3.3 (2.2 to 5.30) | 4.05 (2.4 to 7.35) | 8.50 (4.7 to 17.8) | 5.40 (3.5 to 10.3) | <0.001 * |
| NTS- and NBMS-AECOPD (N = 637) | TS-AECOPD (N = 1100) | BMS-AECOPD (N = 425) | TS- and BMS-AECOPD (N = 32) | p-Value | ||
|---|---|---|---|---|---|---|
| Age in years | Median (IQR) | 68 (61.0 to 75.0) | 66 (60.0 to 73.0) | 66 (58.0 to 74.0) | 65 (61.5 to 68.3) | <0.01 * |
| Male | Yes (n, %) | 498 (78.2) | 1091 (99.2) | 28 (6.6) | 32 (100) | <0.01 † |
| Female | Yes (n, %) | 139 (21.8) | 9 (0.8) | 397 (93.4) | 0 (0) | |
| LOS in days | Median (IQR) | 5.0 (3.0 to 8.0) | 5.0 (3.0 to 7.0) | 6.0 (4.0 to 8.0) | 5.0 (3.0 to 7.0) | 0.019 * |
| Ward | Ward (n, %) | 516 (81.0) | 906 (82.4) | 347 (81.6) | 25 (78.1) | 0.85 † |
| ICU | ICU (n, %) | 121 (19) | 194 (17.6) | 78 (18.4) | 7 (21.9) | |
| Alive | Alive (n, %) | 627 (98.4) | 1070 (97.3) | 395 (92.9) | 30 (93.8) | <0.01 † |
| Dead | Dead (n, %) | 10 (1.6) | 30 (2.7) | 30 (7.1) | 2 (6.3) | |
| CCI | Median (IQR) | 4.0 (3.0 to 5.0) | 4.0 (3.0 to 5.0) | 4.0 (3.0 to 5.0) | 3.0 (3.0 to 4.0) | <0.01 * |
| Bacterial infection | Yes (n, %) | 131 (20.6) | 198 (18.0) | 92 (21.6) | 11 (34.4) | 0.05 † |
| Alcohol consumption | Yes (n, %) | 19 (3.0) | 283 (25.7) | 1 (0.2) | 7 (21.9) | <0.01 † |
| Diabetes mellitus | Yes (n, %) | 184 (28.9) | 223 (20.3) | 110 (25.9) | 5 (15.6) | <0.01 † |
| Heart diseases | Yes (n, %) | 134 (21.0) | 140 (12.7) | 49 (11.5) | 4 (12.5) | <0.01 † |
| Renal diseases | Yes (n, %) | 74 (11.6) | 87 (7.9) | 25 (5.9) | 0 (0) | <0.01 † |
| Liver diseases | Yes (n, %) | 19 (3) | 32 (2.9) | 4 (0.9) | 1 (3.1) | 0.14 † |
| Cor pulmonale | Yes (n, %) | 141 (22.1) | 180 (16.4) | 122 (28.7) | 9 (28.1) | <0.01 † |
| Hypertension | Yes (n, %) | 268 (42.1) | 293 (26.6) | 189 (44.5) | 8 (25.0) | <0.01 † |
| Obesity | Yes (n, %) | 38 (6.0) | 40 (3.6) | 49 (11.5) | 2 (6.3) | <0.01 † |
| OSA | Yes (n, %) | 39 (6.1) | 42 (3.8) | 54 (12.7) | 2 (6.3) | <0.01 † |
