Toxicokinetics, Percutaneous Absorption and Tissue Distribution of Benzophenone-3, an UV Filtering Agent, in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. HPLC-UV and LC-MS/MS Analysis
2.3. In Vitro Skin Permeability
2.4. In Vivo Toxicokinetics
3. Results
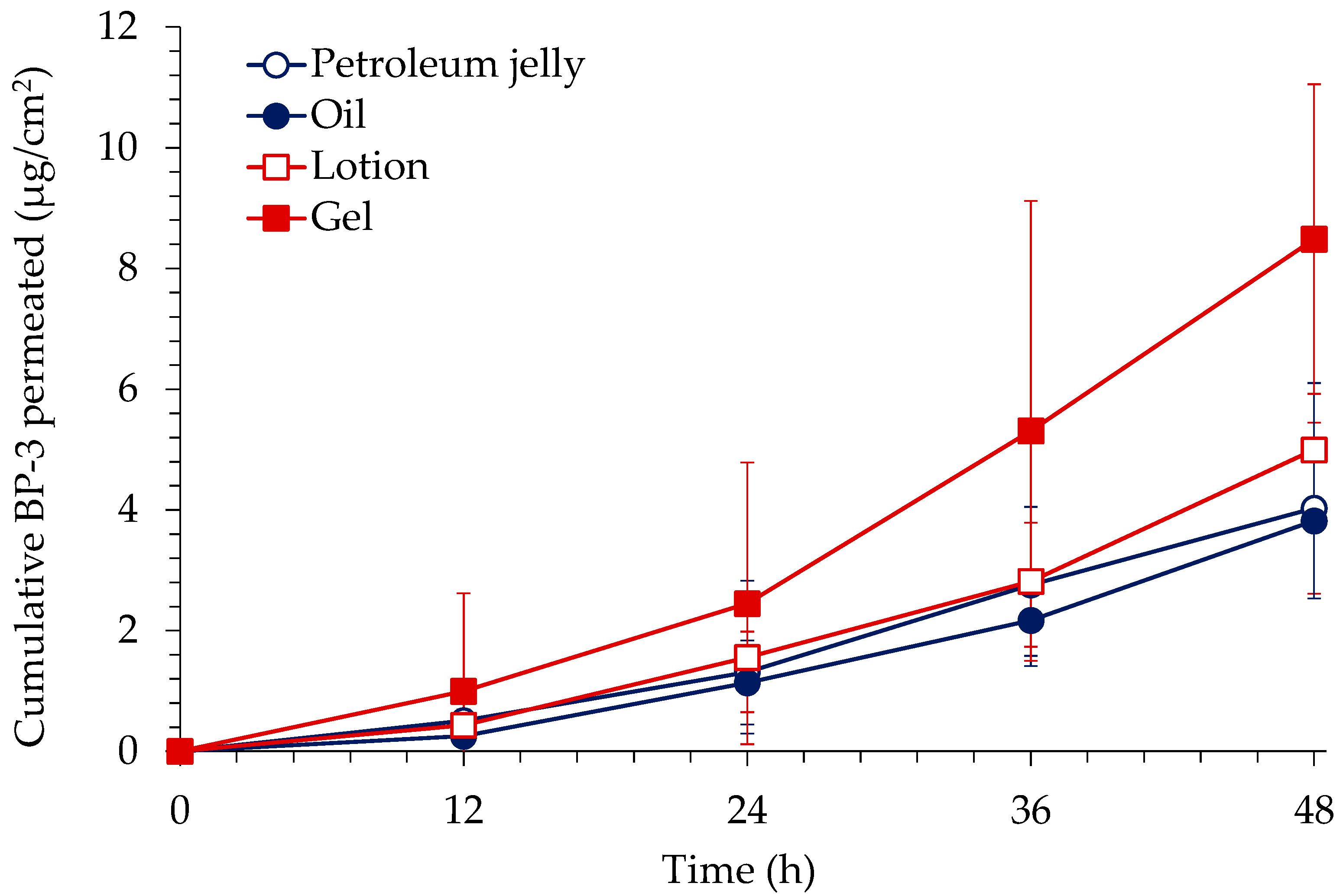
3.1. In Vitro Skin Permeation of BP-3
3.2. In Vivo Percutaneous Absorption
3.3. Tissue Distribution
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Christian, M. Final report on the safety assessment of benzophenones-1,-3,-4,-5,-9, and-11. J. Am. College Toxicol 1983, 2, 35–73. [Google Scholar]
- Clydesdale, G.J.; Dandie, G.W.; Muller, H.K. Ultraviolet light induced injury: Immunological and inflammatory effects. Immunol. Cell Biol. 2001, 79, 547–568. [Google Scholar] [CrossRef]
- De Gruijl, F.R. Photocarcinogenesis: UVA vs. UVB radiation. Ski. Pharmacol. Physiol. 2002, 15, 316–320. [Google Scholar]
- Kaidbey, K.; Gange, R.W. Comparison of methods for assessing photoprotection against ultraviolet A in vivo. J. Am. Acad. Dermatol. 1987, 16, 346–353. [Google Scholar] [CrossRef]
- Gonzalez, H.; Farbrot, A.; Larkö, O.; Wennberg, A.M. Percutaneous absorption of the sunscreen benzophenone-3 after repeated whole-body applications, with and without ultraviolet irradiation. Br. J. Dermatol. 2006, 154, 337–340. [Google Scholar] [CrossRef]
