Impact of Pesticides on Cancer and Congenital Malformation: A Systematic Review
Abstract
:1. Introduction
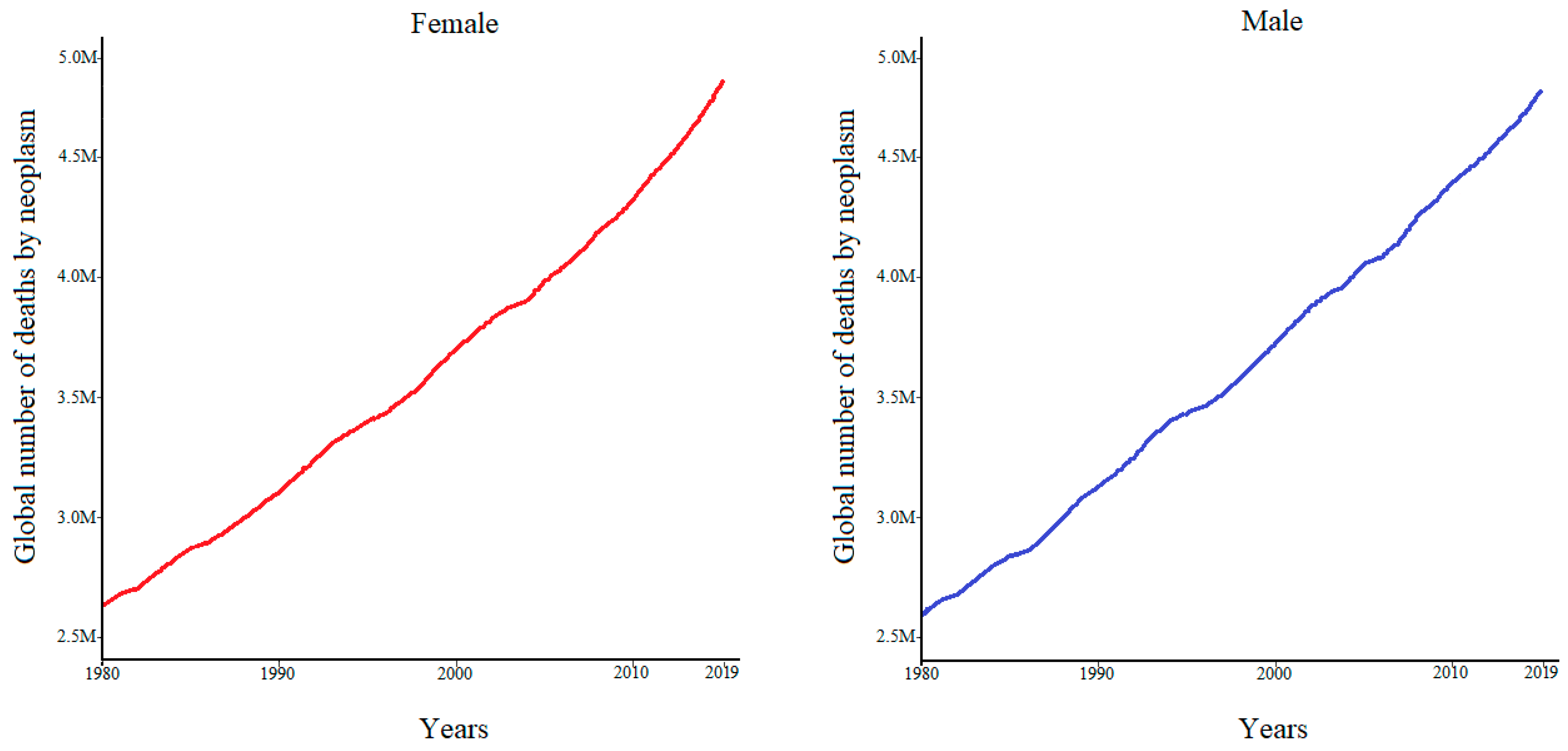
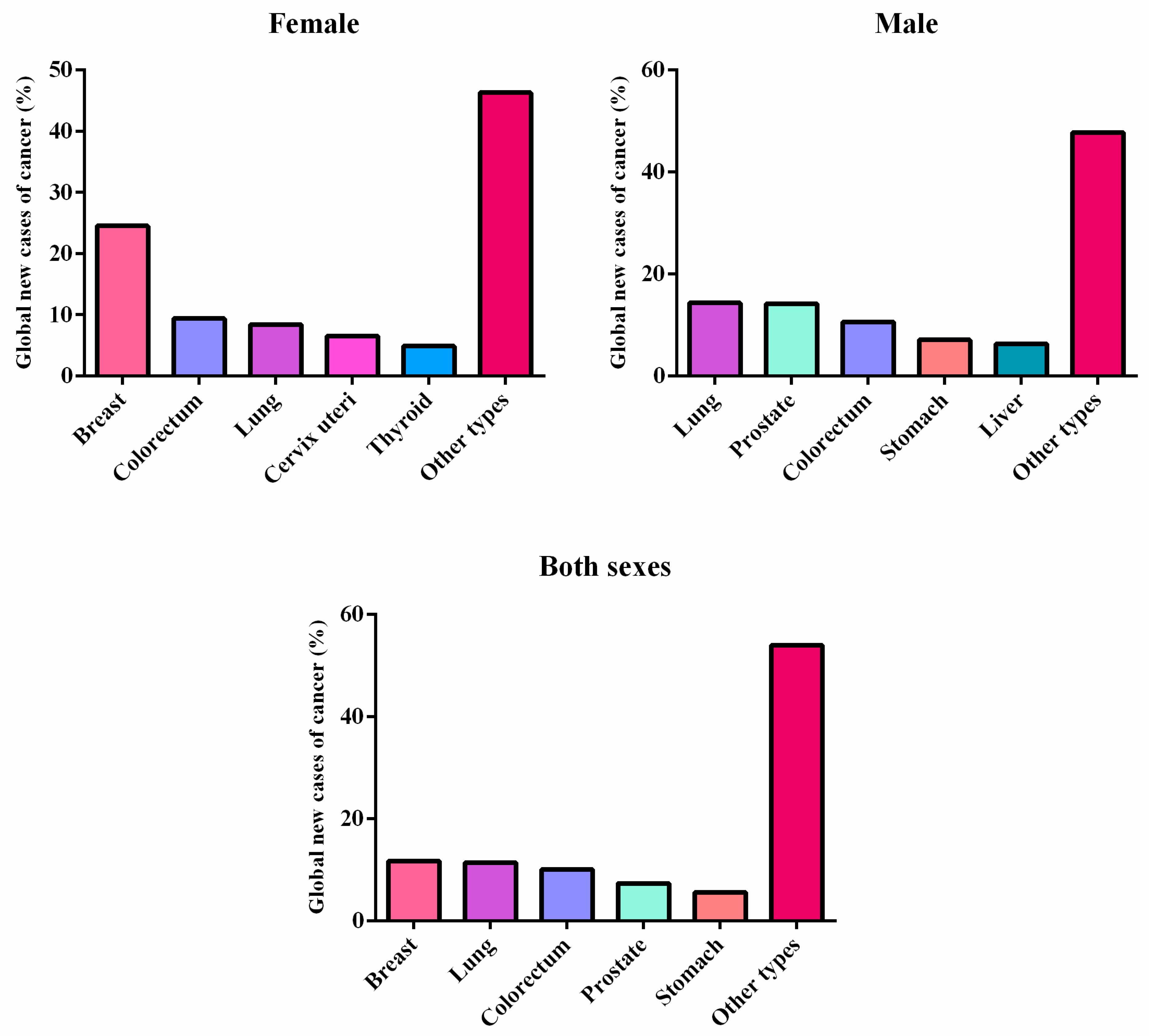
1.1. Cancer
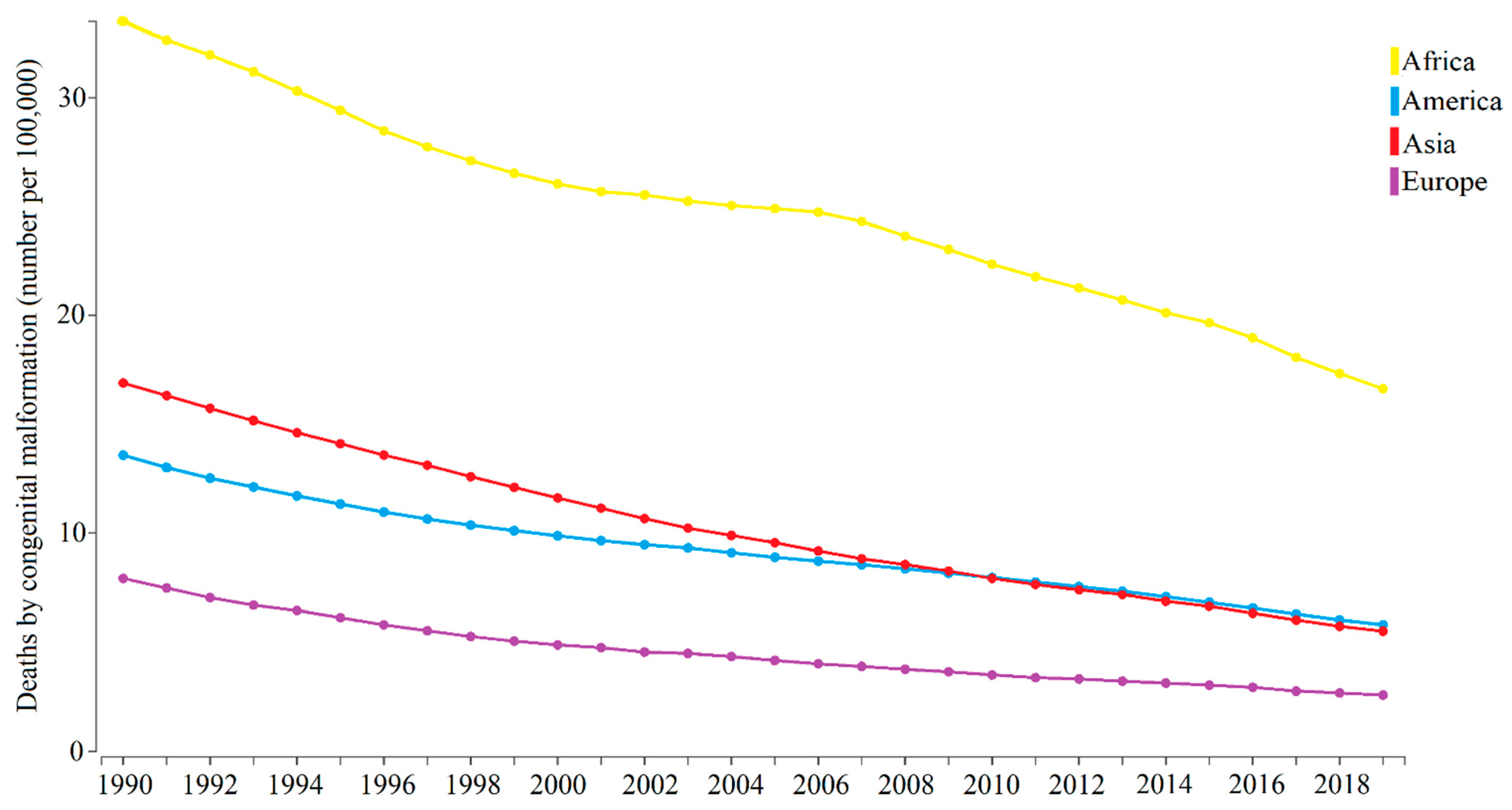
1.2. Congenital Malformations
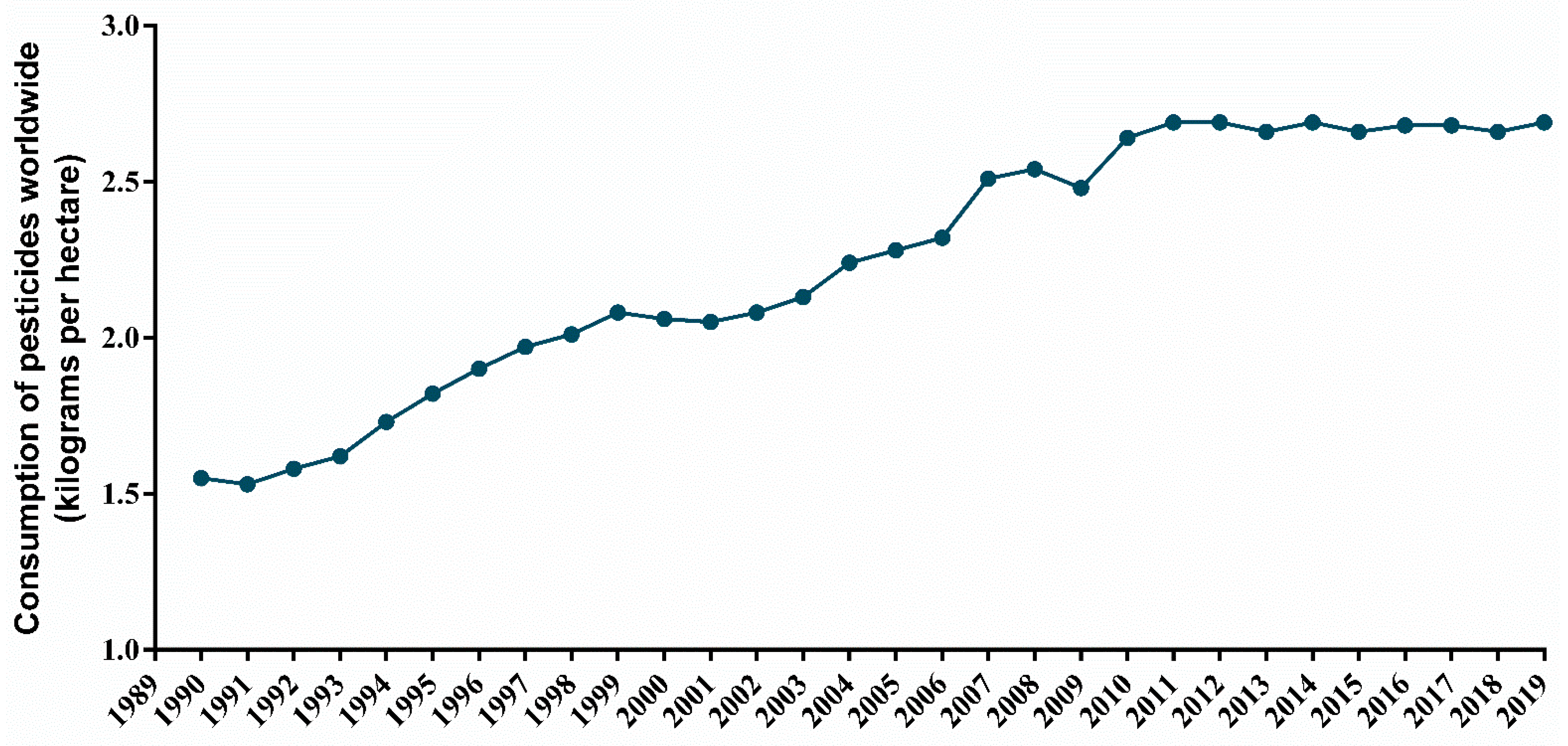
1.3. Pesticides
1.4. Toxicology of Pesticides
1.5. Rationale and Aims of the Systematic Review
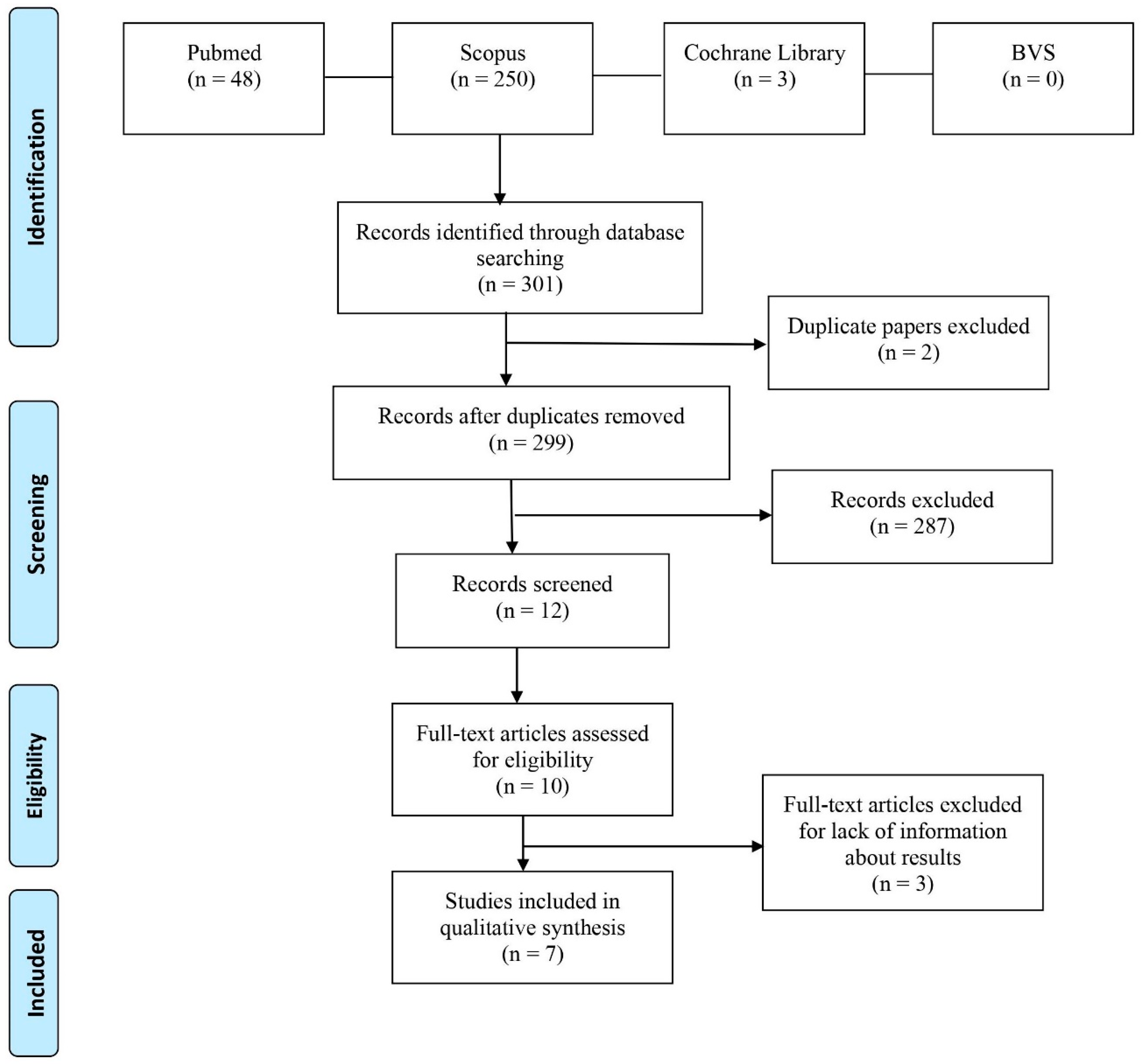
2. Materials and Methods
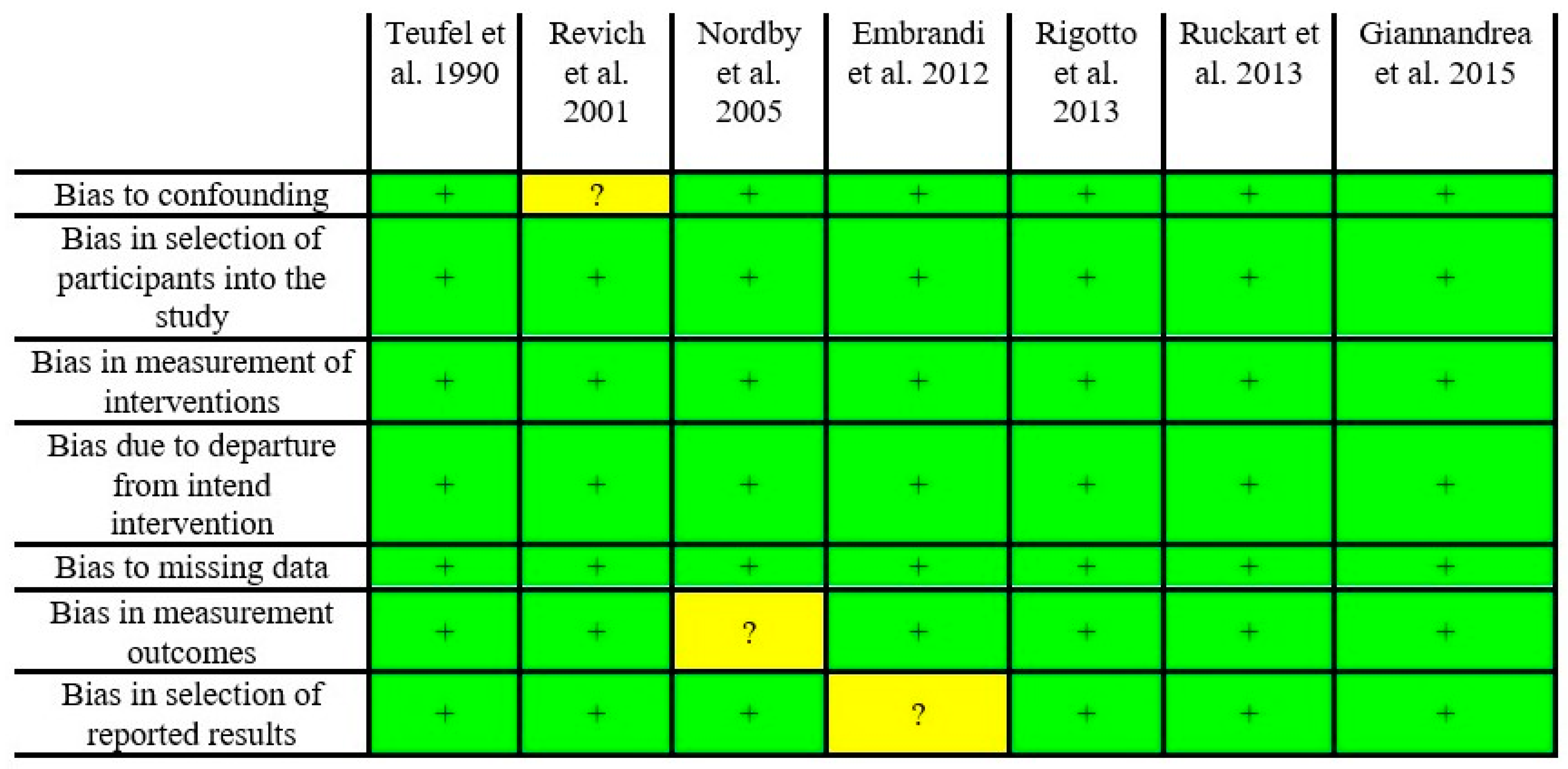
Risk of Bias
3. Results
Risk of Bias
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nicolopoulou-Stamati, P.; Maipas, S.; Kotampasi, C.; Stamatis, P.; Hens, L. Chemical pesticides and human health: The urgent need for a new concept in agriculture. Front. Public Health 2016, 4, 148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopes-Ferreira, M.; Maleski, A.L.A.; Balan-Lima, L.; Bernardo, J.T.G.; Hipolito, L.M.; Seni-Silva, A.C.; Batista-Filho, J.; Falcao, M.A.P.; Lima, C. Impact of pesticides on human health in the last six years in Brazil. Int. J. Environ. Res. Public Health 2022, 19, 3198. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.R.; Corvalán, C.F.; Kjellström, T. How much global ill health is attributable to environmental factors? Epidemiology 1999, 10, 573–584. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization (WHO). Preventing Disease through Healthy Environments: A Global Assessment of the Burden of Disease from Environmental Risks. 2018. Available online: https://www.who.int/publications/i/item/9789241565196 (accessed on 30 March 2022).
- Guo, W.; Pan, B.; Sakkiah, S.; Yavas, G.; Ge, W.; Zou, W.; Tong, W.; Hong, H. Persistent organic pollutants in food: Contamination sources, health effects and detection methods. Int. J. Environ. Res. Public Health 2019, 16, 4361. [Google Scholar] [CrossRef] [Green Version]
- Crain, E.F. Environmental threats to children’s health: A challenge for pediatrics: 2000 Ambulatory Pediatric Association (APA) presidential address. Pediatrics 2000, 106, 871–875. [Google Scholar] [CrossRef] [PubMed]
- Prüss-Ustün, A.; Vickers, C.; Haefliger, P.; Bertollini, R. Knowns and unknowns on burden of disease due to chemicals: A systematic review. Environ. Health 2011, 10, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdollahdokht, D.; Asadikaram, G.; Abolhassani, M.; Pourghadamyari, H.; Abbasi-Jorjandi, M.; Faramarz, S.; Nematollahi, M.H. Pesticide exposure and related health problems among farmworkers’ children: A case-control study in southeast Iran. Environ. Sci. Pollut. Res. 2021, 28, 57216–57231. [Google Scholar] [CrossRef] [PubMed]
- Utyasheva, L.; Bhullar, L. Human rights perspective on pesticide exposure and poisoning in children: A case study of India. Health Hum. Rights 2021, 23, 49–61. [Google Scholar] [PubMed]
- Cheng, Q.; Liu, Q.Q.; Li, K.; Chang, C.H.; Lu, C.A. Assessing dietary pesticide intake and potential health effects: The application of global metabolomics analysis. J. Agric. Food Chem. 2022, 70, 4086–4091. [Google Scholar] [CrossRef] [PubMed]
- Alavanja, M.C.; Ross, M.K.; Bonner, M.R. Increased cancer burden among pesticide applicators and others due to pesticide exposure. CA Cancer J. Clin. 2013, 63, 120–142. [Google Scholar] [CrossRef] [PubMed]
- Ibañez, H.C.; Melanda, V.S.; Gerber, V.K.G.; Licht, O.A.B.; Ibañez, M.V.C.; Júnior, T.R.A.; Mello, R.G.; Komechen, H.; Andrade, D.P.; Picharski, G.L.; et al. Spatial trends in congenital malformations and stream water chemistry in Southern Brazil. Sci. Total Environ. 2019, 650, 1278–1291. [Google Scholar] [CrossRef]
- Altmann, A.E.; Halliday, J.L.; Giles, G.G. Associations between congenital malformations and childhood cancer. A register-based case-control study. Br. J. Cancer 1998, 78, 1244–1249. [Google Scholar] [CrossRef] [Green Version]
- Berbel-Tornero, O.; Ortega-Garcia, J.A.; Ferris-Tortajada, J. Congenital abnormalities and childhood cancer: A cohort record-linkage study. Cancer 2005, 103, 1939–1948. [Google Scholar] [CrossRef] [PubMed]
- American Cancer Society (ACS). Understanding What Cancer Is: Ancient Times to Present. 2018. Available online: https://www.cancer.org/cancer/cancer-basics/history-of-cancer/what-is-cancer.html (accessed on 30 March 2022).
