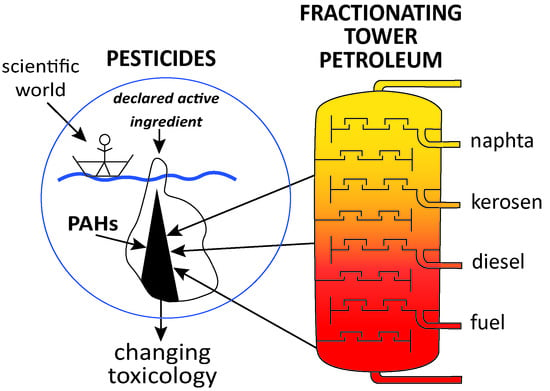
Petroleum in Pesticides: A Need to Change Regulatory Toxicology
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Analyses of PAHs
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Nelson, F.C.; Fiero, G.W. Pesticides formulations, a selected aromatic fraction naturally occurring in petroleum as a pesticide solvent. J. Agri. Food Chem. 1954, 2, 735–737. [Google Scholar] [CrossRef]
- Carson, R. Silent Spring; Mifflin, H., Ed.; Houghton Mifflin Harcourt: Boston, MA, USA, 1962. [Google Scholar]
- Seralini, G.E.; Douzelet, J. The Monsanto Papers, Corruption of Science and Grievous Harm to Public Health; Skyhorse: New York, NY, USA, 2022. [Google Scholar]
- Gad, S.C. Petroleum Hydrocarbons. Encyclopaedia of Toxicology; Elsevier: Amsterdam, NL, USA, 2014. [Google Scholar]
- Colwill, D. Oils in Pesticidal Operations. Int. J. Pest Manag. A 1957, 3, 88–98. [Google Scholar] [CrossRef]
- Melnikov, N. Pesticide formulations. In Chemistry of Pesticides; Springer: Berlin, Germany, 1971; pp. 12–25. [Google Scholar]
- Goeze, J.A.E. Geschichte Einiger, Den Menschen, Thieren, Oekonomie und Gärtnerey Schädlichen Jnsekten: Nebst den Besten Dieselben; Weidmanns Erben und Reich: Leipzig, Germany, 1787. [Google Scholar]
- Eaton, J.K.; Davies, R.G. The toxicity of certain synthetic organic compounds to the fruit-tree red-spider mite. Ann. Appl. Biol. 1950, 37, 471–489. [Google Scholar] [CrossRef]
- Hueper, W. Experimental studies on Cancerigenesis of synthetic liquid Fuels and Petroleum substitutes. Arch. Indust. Hyg. Occup. Med. 1953, 8, 307–327. [Google Scholar]
- Colwill, D. Glossary of Current Surfactants. Int. J. Pest. Manag. A 1957, 3, 100–126. [Google Scholar] [CrossRef]
- Falk, H.L.; Thompson, S.J.; Kotin, P. Carcinogenic potential of pesticides. Arch. Environ. Health 1965, 10, 847–858. [Google Scholar] [CrossRef]
- Eaton, J.K. The Role of Petroleum Oils in the Effective and safe application of Pesticides. In Proceedings of the 7th World Petroleum Congress, Mexico City, Mexico, 2 April 1967. [Google Scholar]
- Wodagenesh, A. The Addition of Oils to Pesticide Formulations in Spraying. Ph.D. Thesis, Imperial College of Science and Technology, London, UK, 1980. [Google Scholar]
- Curcio, L.N. Recent Progress and Issues on Inerts Testing; ASTM International: Philadelphia, PA, USA, 1990. [Google Scholar]
- Nielsen, D.G. Developing Biorational Pesticides for the Landscape Industry; Ornamental. Plants; Ohio State Univ: Wooster, OH, USA, 1990; p. 45. [Google Scholar]
- Zabkiewicz, J.; Beattie, G.A.C.; Watson, D.M.; Stevens, M.L.; Rae, D.J.; Spooner-Hart, R.N. Enhancement of Pesticide Activity by Oil Adjuvants in Spray Oils Beyond 2000: Sustainable Pest and Disease Management; G.A.C. Beattie’s Research; West. Aust. Univ Sydney: Sydney, Australia, 2002; pp. 52–61. [Google Scholar]