- Hayden, C.G.J.; Cross, S.E.; Anderson, C.; Saunders, N.A.; Roberts, M.S. Sunscreen penetration of human skin and related keratinocyte toxicity after topical application. Ski. Pharmacol. Physiol. 2005, 18, 170–174. [Google Scholar] [CrossRef]
- Sarveiya, V.; Risk, S.; Benson, H.A. Liquid chromatographic assay for common sunscreen agents: Application to in vivo assessment of skin penetration and systemic absorption in human volunteers. J. Chromatogr. B 2004, 803, 225–231. [Google Scholar] [CrossRef]
- The Society of Japanese Pharmacopoeia. Japanese standard of cosmetic ingredients, 2nd ed.; Yakuji Nippo Ltd.: Tokyo, Japan, 1985. [Google Scholar]
- U.S. FDA Department of Health and Human Services. Sunscreen Drug Products for Over-the-Counter Human Use; 21 CFR, Subchapter D, part 352; U.S. FDA Department of Health and Human Services: Spring, MD, USA, 2013.
- European Commission (EC). Council Directive 83/574/EEC; European Commission (EC): Brussels, Belgium, 1983. [Google Scholar]
- Korea Food and Drug Administration. Regulation on the Designation of Raw Materials for Cosmetics; Korea Food and Drug Administration: Cheongju, Korea, 2012; Volume 137.