- Instituto Nacional de Câncer José Alencar Gomes da Silva (INCA). ABC do Câncer: Abordagens Básicas para o Controle do Câncer, 2nd ed.; Coordenação Geral de Ações Estratégicas, Coordenação de Educação; organização Luiz Claudio Santos Thuler; Rev. e Atual.: Rio de Janeiro, Brazil, 2020. [Google Scholar]
- Nacional Cancer Institute (NCI). Dictionary of Cancer Terms. 2021. Available online: https://www.cancer.gov/publications/dictionaries/cancer-terms/def/ (accessed on 3 February 2022).
- Ritchie, H.; Roser, M. Causes of Death. 2018. Available online: https://ourworldindata.org/causes-of-death (accessed on 15 January 2022).
- Institute for Health Metrics and Evaluation (IHME). GBD Compare Data Visualization 2020 Seatle: University of Washington. 2020. Available online: https://www.healthdata.org/data-visualization/gbd-compare (accessed on 26 January 2022).
- International Agency for Research on Cancer (IARC). Global Cancer Observatory. 2020. Available online: https://infogram.com/globocan-2020-1h9j6qg7xdp8v4g (accessed on 22 February 2022).
- Baylin, S.B.; Jones, P.A. Epigenetic determinants of cancer. Cold Spring Harb. Perspect. Bio. 2016, 8, a019505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daxinger, L.; Whitelaw, E. Transgenerational epigenetic inheritance: More questions than answers. Genome Res. 2010, 20, 1623–1628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alegría-Torres, J.A.; Baccarelli, A. Epigenetics and lifestyle. Epigenomics 2013, 3, 267–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waterland, R.A.; Jirtle, R.L. Transposable elements: Targets for early nutritional effects on epigenetic gene regulation. Mol. Cell Biol. 2003, 23, 5293–5300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Humphrey, K.M.; Pandey, S.; Martin, J.; Hagoel, T.; Grand’Maison, A.; Ohm, J.E. Establishing a role for environmental toxicant exposure induced epigenetic remodeling in malignant transformation. Semin. Cancer Biol. 2019, 57, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Nicolella, H.D.; Assis, S. Epigenetic inheritance: Intergenerational effects of pesticides and other endocrine disruptors on cancer development. Int. J. Mol. Sci. 2022, 23, 4671. [Google Scholar] [CrossRef] [PubMed]
- Ruckart, P.Z.; Bove, F.J.; Maslia, M. Evaluation of exposure to contaminated drinking water and specific birth defects and childhood cancers at Marine Corps Base Camp Lejeune, North Carolina: A case-control study. Environ. Health 2013, 12, 104. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Birth Defects Surveillance Atlas of Selected Congenital Anomalies. 2014. Available online: https://apps.who.int/nutrition/publications/birthdefects_atlas/en/index.html (accessed on 30 March 2022).
- Global Health Observatory (GHO). Child Mortality and Causes of Death. 2021. Available online: https://www.who.int/data/gho/data/themes/topics/topic-details/GHO/child-mortality-and-causes-of-death (accessed on 27 January 2022).
- Center for Disease Control and Prevention (CDC). What Are Birth Defects? 2021. Available online: https://www.cdc.gov/ncbddd/birthdefects/facts.html#ref (accessed on 25 January 2022).