- Agnello, A.M. Petroleum-derived spray oils: Chemistry, history, refining and formulation. In Spray Oils Beyond 2000; G.A.C. Beattie’s Research; West. Aust. Univ Sydney: Sydney, Australia, 2002; pp. 2–18. [Google Scholar]
- Richard, S.; Moslemi, S.; Sipahutar, H.; Benachour, N.; Seralini, G.E. Differential effects of glyphosate and roundup on human placental cells and aromatase. Environ. Health Perspect. 2005, 113, 716–720. [Google Scholar] [CrossRef] [Green Version]
- Bogran, C.E.; Ludwig, S.; Metz, B. Using oils as pesticides. In Texas Farmer Collection; Texas Agrilife Extension: Tamu, TX, USA, 2006. [Google Scholar]
- Buteler, M.; Stadler, T. A review on the mode of action and current use of petroleum distilled spray oils. In Pesticides in the Modern World; Intech Open: London, UK, 2011. [Google Scholar]
- Mesnage, R.; Bernay, B.; Seralini, G.E. Ethoxylated adjuvants of glyphosate-based herbicides are active principles of human cell toxicity. Toxicology 2013, 313, 122–128. [Google Scholar] [CrossRef]
- Defarge, N.; Takacs, E.; Lozano, V.L.; Mesnage, R.; Spiroux de Vendomoix, J.; Seralini, G.E.; Szekacs, A. Co-formulants in glyphosate-based herbicides disrupt aromatase activity in human cells below toxic levels. Int. J. Environ. Res. Public Health 2016, 13, 264. [Google Scholar] [CrossRef] [Green Version]
- Seralini, G.E.; Jungers, G. Toxic Compounds in herbicides without glyphosate. Food Chem. Tox. 2020, 146, 111770. [Google Scholar] [CrossRef]
- EFSA. Data collection on co-formulants used in representative plant protection product formulations in the context of the EFSA peer review process for approval/renewal of approval of active substances. Tech. Rep. 2022. [Google Scholar] [CrossRef]
- Yunker, M.B.; Macdonald, R.W.; Vingarzan, R.; Mitchell, R.H.; Goyette, D.; Sylvestre, S. PAHs in the Fraser River basin: A critical appraisal of PAH ratios as inicators of PAH source and composition. Org. Geochem. 2002, 33, 489–515. [Google Scholar] [CrossRef]
- Liu, Q.; Xia, C.; Wang, L.; Tang, J. Fingerprint analysis reveals sources of petroleum hydrocarbons in soils of different geographical oilfields of China and its ecological assessment. Sci. Rep. 2022, 12, 4808. [Google Scholar] [CrossRef] [PubMed]
- Halfadji, A.; Touabet, A.; Portet-Koltalo, F.; Le Derf, F.; Merlet-Machour, N. Concentrations and source identification of polycyclic aromatic hydrocarbons (PAHs) and polychlorinated biphenyls (PCBs) in agricultural, urban/residential, and industrial soils, east of Oran (Northwest Algeria). Polycyclic. Arom. Comp. 2019, 39, 299–310. [Google Scholar] [CrossRef]
- Evans, M.; Davies, M.; Janzen, K.; Muir, D.; Hazewinkel, R.; Kirk, J.; de Boer, D. PAH distributions in sediments in the oil sands monitoring area and western Lake Athabasca: Concentration, composition and diagnostic ratios. Environ. Pollut. 2016, 213, 671–687. [Google Scholar] [CrossRef] [PubMed]