- Fent, K.; Kunz, P.Y.; Gomez, E. UV filters in the aquatic environment induce hormonal effects and affect fertility and reproduction in fish. CHIMIA Int. J. Chem. 2008, 62, 368–375. [Google Scholar]
- Heneweer, M.; Muusse, M.; van den Berg, M.; Sanderson, J.T. Additive estrogenic effects of mixtures of frequently used UV filters on pS2-gene transcription in MCF-7 cells. Toxicol. Appl. Pharmacol. 2005, 208, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Kunz, P.Y.; Fent, K. Estrogenic activity of UV filter mixtures. Toxicol. Appl. Pharmacol. 2006, 217, 86–99. [Google Scholar] [CrossRef]
- Schreurs, R.; Lanser, P.; Seinen, W.; van der Burg, B. Estrogenic activity of UV filters determined by an in vitro reporter gene assay and an in vivo transgenic zebrafish assay. Arch. Toxicol. 2002, 76, 257–261. [Google Scholar] [CrossRef]
- Sieratowicz, A.; Kaiser, D.; Behr, M.; Oetken, M.; Oehlmann, J. Acute and chronic toxicity of four frequently used UV filter substances for Desmodesmus subspicatus and Daphnia magna. J. Environ. Sci. Health A 2011, 46, 1311–1319. [Google Scholar] [CrossRef] [PubMed]
- Schlumpf, M.; Cotton, B.; Conscience, M.; Haller, V.; Steinmann, B.; Lichtensteiger, W. In vitro and in vivo estrogenicity of UV screens. Environ. Health Perspect. 2001, 109, 239–244. [Google Scholar] [CrossRef]
- Molina-Molina, J.M.; Escande, A.; Pillon, A.; Gomez, E.; Pakdel, F.; Cavaillès, V.; Balaguer, P. Profiling of benzophenone derivatives using fish and human estrogen receptor-specific in vitro bioassays. Toxicol. Appl. Pharmacol. 2008, 232, 384–395. [Google Scholar] [CrossRef]
- Schreurs, R.H.; Sonneveld, E.; Jansen, J.H.; Seinen, W.; van der Burg, B. Interaction of polycyclic musks and UV filters with the estrogen receptor (ER), androgen receptor (AR), and progesterone receptor (PR) in reporter gene bioassays. Toxicol. Sci. 2005, 83, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Zeiger, E.; Anderson, B.; Haworth, S.; Lawlor, T.; Mortelmans, K.; Speck, W. Salmonella mutagenicity tests: III. Results from the testing of 255 chemicals. Environ. Mutagenesis 1987, 9, 61–109. [Google Scholar] [CrossRef]
- French, J.E. NTP technical report on the toxicity studies of 2-Hydroxy-4-methoxybenzophenone (CAS No. 131-57-7) Administered Topically and in Dosed Feed to F344/N Rats and B6C3F1 Mice. Toxic. Rep. Ser. 1992, 21, 1-E14. [Google Scholar]
- Schlecht, C.; Klammer, H.; Jarry, H.; Wuttke, W. Effects of estradiol, benzophenone-2 and benzophenone-3 on the expression pattern of the estrogen receptors (ER) alpha and beta, the estrogen receptor-related receptor 1 (ERR1) and the aryl hydrocarbon receptor (AhR) in adult ovariectomized rats. Toxicology 2004, 205, 123–130. [Google Scholar] [CrossRef]
- Suzuki, T.; Kitamura, S.; Khota, R.; Sugihara, K.; Fujimoto, N.; Ohta, S. Estrogenic and antiandrogenic activities of 17 benzophenone derivatives used as UV stabilizers and sunscreens. Toxicol. Appl. Pharmacol. 2005, 203, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Blair, R.; Fang, H.; Branham, W.S.; Hass, B.; Dial, S.L.; Moland, C.L.; Tong, W.; Shi, L.; Perkins, R.; Sheehan, D.M. Estrogen receptor relative binding affinities of 188 natural and xenochemicals: Structural diversity of ligands. Toxicol. Sci. 2000, 54, 138–153. [Google Scholar] [CrossRef]