- Nordby, K.-C.; Andersen, A.; Irgens, L.M.; Kristensen, P. Indicators of mancozeb exposure in relation to thyroid cancer and neural tube defects in farmers’ families. Scand. J. Work Environ. Health 2005, 31, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Christianson, A.; Howson, C.P.; Modell, B. Global Report on Birth Defects the Hidden Toll of Dying and Disabled Children Executive Summary, 1st ed.; White Plains: New York, NY, USA, 2006. [Google Scholar]
- Centers for Disease Control and Prevention (CDC). Infant Mortality. 2021. Available online: https://www.cdc.gov/reproductivehealth/maternalinfanthealth/infantmortality.htm#causes (accessed on 3 March 2022).
- World Health Organization (WHO). Congenital Anomalies: Key Facts. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/congenital-anomalies (accessed on 30 March 2022).
- Boyle, B.; Addor, M.C.; Arriola, L.; Barisic, I.; Bianchi, F.; Csáky-Szunyogh, M.; Walle, H.E.K.; Dias, C.M.; Draper, E.; Gatt, M.; et al. Estimating global burden of disease due to congenital anomaly: An analysis of European data. Arch. Dis. Child. Fetal Neonatal Ed. 2018, 103, 22–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization (WHO). Birth Defects Surveillance a Manual for Programme Managers. 2014. Available online: https://www.who.int/publications/i/item/9789241548724 (accessed on 27 January 2022).
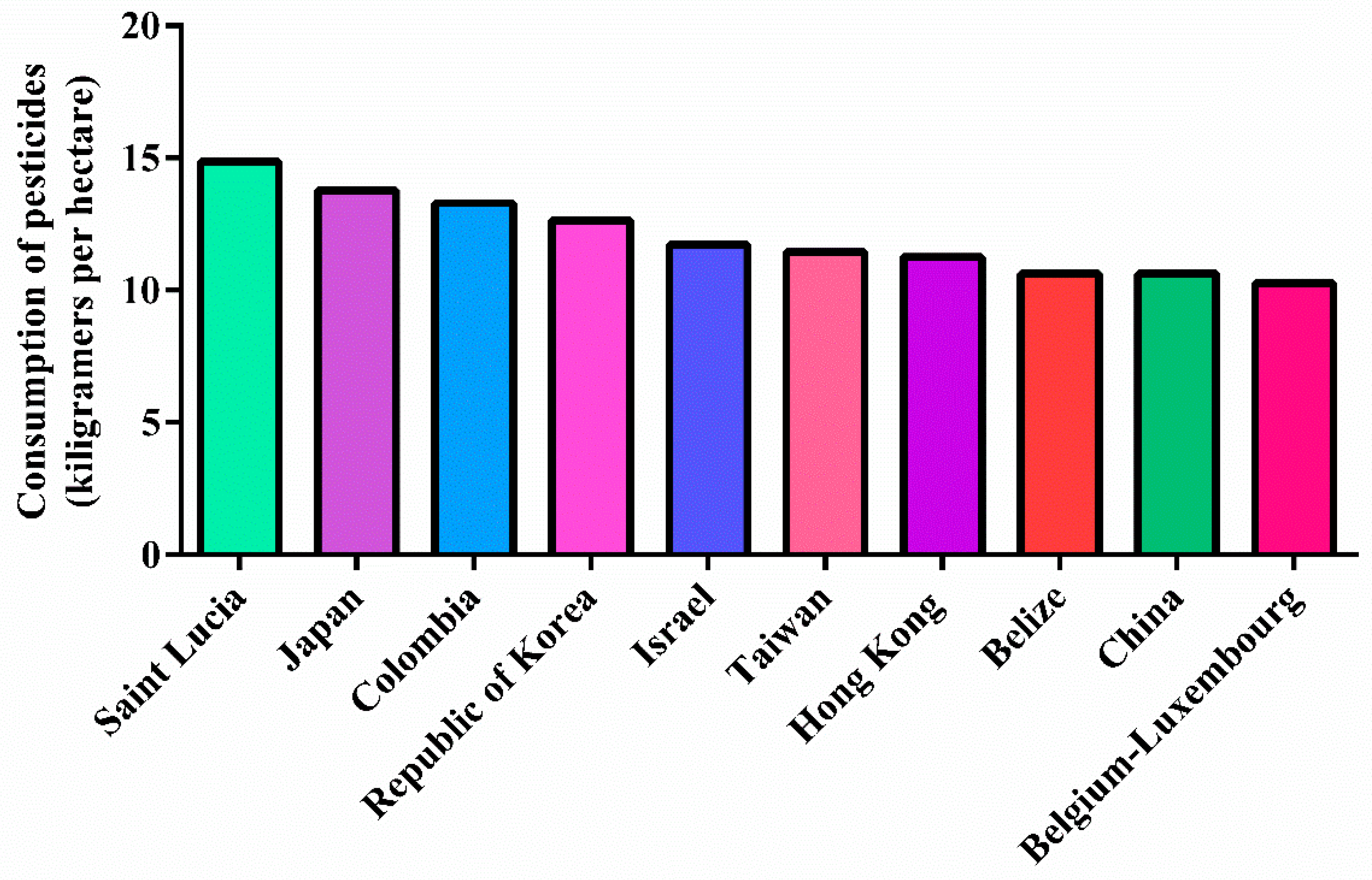
- Sharma, A.; Kumar, V.; Shahzad, B.; Tanveer, M.; Sidhu, G.P.S.; Handa, N.; Kohli, S.K.; Yadav, P.; Bali, A.S.; Parihar, R.D.; et al. Worldwide pesticide usage and its impacts on ecosystem. SN Appl. Sci. 2019, 1, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Gonçalves, L.F.; Jeanty, P.; Piper, J. The accuracy of prenatal ultrasonography in detecting congenital anomalies. Am. J. Obstet. Gynecol. 1994, 171, 1606–1612. [Google Scholar] [CrossRef]
- Borsellino, A.; Zaccara, A.; Nahom, A.; Trucchi, A.; Aite, L.; Giorlandino, C.; Bagolan, P. False-positive rate in prenatal diagnosis of surgical anomalies. J. Pediatr. Surg. 2006, 41, 826–829. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Zamora, M.A.; Borrell, A.; Borobio, V.; Gonce, A.; Perez, M.; Botet, F.; Nadal, A.; Albert, A.; Puerto, B.; Fortuny, A. False positives in the prenatal ultrasound screening of fetal structural anomalies. Prenat. Diagn. 2006, 27, 18–22. [Google Scholar] [CrossRef]
- Ibrahim, R.S.M.; Emad-Eldin, S. Beyond fetal magnetic resonance diagnosis of corpus callosum agenesis. Egypt. J. Radiol. Nucl. Med. 2020, 51, 75. [Google Scholar] [CrossRef]
- Aktar, M.W.; Sengupta, D.; Chowdhury, A. Impact of pesticides use in agriculture: Their benefits and hazards. Interdiscip. Toxicol. 2009, 2, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Siqueira, D.F.; Moura, R.M.; Laurentino, G.E.C.; Araújo, A.J.; Cruz, S.L. Análise da exposição de trabalhadores rurais a agrotóxicos. Rev. Bras. Promoç. Saúde 2013, 26, 182–191. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Chemical Safety: Pesticides. 2020. Available online: https://www.who.int/news-room/q-a-detail/chemical-safety-pesticides (accessed on 14 January 2022).
- Pelaez, V.; Teodorovicz, T.; Guimarães, T.A.; Silva, L.R.; Moreau, D.; Mizukawa, G. A dinâmica do comércio internacional de agrotóxicos. Rev. Política Agrícola 2016, 25, 39–52. [Google Scholar]
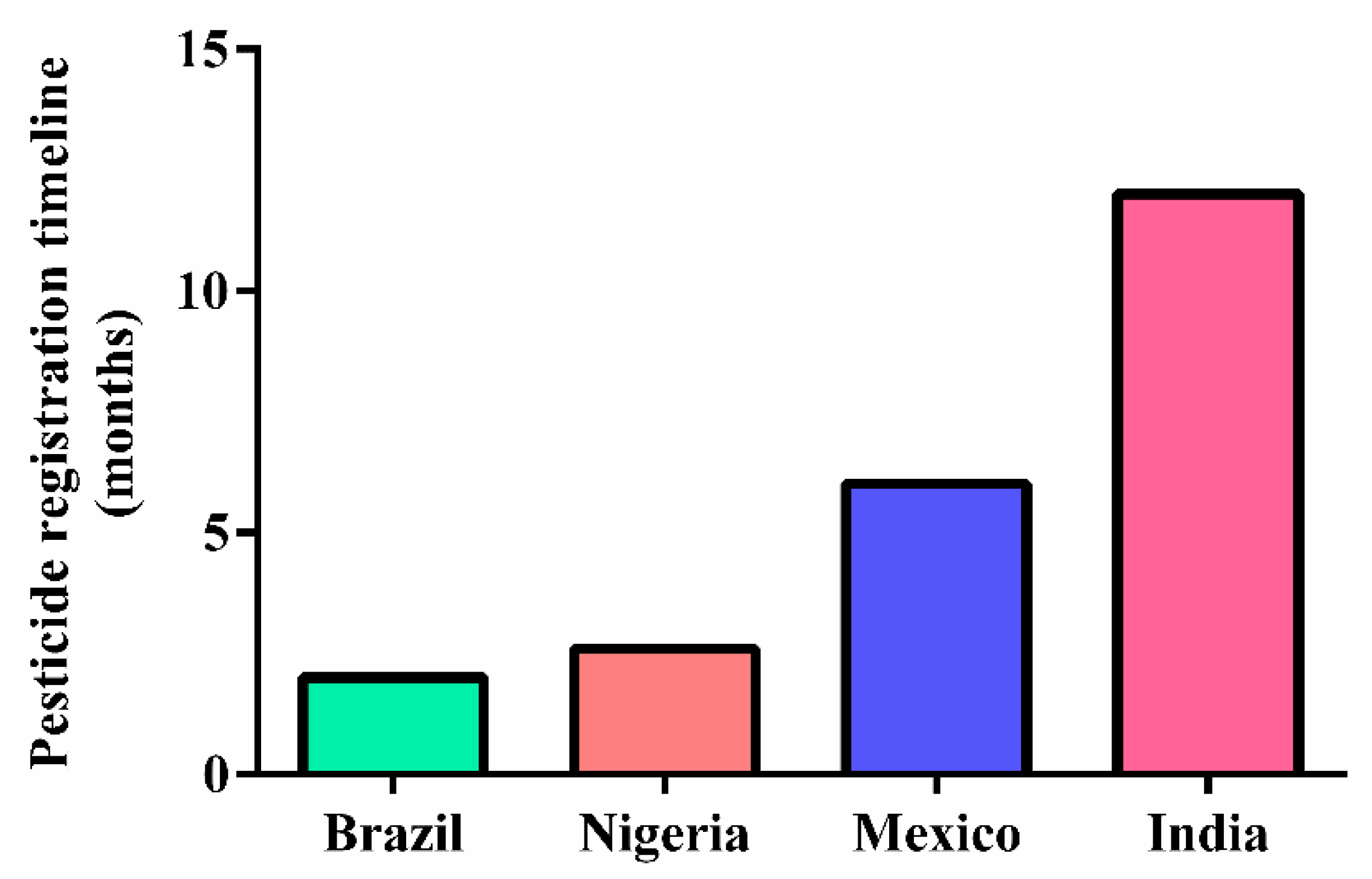
- Food and Agriculture Organization of the United Nations (FAO). The Pesticide Registration Process. 2020. Available online: https://www.fao.org/pesticide-registration-toolkit/about/quick-start-guide/en/ (accessed on 25 February 2022).