| PAH | Yes (n, %) | 92 (14.4) | 195 (17.7) | 88 (20.7) | 8 (25.0) | 0.04 † |
| Sepsis | Yes (n, %) | 26 (4.1) | 46 (4.2) | 20 (4.7) | 2 (6.3) | 0.90 † |
| T1RF | Yes (n, %) | 16 (2.5) | 47 (4.3) | 17 (4.0) | 0 (0) | 0.18 † |
| T2RF | Yes (n, %) | 100 (15.7) | 166 (15.1) | 96 (22.6) | 9 (28.1) | <0.01 † |
| AECOPD-NBE | Yes (n, %) | 295 (46.3) | 560 (50.9) | 164 (38.6) | 6 (18.8) | <0.01 † |
| AECOPD-E | Yes (n, %) | 128 (20.1) | 179 (16.3) | 77 (18.1) | 4 (12.5) | |
| AECOPD-P | Yes (n, %) | 76 (11.9) | 165 (15.0) | 66 (15.5) | 2 (6.3) | |
| AECOPD-BC | Yes (n, %) | 46 (7.2) | 61 (5.5) | 51 (12.0) | 10 (31.3) | |
| AECOPD-B | Yes (n, %) | 75 (11.8) | 114 (10.4) | 54 (12.7) | 8 (25.0) | |
| AECOPD-BE | Yes (n, %) | 17 (2.7) | 21 (1.9) | 13 (3.1) | 2 (6.3) | |
| Lymphocytes % | Median (IQR) | 13.5 (7.45 to 22.1) | 12.9 (6.8 to 20) | 15.25 (9.6 to 22.1) | 12.8 (6.6 to 23.9) | <0.001 * |
| Eosinophils % | Median (IQR) | 1.3 (0.2 to 3.6) | 0.85 (0.1 to 3.0) | 1.3 (0.2 to 3.6) | 2.4 (0.4 to 3.9) | 0.001 * |
| Monocytes % | Median (IQR) | 4.6 (3.3 to 6.2) | 4.5 (3.0 to 6.0) | 4.2 (3.1 to 5.4) | 5.2 (3.2 to 6.4) | 0.068 * |
| Basophils % | Median (IQR) | 0.3 (0.2 to 0.5) | 0.3 (0.2 to 0.5) | 0.3 (0.2 to 0.5) | 0.3 (0.2 to 0.5) | 0.811 * |
| RBC count (million/cumm) | Median (IQR) | 4.5 (4.0 to 5.1) | 4.7 (4.3 to 5.3) | 4.46 (4.1 to 4.9) | 4.83 (4.6 to 5.3) | <0.001 * |
| Platelet count (lakh cells/cumm) | Median (IQR) | 2.47 (1.91 to 3.1) | 2.46 (1.86 to 3.18) | 2.65 (2.16 to 3.32) | 2.090 (1.95 to 2.5) | < 0.001 * |
| NLR | Median (IQR) | 5.90 (3.0 to 11.6) | 6.10 (3.5 to 13.2) | 5.15 (3.2 to 8.77) | 6.10 (3.0 to 13.4) | 0.002 * |
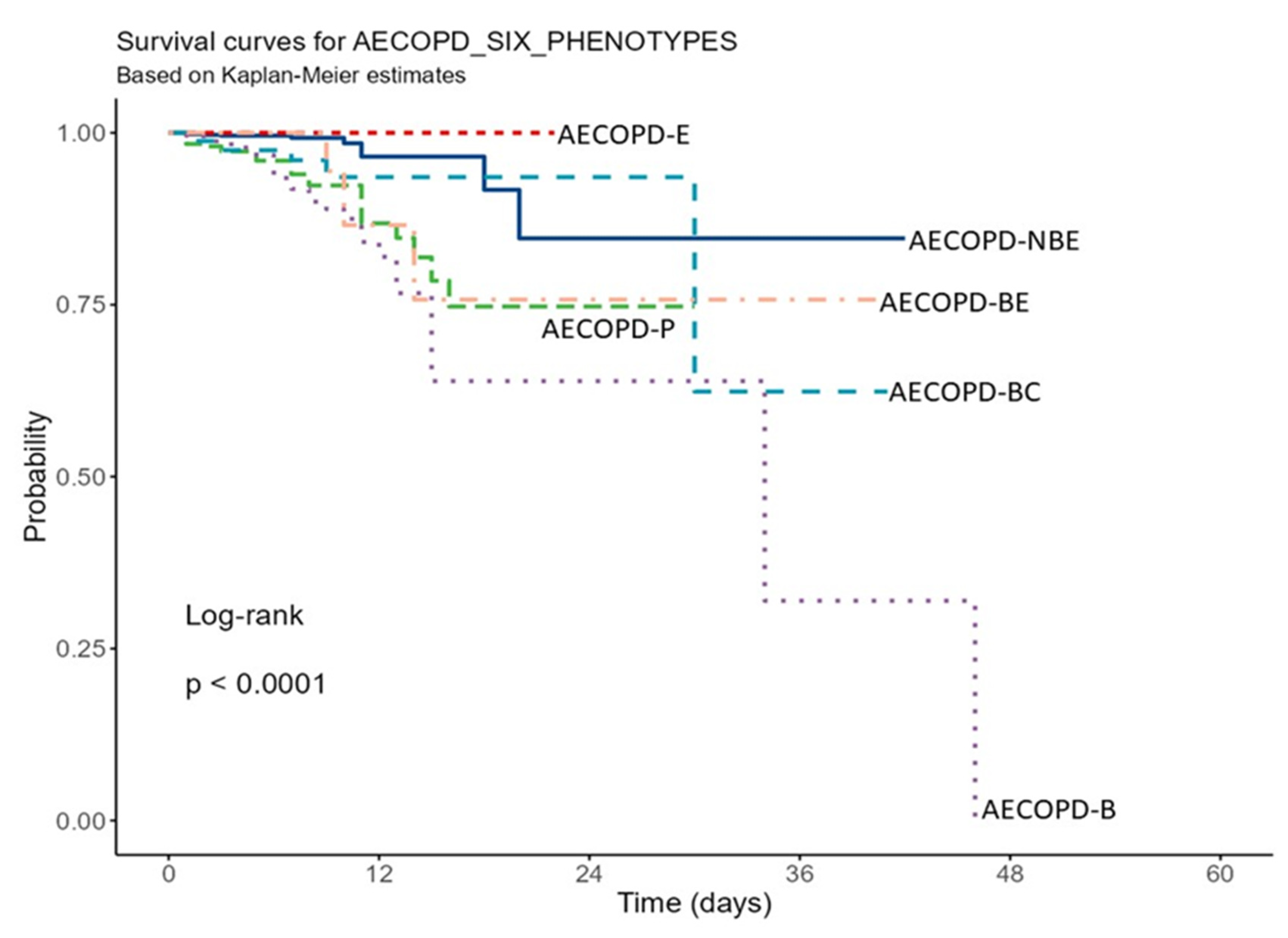
| Levels | Levels | p-Value |
|---|---|---|
| AECOPD-E | AECOPD-NBE | 0.529 |
| AECOPD-P | AECOPD-NBE | <0.001 |
| AECOPD-P | AECOPD-E | <0.001 |
| AECOPD-BC | AECOPD-NBE | 0.146 |
| AECOPD-BC | AECOPD-E | 0.008 |
| AECOPD-BC | AECOPD-P | 1.000 |
| AECOPD-B | AECOPD-NBE | <0.001 |