- Tobiszewski, M.; Namiesnik, J. PAH diagnostic ratios for the identification of pollution emission sources. Environ. Pollut. 2012, 162, 110–119. [Google Scholar] [CrossRef]
- Portet-Koltalo, F.; Machour, N. Analytical Methodologies for the Control of Particle-Phase Polycyclic Aromatic Compounds from Diesel Engines Exhausts; Bari, S., Ed.; Intech Open: London, UK, 2012. [Google Scholar]
- Tush, D.; Loftin, K.A.; Meyer, M.T. Characterization of polyoxyethylene tallow amine surfactants in technical mixtures and glyphosate formulations using ultra-high performance liquid chromatography and triple quadrupole mass spectrometry. J. Chrom. A 2013, 1319, 80–87. [Google Scholar] [CrossRef]
- Motelay-Massei, A.; Ollivon, D.; Garban, B.; Tiphagne-Larcher, K.; Zimmerlin, I.; Chevreuil, M. PAHs in the bulk atmospheric deposition of the Seine river basin: Source identification and apportionment by ratios, multivariate statistical techniques and scanning electron microscopy. Chemosphere 2007, 67, 312–321. [Google Scholar] [CrossRef]
- Defarge, N.; de Vendômois, J.S.; Seralini, G.E. Toxicity of formulants and Heavy metals in glyphosate-based herbicides and other pesticides. Tox. Rep. 2018, 5, 156–163. [Google Scholar] [CrossRef]
- Seralini, G.E.; Douzelet, J.; Jungers, G. Detection of Pollutants in Organic and Non-Organic Food: Are PAHs Coming from Pesticides? Food Nutr. 2022, J7, 238. [Google Scholar]
- Seralini, G.E.; Jungers, G. Endocrine disruptors also function as nervous disruptors and can be renamed endocrine and nervous disruptors (ENDs). Tox. Rep. 2021, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Perono, G.A.; Petrik, J.J.; Thomas, P.J.; Holloway, A.C. The effects of polycyclic aromatic compounds on mammalian ovarian function. Curr. Res. Tox. 2022, 3, 100070. [Google Scholar] [CrossRef] [PubMed]
- Priyadarshanee, M.; Mahto, U.; Das, S. Mechanism of toxicity and adverse health effects of environmental pollutants. Microb. Biodegr. Biorem. 2022, 33–53. [Google Scholar] [CrossRef]


| Year | Group | Comment on Petroleum Oil Products: | References |
|---|---|---|---|
| 1787 | Colonial pesticides information, UK | Used as an insecticide as long ago as 1787 | Goeze [7], cited in Colwill [5], 1957 |
| 1950 | Shell Research, UK | Insecticidal from 0.1–3% at least | Eaton and Davis [8] |
| 1953 | National Cancer Institute USA | Crude and processed oils possess carcinogenic properties | Hueper [9] |
| 1954 | Esso Standard Oil USA | Excellent solvent for pesticide formulations | Nelson and Fiero [1] |
| 1957 | Colonial Pesticides Information Service UK | Frequently employed with insecticidal and fungicidal agents–herbicidal activity increases with the aromaticity of the oil–solvent carriers of pesticides | Colwill [10] |
| 1965 | USA Public Health Service | Carcinogenic potential of pesticides | Falk et al. [11] |
| 1967 | Shell Research Ltd. UK | Petroleum blended with pesticides | Eaton [12] |