- European Parliament (EP). The Use of Pesticides in Developing Countries and Their Impact on Health and the Right to Food; Policy Department for External Relations: Brussels, Belgium, 2021. [Google Scholar]
- Food and Agriculture Organization of the United Nations (FAO). Global Situation of Pesticide Management in Agriculture and Public Health. 2018. Available online: http://www.fao.org/3/ca7032en/ca7032en.pdf (accessed on 30 March 2022).
- Donley, N. The USA lags behind other agricultural nations in banning harmful pesticides. Environ. Health 2019, 18, 1–12. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Sixty-Third World Health Assembly; World Health Organization: Geneva, Switzerland, 2010; pp. 17–21. [Google Scholar]
- Zaidon, S.Z.; Ho, Y.B.; Hashim, Z.; Saari, N.; Praveena, S.M. Pesticides Contamination and Analytical Methods of Determination in Environmental Matrices in Malaysia and Their Potential Human Health Effects—A Review. Malays. J. Med. Health Sci. 2018, 4, 81–88. [Google Scholar]
- Godinho, A.F.; Almeida, A.A. Toxicologia de Praguicidas e Metais Pesados. In Ensaios em Biociências, 1st ed.; Instituto de Biociências de Botucatu—UNESP: Botucatu, Brazil, 2019; pp. 71–82. [Google Scholar]
- Shah, R. Pesticides and Human Health. In Emerging Contaminants; Nuro, A., Ed.; E-book: IntechOpen: London, UK, 2020; p. 23. [Google Scholar]
- Ministério da Saúde (MS). Diretrizes Metodológicas: Elaboração de Revisão Sistemática e Metanálise de Ensaios Clínicos Randomizados. Ministério da Saúde. 2012. Available online: https://bvsms.saude.gov.br/bvs/publicacoes/diretrizes_metodologicas_elaboracao_sistematica.pdf (accessed on 5 April 2022).
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-p) 2015: Elaboration and explanation. BMJ 2015, 350, g7647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Latorraca, C.O.C.; Rodrigues, M.; Pacheco, R.L.; Martimbianco, A.L.C.; Riera, R. Busca em bases de dados eletrônicas da área da saúde: Por onde começar. Diagn Tratamento. 2019, 24, 59–63. [Google Scholar]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; John Wiley & Sons: Chichester, UK, 2019. [Google Scholar]
- Teufel, M.; Niessen, K.H.; Sartoris, J.; Brands, W. Chlorinated hydrocarbons in fat tissue: Analyses of residues in healthy children, tumor patients, and malformed children. Arch. Environ. Contam. Toxicol. 1990, 19, 646–652. [Google Scholar] [CrossRef]
- Revich, B.; Aksel, E.; Ushakova, T.; Ivanova, I.; Zhuchenko, N.; Klyuev, N.; Brodsky, B.; Sotskov, Y. Dioxin exposure and public health in Chapaevsk, Russia. Chemosphere 2001, 43, 951–966. [Google Scholar] [CrossRef]
- Embrandiri, A.; Singh, R.P.; Ibrahim, M.H.; Khan, A.B.S. An epidemiological study on the health effects of endosulfan spraying on cashew plantations in Kasaragod District, Kerala, India. Asian J. Epidemiol. 2012, 5, 22. [Google Scholar] [CrossRef] [Green Version]
- Rigotto, R.M.; Silva, A.M.C.; Ferreira, M.J.M.; Rosa, I.F.; Aguiar, A.C.P. Trends of chronic health effects associated to pesticide use in fruit farming regions in the state of Ceará, Brazil. Rev. Bras. Epidemiol. 2013, 16, 763–773. [Google Scholar] [CrossRef] [Green Version]
- Paoli, D.; Giannandrea, F.; Gallo, M.; Turci, R.; Cattaruzza, M.S.; Lombardo, F.; Lenzi, A.; Gandini, L. Exposure to polychlorinated biphenyls and hexachlorobenzene, semen quality and testicular cancer risk. J. Endocrinol. Investigat. 2015, 38, 745–752. [Google Scholar] [CrossRef] [Green Version]
- Environmental Protection Agency (EPA). Recognition and Management of Pesticides Poisonings. 2013. Available online: https://www.epa.gov/sites/default/files/2015-01/documents/rmpp_6thed_final_lowresopt.pdf (accessed on 5 April 2022).
- Stillerman, K.P.; Mattison, D.R.; Giudice, L.C.; Woodruff, T.J. Environmental exposures and adverse pregnancy outcomes: A review of the science. Reprod. Sci. 2008, 15, 631–650. [Google Scholar] [CrossRef]
- Anwar, W.A. Biomarkers of human exposure to pesticides. Environ. Health Perspect. 1997, 105, 801–806. [Google Scholar] [PubMed]
- European Union (EU). Commission Implementing Regulation. Official Journal of the European Union. 2020. Available online: https://www.legislation.gov.uk/eur/2020/2087/contents (accessed on 5 April 2022).
- National Institute of Environmental Health Sciences (NIEHS). Dioxins. 2021. Available online: https://www.niehs.nih.gov/health/topics/agents/dioxins/index.cfm (accessed on 3 February 2022).
- World Health Organization (WHO). Global Situation of Pesticide Management in Agriculture and Public Health. 2018. Available online: https://apps.who.int/iris/handle/10665/329971 (accessed on 3 February 2022).