- National Toxicity Program. NTP Technical Report on the Toxicity Studies of 2-Hydroxy-4-Methoxylbenzophenone in F244/N Rats and B6C3F1 Mice (Dosed Feed and Dermal Studies); NTP. Tox. 21; NTP Research: Triangle Park, NC, USA, 1991. [Google Scholar]
- Treffel, P.; Gabard, B. Skin penetration and sun protection factor of ultra-violet filters from two vehicles. Pharm. Res. 1996, 13, 770–774. [Google Scholar] [CrossRef] [PubMed]
- Benson, H.A.; Sarveiya, V.; Risk, S.; Roberts, M.S. Influence of anatomical site and topical formulation on skin penetration of sunscreens. Ther. Clin. Risk Manag. 2005, 1, 209–218. [Google Scholar]
- Hayden, C.G.; Roberts, M.S. Systemic absorption of sunscreen after topical application. Lancet 1997, 350, 863–864. [Google Scholar] [CrossRef]
- Gustavsson Gonzalez, H.; Farbrot, A.; Larkö, O. Percutaneous absorption of benzophenone-3, a common component of topical sunscreens. Clin. Exp. Dermatol. 2002, 27, 691–694. [Google Scholar] [CrossRef]
- Janjua, N.R.; Mogensen, B.; Andersson, A.M.; Petersen, J.H.; Henriksen, M.; Skakkebæk, N.E.; Wulf, H.C. Systemic absorption of the sunscreens benzophenone-3, octyl-methoxycinnamate, and 3-(4-methyl-benzylidene) camphor after whole-body topical application and reproductive hormone levels in humans. J. Investig. Dermatol. 2004, 123, 57–61. [Google Scholar] [CrossRef]
- Matta, M.K.; Zusterzeel, R.; Pilli, N.R.; Patel, V.; Volpe, D.A.; Florian, J.; Oh, L.; Bashaw, E.; Zineh, I.; Sanabria, C.; et al. Effect of Sunscreen Application Under Maximal Use Conditions on Plasma Concentration of Sunscreen Active Ingredients: A Randomized Clinical Trial. JAMA 2019, 321, 2082–2091. [Google Scholar] [CrossRef]
- Matta, M.K.; Florian, J.; Zusterzeel, R.; Pilli, N.R.; Patel, V.; Volpe, D.A.; Yang, Y.; Oh, L.; Bashaw, E. Effect of Sunscreen Application on Plasma Concentration of Sunscreen Active Ingredients. JAMA 2020, 323, 256–267. [Google Scholar] [CrossRef]
- El Dareer, S.M.; Kalin, J.R.; Tillery, K.F.; Hill, D.L. Disposition of 2-hydroxy-4-methoxybenzophenone in rats dosed orally, intravenously, or topically. J. Toxicol. Environ. Health Part A Curr. Issues 1986, 19, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Kadry, A.M.; Okereke, C.S.; Abdel-Rahman, M.S.; Friedman, M.A.; Davis, R.A. Pharmacokinetics of benzophenone-3 after oral exposure in male rats. J. Appl. Toxicol. 1995, 15, 97–102. [Google Scholar] [CrossRef]
- Kasichayanula, S.; House, J.D.; Wang, T.; Gu, X. Percutaneous characterization of the insect repellent DEET and the sunscreen oxybenzone from topical skin application. Toxicol. Appl. Pharmacol. 2007, 223, 187–194. [Google Scholar] [CrossRef]
- Okereke, C.S.; Kadry, A.M.; Abdel-Rahman, M.S.; Davis, R.A.; Friedman, M.A. Metabolism of benzophenone-3 in rats. Drug Metab. Dispos. 1993, 21, 788–791. [Google Scholar] [PubMed]