| 1971 | University of Moscow, USSR | The successful use of pesticides depends to a large degree on the formulation, mainly with petroleum products | Melnikov [6] |
| 1980 | University of London, UK | The addition of oil gives greater efficiency of active ingredient | Wodagenesh [13] |
| 1990 | American Society for Testing and Materials, Philadelphia, USA | Petroleum solvents are used as inerts in pesticide formulations | Curcio [14] |
| 1990 | Ohio State University USA | Horticultural (petroleum) oils in combination with insecticides have been used for decades | Nielsen [15] |
| 2002 | Forest Research Australia | Enhancement of pesticide activity by oil adjuvants Necessity of global evaluation | Zabkiewicz [16] |
| 2002 | Agricultural Experiment Station, New York USA | Petroleum distilled oils used for pest control over a century | Agnello [17] |
| 2005 | University of Caen Normandy, France | Toxicity and endocrine disruption of glyphosate amplified at least 100 times by formulations | Richard et al. [18] |
| 2006 | Texas AgriLife Extension, USA | Oils used as pesticides for centuries, include distillation products from petroleum | Bogran et al. [19] |
| 2011 | Toxicology Argentina | Mode of action of petroleum oils as pesticides | Buteler and Stadler [20] |
| 2013 | University of Caen Normandy, France | Polyethoxylated-petroleum derived products toxic in pesticides | Mesnage et al. [21] |
| 2016 | University of Caen Normandy, France | Endocrine disruption and human cell toxicity by pesticide co-formulants | Defarge et al. [22] |
| 2020 | University of Caen Normandy, France | Oil residues in herbicides without glyphosate | Seralini and Jungers [23] |
| 2022 | European Union | Petroleum is a co-formulant of pesticides | EFSA [24] |
| Sample | Herbicide Name | Made Date | Declared Ingredient | % | Authorization | Holder | Provider | Lot Number |
|---|---|---|---|---|---|---|---|---|
| A | Roundup Speed-Evergreen Monsanto | 2018 | Acetic acid | 6 | 2130153 | Monsanto technology LLC | Evergreen Garden Care France SAS (69) | C8N515 |
| B | Fertiligene-Herbatak Contact Scotts | 2018 | 6 | 2130153 | SCOTTS France SAS | SCOTTS FRANCE SAS (69) | 338 18 07:16L23 | |
| C | Biocontrole Jardin d’Eden-Starnet Jade | 2018 | Pelargonic acid | 51.9 | 2170243 n°CAS 112-05-0 | JADE | START (37) JE_DBIO250 | V050CB |
| D | Fertiligene-Herbatak Express Scotts Jade | 2018 | 51.9 | 2170243 | JADE | SCOTTS France | 353 18 08:14 L25 | |
| E1 | Clairland-Herbistop Compo | 2018 | 24.3 | 2140121 | COMPO France SAS | COMPO France SAS | 21/11/2018/A | |
| E2 | 2019 | 04/04/19/A | ||||||
| E3 | 2019 | 17/9/2019/A | ||||||
| F | Clairland express-Herbistop spray | 2019 | 3.1 | 2160115 | COMPO France SAS | COMPO France SAS | 190508 | |
| G | Solabiol-Beloukha Garden | 2019 | 51.9 | 2170243 | JADE | SBM Life Sciences SAS | 19031 | |