| AECOPD-B | AECOPD-E | <0.001 |
| AECOPD-B | AECOPD-P | 1.000 |
| AECOPD-B | AECOPD-BC | 0.389 |
| AECOPD-BE | AECOPD-NBE | 0.356 |
| AECOPD-BE | AECOPD-E | 0.090 |
| AECOPD-BE | AECOPD-P | 1.000 |
| AECOPD-BE | AECOPD-BC | 1.000 |
| AECOPD-BE | AECOPD-B | 1.000 |
| Levels | Levels | p-Value |
|---|---|---|
| TS-AECOPD | NTS and NBMS-AECOPD | 0.095 |
| BMS-AECOPD | NTS and NBMS-AECOPD | <0.001 |
| BMS-AECOPD | TS-AECOPD | 0.009 |
| TS and BMS-AECOPD | NTS and NBMS-AECOPD | 0.034 |
| TS and BMS-AECOPD | TS-AECOPD | 0.210 |
| TS and BMS-AECOPD | BMS-AECOPD | 0.838 |
| Model 1: Pathology | Model 2: Exposure | ||||
|---|---|---|---|---|---|
| HR (Univariable) | HR (Multivariable) | HR (Univariable) | HR (Multivariable) | ||
| Sex | Male | Reference | Reference | - | - |
| Female | 2.47 (1.55–3.93) *** | 3.05 (1.83–5.09) *** | - | - | |
| Age | Mean (SD) | 1.02 (1.00–1.05) * | 1.03 (1.00–1.06) | 1.02 (1.00–1.05) * | 1.03 (1.00–1.06) |
| Cor pulmonale | Yes | 1.37 (0.83–2.26) | 0.76 (0.44–1.32) | 1.37 (0.83–2.26) | 0.91 (0.54–1.54) |
| T1RF | Yes | 0.83 (0.20–3.40) | 0.88 (0.21–3.66) | 0.83 (0.20–3.40) | 1.01 (0.24–4.21) |
| T2RF | Yes | 2.21 (1.37–3.56) *** | 1.74 (1.04–2.91) * | 2.21 (1.37–3.56) *** | 1.75 (1.04–2.93) * |
| Hypertension | Yes | 1.00 (0.62–1.62) | 0.70 (0.41–1.20) | 1.00 (0.62–1.62) | 0.80 (0.47–1.34) |
| PAH | Yes | 1.07 (0.60–1.92) | 0.86 (0.46–1.58) | 1.07 (0.60–1.92) | 0.85 (0.47–1.55) |
| Sepsis | Yes | 5.65 (3.29–9.72) *** | 3.13 (1.72–5.71) *** | 5.65 (3.29–9.72) *** | 5.23 (2.96–9.25) *** |
| CCI | Mean (SD) | 1.10 (0.95–1.27) | 1.07 (0.91–1.26) | 1.10 (0.95–1.27) | 0.97 (0.78–1.22) |
| AECOPD phenotypes based on Exposure type | NTS and NBMS-AECOPD | - | - | Reference | Reference |
| TS-AECOPD | - | - | 2.18 (1.04–4.61) * | 2.17 (1.02–4.63) * | |
| BMS-AECOPD | - | - | 4.82 (2.29–10.16) *** | 5.28 (2.46–11.35) *** | |
| TS and BMS-AECOPD | - | - | 6.35 (1.36–29.51) * | 7.24 (1.53–34.29) * | |