- Kim, T.H.; Shin, B.S.; Kim, K.-B.; Shin, S.W.; Seok, S.H.; Kim, M.K.; Kim, E.J.; Kim, D.; Kim, M.G.; Park, E.-S.; et al. Percutaneous Absorption, Disposition, and Exposure Assessment of Homosalate, a UV Filtering Agent, in Rats. J. Toxicol Environ. Health A 2014, 77, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Organization for Economic Cooperation and Development. OECD Guideline for the Testing of Chemicals: Skin Absorption: In Vitro Method; OECD: Paris, France, 2004. [Google Scholar]


| Petroleum Jelly | Oil | Lotion | Gel |
|---|---|---|---|
| Benzophenone-3 (5%) | Benzophenone-3 (5%) | Benzophenone-3 (5%) | Benzophenone-3 (5%) |
| Petroleum jelly (95%) | Miglyol (18.7%) | Miglyol (18.7%) | Miglyol (18.7%) |
| Mineral oil (72.3%) | Marketed body emulsion (75.8%) | Carbomer 940 (1.1%) | |
| Bees wax (4.0%) | Surfactant (0.5%) | Poloxamer 188 (0.2%) | |
| EDTA Na (0.1%) | |||
| Methyl paraben (0.1%) | |||
| NaOH (10.0%) | |||
| Surfactant (0.5%) | |||
| DW (64.3%) |
| Formulation | Epidermis/Dermis (%) | Stratum Corneum (%) |
|---|---|---|
| Petroleum jelly | 2.1 ± 0.3 | 1.0 ± 0.5 |
| Oil | 2.1 ± 0.6 | 2.8 ± 1.6 |
| Lotion | 3.3 ± 0.9 | 3.4 ± 1.3 |
| Gel | 4.7 ± 1.0 | 17.0 ± 4.5 * |
| Parameters | i.v. (n = 4) | Transdermal (n = 5) |
|---|---|---|
| t1/2 (h) * | 3.1 ± 1.6 | 18.3 ± 5.8 |
| Tmax (h) | - | 4.8 ± 3.5 |
| C0 (ng/mL) | 577.8 ± 121.7 | - |
| Cmax (ng/mL) | - | 26.4 ± 4.6 |
| AUClast (ng·h/mL) | 208.2 ± 23.6 | 550 ± 155.4 |
| AUCinfinity (ng·h/mL) | 219.2 ± 25.9 | 781.4 ± 311.6 |
| F (%) | - | 6.9 ± 1.8 |
| Tissues | Concentration (ng/mL or ng/g) | kp |
|---|---|---|
| Plasma | 180.50 ± 52.29 | - |
| Kidney | 620.68 ± 297.70 | 4.00 ± 2.87 |
| Liver | 269.19 ± 52.14 | 1.54 ± 0.27 |
| Spleen | 155.71 ± 27.63 | 0.89 ± 0.13 |
| Lung | 710.00 ± 326.77 | 4.50 ± 2.87 |
| Heart | 363.14 ± 132.57 | 2.11 ± 0.87 |
| Testis | 136.74 ± 70.88 | 0.73 ± 0.16 |
| Stomach | 476.16 ± 99.72 | 2.70 ± 0.44 |
| Small intestine | 532.22 ± 296.66 | 2.92 ± 1.35 |
| Large intestine | 1174.44 ± 972.83 | 6.39 ± 5.77 |
| Brain | 352.75 ± 95.81 | 2.07 ± 0.81 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, W.; Seok, S.H.; Shin, S.; Ryu, S.H.; Kim, K.-B.; Shin, B.S.; Kim, T.H. Toxicokinetics, Percutaneous Absorption and Tissue Distribution of Benzophenone-3, an UV Filtering Agent, in Rats. Toxics 2022, 10, 672. https://doi.org/10.3390/toxics10110672
Jung W, Seok SH, Shin S, Ryu SH, Kim K-B, Shin BS, Kim TH. Toxicokinetics, Percutaneous Absorption and Tissue Distribution of Benzophenone-3, an UV Filtering Agent, in Rats. Toxics. 2022; 10(11):672. https://doi.org/10.3390/toxics10110672
Chicago/Turabian StyleJung, Woohyung, Su Hyun Seok, Soyoung Shin, Sung Ha Ryu, Kyu-Bong Kim, Beom Soo Shin, and Tae Hwan Kim. 2022. "Toxicokinetics, Percutaneous Absorption and Tissue Distribution of Benzophenone-3, an UV Filtering Agent, in Rats" Toxics 10, no. 11: 672. https://doi.org/10.3390/toxics10110672
APA StyleJung, W., Seok, S. H., Shin, S., Ryu, S. H., Kim, K.-B., Shin, B. S., & Kim, T. H. (2022). Toxicokinetics, Percutaneous Absorption and Tissue Distribution of Benzophenone-3, an UV Filtering Agent, in Rats. Toxics, 10(11), 672. https://doi.org/10.3390/toxics10110672