| H | Neudorff-Finalsan | 2018 | 18.8 | 2170355 CAS 112-05-0 | W.Neudorff GmbH KG | Or Brun (85) | 11806086 | |
| I | Roundup-Unkrautfrei Germany | 2019 | 51.92 | Nr 008529-62 | Belchim Crop Protection NV | Evergreen Garden Care Deutschland | NR.008529-62 1088/3285-CLP 12892398 C9N907 | |
| J | Target-Poland | 2017 | 71.7 | MRiRW nrR-140/2017 | Belchim Crop Protection | Target SA | MriRWnrR-140/2017 z | |
| K | Compo-Poland | 2016 | 24.26 | MRiRW nrR-34/2016 | COMPO GmbH | COMPO Polska | MRiRW nr R-34/2016 wu z | |
| L | Solabiol-Herbiclean | 2018 | Caprylic and Capric acids | 3 | 2140167 | SBM Développement SAS | SBM Life Sciences SAS | LOT 147MC38 |
| M | Solabiol-Herbiclean | 2015 | 1.8 + 1.2 | 2140167 | SBM Développement SAS | SBM Life Sciences SAS | LOT 43135 | |
| N | Bros-Poland | 2019 | Benzalkonium Chloride | 1.25 | 1000/04 | BROS Sp. Zo.o. sp.k. Polska | BROS Sp. Zo.o. sp.k. Polska | DW/EXP 03 2022 UFI: 2JFA-40VN-G008-8JWH |
| O | Domodev | <2008 | Glyphosate | 36 | 9900028 | Domodev | Domodev | |
| P | Burren | 1985 | 36 | Barclay chemicals LTD Dublin | BHS | |||
| Q | Roundup 6H | <2000 | Glyphosate + Pelargonic acid | 0.72 + 0.204 | 2120157 | Monsanto | SCOTT France SAS | C4001 B1001 |
| R | KB desherbant liquide | <2010 | Glufosinate | 6 | 8900339 | Hoechst | Rhone poulenc | 0 05 V 136 |
| S | Cora desherbant gazon | <2000 | Mecoprop.P+2.4MCPA+Dicamba | 20 + 10 + 2.4 | 9000662 | SCOTTS France SAS | CORA | |
| T | Round up express | <2010 | Glyphosate | 7.2 | 2010321 | Monsanto agriculture France SAS | SCOTT France SAS | C3029 B071P |
| U | Burren | <2010 | 36 | 2000499 | Barclay chemicals LTD Dublin | BHS | PG-BN.448843.SEG | |
| V | STARANE 200 | <2010 | Fluoroxypyr | 20 | 8400600 | DOW agro sciences SAS | DOW Agro sciences | |
| W | Likid allees | <2015 | Glyphosate+Diflufenicanil | 25 | 9800107 | SCOTTS France SAS | Fertiligene |
| Compounds/ | Chemical Structure | Number of Cycles | Molecular Mass | Boiling Point (°C) | Vapour Pressure | Log Kow | Aqueous Solubility (25 °C) (mg/ L) | MaxPAHs | Toxicity PAHs Standards * | Max in | Max/ Standard * |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Abbreviations | (g/mol) | (Pa at 25 °C) | (µg/L) | (µg/kg) | Samples * | ||||||
| Naphthalene |  | 2 | 128.2 | 218 | 10.4 | 3.4 | 31.7 | 450 | 40, K | E2 | 11.25 |
| NAPH | |||||||||||
| Acenaphtylene |  | 3 | 152.2 | 280 | 0.89 | 4.07 | - | 653.4 | 10 | O | 65.34 |
| ACY | |||||||||||
| Acenaphthene |  | 3 | 154.2 | 279 | 0.29 | 3.92 | 3.9 | 130 | 60 | E2 | 2.16 |
| ACE | |||||||||||
| Fluorene |  | 3 | 166.2 | 295 | 0.08 | 4.18 | 1.68 | 2200 | 40, K | E1 | 55 |
| FLUO | |||||||||||
| Anthracene |  | 3 | 178.2 | 340 | 8.0 10−4 | 4.5 | 0.073 | 883.3 | 40 | V | 22.08 |
| ANT | |||||||||||
| Phenanthrene |  | 3 | 178.2 | 340 | 0.016 | 4.52 | 1.29 | 1046.7 | 20, K? | O | 52.33 |
| PHEN | |||||||||||
| Fluoranthene |  | 4 | 202.3 | 375 | 0.00123 | 5.20 | 0.26 | 828.8 | 0.1, K | O | 8288 |