| AECOPD phenotypes based on pathology | AECOPD-NBE | Reference | Reference | - | - |
| AECOPD-E | 0.00 (0.00-Inf) | 0.00 (0.00-Inf) | - | - | |
| AECOPD-B | 7.21 (3.47–14.97) *** | 6.42 (3.06–13.46) *** | - | - | |
| AECOPD-BE | 3.53 (0.96–12.89) | 3.84 (1.04–14.20) * | - | - | |
| AECOPD-P | 5.34 (2.54–11.20) *** | 4.33 (2.01–9.30) *** | - | - | |
| AECOPD-BC | 3.30 (1.25–8.72) * | 2.72 (1.00–7.38) * | - | - | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaleem Ullah, M.; Parthasarathi, A.; Biligere Siddaiah, J.; Vishwanath, P.; Upadhyay, S.; Ganguly, K.; Anand Mahesh, P. Impact of Acute Exacerbation and Its Phenotypes on the Clinical Outcomes of Chronic Obstructive Pulmonary Disease in Hospitalized Patients: A Cross-Sectional Study. Toxics 2022, 10, 667. https://doi.org/10.3390/toxics10110667
Kaleem Ullah M, Parthasarathi A, Biligere Siddaiah J, Vishwanath P, Upadhyay S, Ganguly K, Anand Mahesh P. Impact of Acute Exacerbation and Its Phenotypes on the Clinical Outcomes of Chronic Obstructive Pulmonary Disease in Hospitalized Patients: A Cross-Sectional Study. Toxics. 2022; 10(11):667. https://doi.org/10.3390/toxics10110667
Chicago/Turabian StyleKaleem Ullah, Mohammed, Ashwaghosha Parthasarathi, Jayaraj Biligere Siddaiah, Prashant Vishwanath, Swapna Upadhyay, Koustav Ganguly, and Padukudru Anand Mahesh. 2022. "Impact of Acute Exacerbation and Its Phenotypes on the Clinical Outcomes of Chronic Obstructive Pulmonary Disease in Hospitalized Patients: A Cross-Sectional Study" Toxics 10, no. 11: 667. https://doi.org/10.3390/toxics10110667
APA StyleKaleem Ullah, M., Parthasarathi, A., Biligere Siddaiah, J., Vishwanath, P., Upadhyay, S., Ganguly, K., & Anand Mahesh, P. (2022). Impact of Acute Exacerbation and Its Phenotypes on the Clinical Outcomes of Chronic Obstructive Pulmonary Disease in Hospitalized Patients: A Cross-Sectional Study. Toxics, 10(11), 667. https://doi.org/10.3390/toxics10110667