| FLT | |||||||||||
| Pyrene |  | 4 | 202.3 | 404 | 0.0006 | 5.18 | 0.135 | 411.5 | 30, K? | S | 13.71 |
| PYR | |||||||||||
| Chrysene |  | 4 | 228.3 | 438 | - | 5.86 | 0.00179 | 540 | 50, K | E2 | 10.8 |
| CHRYS | |||||||||||
| Benz[a] |  | 4 | 228.3 | 448 | 2.8 10−5 | 5.61 | 0.014 | 67 | 0.01, K | K | 6700 |
| Anthracene | |||||||||||
| B[a]ANT | |||||||||||
| Benzo[b] |  | 5 | 252.3 | 481 | - | 5.78 | 0.0015 | 97.9 | 0.1, K | O | 979 |
| Fluoranthene | |||||||||||
| B[b]FLT | |||||||||||
| Benzo[k] |  | 5 | 252.3 | 480 | - | 6.11 | 0.0008 | 91.4 | 1, K | W | 91.4 |
| Fluoranthene | |||||||||||
| B(k)FLT | |||||||||||
| Benzo[a]pyrene |  | 5 | 252.3 | 495 | 7.3 10−7 | 6.50 | 0.004 | 45 | 0.01, K | E2 | 4500 |
| B[a]PYR | |||||||||||
| Benzo[g,h,i] |  | 6 | 276.3 | 536 | 1.4 10−8 | 7.10 | 0.00026 | 48 | 0.1, K | E2 | 480 |
| Perylene | |||||||||||
| B(ghi)PER | |||||||||||
| Indeno[1,2,3-cd] |  | 6 | 276.3 | 524 | - | - | 0.00019 | 68 | 0.2, K | E2 | 340 |
| Pyrene | |||||||||||
| InPYR | |||||||||||
| Dibenz[a,h] |  | 5 | 278.4 | 550 | - | 6.75 | 0.00050 | 33 | 0.5, K | E2 | 66 |
| Anthracene | |||||||||||
| DB[ah]ANT |
| Compounds/ | A | B | C | D | E1 | E2 | E3 | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abbreviations | |||||||||||||||||||||||||
| Naphthalene | 2.1 | <5 | 24.0 | <2 | 11.0 | 450.0 | <22 | <10 | <2 | 25.0 | <2 | <2 | <10 | <10 | <10 | 69.0 | <22 | 42.3 | <4.4 | <22 | <2.2 | <2.2 | <2.2 | <2.2 | <22 |
| NAPH | |||||||||||||||||||||||||
| Acenaphtylene | <5 | <5 | <15 | <2 | <10 | 210.0 | 22.3 | <10 | <2 | <5 | <2 | <2 | 10.0 | <10 | <10 | <3 | 653.4 | 88.8 | 131.1 | <36.5 | 15.3 | <3.7 | <3.7 | 54.9 | 30.8 |
| ACY | |||||||||||||||||||||||||
| Acenaphthene | <5 | <5 | 88.0 | <2 | 29.0 | 130.0 | <3.1 | <10 | <2 | <5 | <2 | <2 | <2 | <10 | <10 | 3.0 | <31 | <6.2 | <6.2 | <31 | <3.1 | <3.1 | 7.5 | <3.1 | <31 |
| ACE | |||||||||||||||||||||||||
| Fluorene | 2.2 | 5.0 | 660.0 | 3.3 | 2200.0 | 54.0 | <3.4 | <10 | 3.3 | <5 | 12.0 | 21.0 | 2100.0 | <10 | <10 | <3 | 197 | 25.2 | <6.8 | <34 | 11.7 | <3.4 | 50.6 | 2.2 | 179.4 |
| FLUO | |||||||||||||||||||||||||
| Anthracene | 2.9 | <5 | 20.0 | <2 | 16.0 | 37.0 | <35.5 | <10 | <2 | <5 | <2 | 6.0 | 11.0 | <10 | <10 | <3 | 91.1 | 16.5 | 4.9 | <35.5 | 7.2 | 8.8 | 10.5 | 883.3 | 34.2 |
| ANT | |||||||||||||||||||||||||
| Phenanthrene | 16.0 | 10.0 | 76.0 | 30.0 | 68.0 | 56.0 | <27.5 | 26.0 | 5.3 | 8.4 | 16.0 | 49.0 | 71.0 | <10 | <10 | 6.0 | 1046.7 | 238.9 | 109.6 | 265.8 | 71.1 | 31.9 | 240 | 8.5 | 216.7 |
| PHEN | |||||||||||||||||||||||||
| Fluoranthene | 11.0 | 7.1 | <15 | 14.0 | 46.0 | 30.0 | <23.5 | 20.0 | 5.5 | <5 | 8.9 | 3.8 | 55.0 | <10 | <10 | <3 | 828.8 | 46.1 | 157 | <23.5 | 50.7 | 53.9 | 95.6 | 18.9 | 442.4 |
| FLT | |||||||||||||||||||||||||
| Pyrene | 8.7 | 6.0 | <15 | 4.0 | 33.0 | <15 | <34 | 17.0 | 2.9 | <5 | 6.1 | <2 | 26.0 | <10 | <10 | <3 | 111.6 | 18.1 | 17.8 | <34 | 411.5 | <3.4 | 6.2 | 0.6 | <34 |
| PYR | |||||||||||||||||||||||||
| Chrysene | 5.6 | <5 | <15 | 9.6 | 19.0 | 540.0 | <81 | 10.0 | 4.7 | <5 | <2 | 11.0 | 28.0 | <10 | <10 | <3 | <81 | <16.2 | <16.2 | <81 | <8.1 | <8.1 | <8.1 | <8.1 | <81 |
| CHRYS | |||||||||||||||||||||||||
| Benz[a] | <5 | <5 | <15 | 2.5 | 39.0 | 35.0 | <88 | 11.0 | 4.00 | <5 | <2 | 11.0 | 67.0 | <10 | <10 | <3 | <88 | <17.6 | <17.6 | <88 | <8.8 | <8.8 | <8.8 | <8.8 | <88 |
| Anthracene | |||||||||||||||||||||||||
| B[a]ANT | |||||||||||||||||||||||||
| Benzo[b] | <5 | <5 | <15 | 9.3 | 17.0 | 32.0 | <133 | <10 | 6.00 | <5 | <2 | 16.0 | 22.0 | <10 | <10 | <3 | 97.9 | 5.5 | 4.3 | <133 | <13.3 | <13.3 | <13.3 | <13.3 | 97.6 |
| Fluoranthene | |||||||||||||||||||||||||
| B(b)FLT | |||||||||||||||||||||||||
| Benzo[k] | <5 | <5 | <15 | <2 | <10 | <15 | <154 | <10 | <2 | <5 | <2 | 9.1 | <10 | <10 | <10 | <3 | <1540 | 6.6 | <30.8 | <154 | <15.4 | <15.4 | <15.4 | <15.4 | 91.4 |
| Fluoranthene | |||||||||||||||||||||||||
| B(k)FLT | |||||||||||||||||||||||||
| Benzo[a]pyrene | 6.4 | <5 | <15 | 2.3 | 16.0 | 45.0 | <409 | <10 | <2 | <5 | <2 | <2 | 17.0 | <10 | <10 | <3 | <409 | <81.8 | <81.8 | <409 | <40.9 | <40.9 | <40.9 | <40.9 | <409 |
| B(a)PYR | |||||||||||||||||||||||||
| Benzo[g,h,i] | <5 | <5 | <15 | <2 | 11.0 | 48.0 | <1500 | <10 | <2 | <5 | <2 | <2 | 12.0 | <10 | <10 | <3 | <1500 | <300 | <3 00 | <1500 | <150 | <150 | <150 | <150 | <1500 |
| Perylene | |||||||||||||||||||||||||
| B(ghi)PER | |||||||||||||||||||||||||
| Indeno[1,2,3-cd] | <5 | <5 | <15 | <2 | 11.0 | 68.0 | <1800 | <10 | <2 | <5 | <2 | <2 | 11.0 | <10 | <10 | <3 | <1800 | <360 | <360 | <1800 | <1800 | <180 | <180 | <180 | <1800 |
| Pyrene | |||||||||||||||||||||||||
| InPYR | |||||||||||||||||||||||||
| Dibenz[a,h] | <5 | <5 | <15 | <2 | <10 | 33.0 | <1800 | <10 | <2 | <5 | <2 | <2 | <10 | <10 | <10 | <3 | <1800 | <360 | <360 | <1800 | <180 | <180 | <180 | <180 | <1800 |
| Anthracene | |||||||||||||||||||||||||
| DB[ah]ANT | |||||||||||||||||||||||||
| Total PAHs | 54.9 | 28.1 | 868.0 | 75.0 | 2516.0 | 1768.0 | 22.3 | 84.0 | 31.7 | 33.4 | 43.0 | 126.9 | 2430.0 | <10 | <10 | 78.0 | 3026.5 | 488.0 | 424.7 | 265.8 | 567.5 | 94.6 | 410.4 | 968.4 | 1092.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jungers, G.; Portet-Koltalo, F.; Cosme, J.; Seralini, G.-E. Petroleum in Pesticides: A Need to Change Regulatory Toxicology. Toxics 2022, 10, 670. https://doi.org/10.3390/toxics10110670
Jungers G, Portet-Koltalo F, Cosme J, Seralini G-E. Petroleum in Pesticides: A Need to Change Regulatory Toxicology. Toxics. 2022; 10(11):670. https://doi.org/10.3390/toxics10110670
Chicago/Turabian StyleJungers, Gérald, Florence Portet-Koltalo, Julie Cosme, and Gilles-Eric Seralini. 2022. "Petroleum in Pesticides: A Need to Change Regulatory Toxicology" Toxics 10, no. 11: 670. https://doi.org/10.3390/toxics10110670