- Valadares-Inglis, M.C.; Fontes, E.M.G. Novas tecnologias aplicáveis ao controle biológico. In Controle Biológico de Pragas da Agricultura, 1st ed.; Embrapa: Brasília, Brazil, 2020; p. 510. [Google Scholar]
- Panis, C.; Candiotto, L.Z.P.; Gaboardi, S.C.; Gurzenda, S.; Cruz, J.; Castro, M.; Lemos, B. Widespread pesticide contamination of drinking water and impact on cancer risk in Brazil. Environ. Int. 2022, 165, 107321. [Google Scholar] [CrossRef] [PubMed]
- Kalra, S.; Resident, S.; Dewan, P. Organochlorine pesticide exposure in mothers and neural tube defects in offsprings. Reprod. Toxicol. 2016, 66, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Lim, L.; Bolstad, H.M. Organophosphate Insecticides: Neurodevelopmental Effects; NRIAGU; Elsevier: Oxford, UK, 2019; pp. 785–791. [Google Scholar]
- Ren, A.; Qiu, X.; Jin, L.; Ma, J.; Li, Z.; Zhang, L.; Zhu, H.; Finnell, R.H.; Zhu, T. Association of selected persistent organic pollutants in the placenta with the risk of neural tube defects. Proc. Natl. Acad. Sci. USA 2011, 108, 12770–12775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, K.E.M.; Frøysa, H.G.; Karlsen, O.A.; Blaser, N.; Zimmer, K.E.; Berntsen, H.F.; Verhaegen, S.; Ropstad, E.; Kellmann, R.; Goksøyr, A. Effects of defined mixtures of POPs and endocrine disruptors on the steroid metabolome of the human H295R adrenocortical cell line. Chemosphere 2019, 218, 328–339. [Google Scholar] [CrossRef] [Green Version]
- Kalofiri, P.; Balias, G.; Tekos, F. The EU endocrine disruptors’ regulation and the glyphosate controversy. Toxicol. Rep. 2021, 8, 1193–1199. [Google Scholar] [CrossRef]
- Suzawa, M.; Ingraham, H.A. The Herbicide Atrazine Activates Endocrine Gene Networks via Non-Steroidal. PLoS ONE 2017, 3, e2117. [Google Scholar]
- Daltveit, D.S.; Klungsøyr, K.; Engeland, A.; Ekbom, A.; Gissler, M.; Glimelius, I.; Grotmol, T.; Madanat-Harjuoja, L.; Ording, A.G.; Sæther, S.M.M.; et al. Cancer risk in individuals with major birth defects: Large Nordic population based case-control study among children, adolescents, and adults. BMJ 2020, 371, m4060. [Google Scholar] [CrossRef]
- Garci, A.M. Pesticide Exposure and Women’s Health. Am. J. Ind. Med. 2023, 594, 584–594. [Google Scholar]
- Hutter, H.; Poteser, M.; Lemmerer, K.; Wallner, P.; Sanavi, S.S.; Kundi, M.; Moshammer, H.; Weitensfelder, L. Indicators of Genotoxicity in Farmers and Laborers of Ecological and Conventional Banana Plantations in Ecuador. Int. J. Environ. Res. Public Health 2020, 17, 1435. [Google Scholar] [CrossRef] [Green Version]
- Togawa, K.; Leon, M.E.; Lebailly, P.; Freeman, L.E.B.; Nordby, K.-C.; Baldi, I.; MacFarlane, E.; Shin, A.; Park, S.; Greenlee, R.T.; et al. Cancer incidence in agricultural workers: Findings from an international consortium of agricultural cohort studies (AGRICOH). Environ. Int. 2021, 157, 1–11. [Google Scholar] [CrossRef] [PubMed]
| Chemical Classification | Group | Mechanism of Toxicity | Effects on the Organism |
|---|---|---|---|
| Insecticides | Organochlorines | Prolongation of the opening time of sodium channels; competitive inhibitors of GABA-regulated chlorine flux; interference with cellular calcium by modification of membrane Ca++/Mg++ ATPase and calmodulin enzyme activity; organic bioaccumulation capacity; induction of liver enzymes. | Neurological disorders: paresthesia of the tongue, face and lips, tremors, mental confusion, coma, etc.; gastrointestinal changes such as: vomiting, abdominal colic, diarrhea, salivation, etc.; hepatic alterations: hepatic enzyme stimulation; carcinogenesis; mutagenesis; weight loss; general: anxiety, heart rhythm changes, arthralgia, memory loss, etc. |
| Organophosphates | Blood cholinesterase inhibition. | Sialorrhea, tearing, nausea, vomiting, diarrhea, increased bronchial secretion, bradycardia, sweating, dyspnea, respiratory depression, miosis, hyperactivity, seizures, coma, and death. | |
| Pyrethroids | Leading to cholinergic crisis; inhibition of GABA-regulated chlorine flux; modification of membrane Ca++/Mg++ ATPase enzyme activity. | T syndrome: involuntary tremors; CS syndrome: choreoathetosis and salivation; excitatory neurotoxicity in: brain system, spinal cord, and peripheral nervous system; general: cutaneous, ocular, and gastrointestinal effects, convulsions, coma, respiratory paralysis and death. | |
| Herbicides | Chlorophenoxyacetic acid | Intense lipid proliferation and depletion of the cofactor NADPH, causing cell death. | General: burning sensation in the mouth, nausea and vomiting, abdominal pain, diarrhea, dyspnea and hypoxemia, eye irritation, changes in level of consciousness, drowsiness, pulmonary edema, etc. |
| Rodenticides | Coumarins | Inhibition of the synthesis of vitamin K dependent factors; inhibition of prothrombin (factor II) synthesis in the liver; plasma thrombin precursors; vaso destructive action with capillary damage. | Hemorrhagic phenomena such as: epistaxis, purpura, petechiae, hematuria, and skin pallor. |
| Ureics | Inhibition of complex I NADH ubiquinone reductase activity in mammalian mitochondria: complex I activity is related to insulin release in insulinoma cells and pancreatic islets. Affects the mitochondrial respiration of NAD-bound substrates in energy-demanding pancreatic islet cells. | General: dyspnea, cyanosis, pulmonary edema, increased bronchial and tracheal secretion, pleural effusion, etc. | |
| Fluorine compounds | Mimicking the action of acetate, incorporating it into the Krebs cycle. It turns into fluorine citrate; fluoride citrate is an inhibitor of aconitase and succinate dehydrogenase. Leading to blockage in the tricarboxylic acid cycle; blockade of tricarboxylic acids causes low energy production, leading to a reduction in oxygen consumption and cellular ATP concentration (mainly in cardiac and central nervous system cells). | Respiratory depression; cardiovascular system: hypotension, cardiac depression, ventricular tachycardia, fibrillation; general: nausea, vomiting, abdominal pain, agitation, anxiety, muscle spasm, stupor, reversible acute renal failure, coma, etc. | |
| Fungicides | Heavy metal-based fungicides | The heavy metal binds to chemical groups such as carboxyl, amine, sulfhydryl, phosphoryl, imino, hydroxyl, interfering with cellular oxygen transport and energy production. | General: erythema, edema and vesicular eruptions, skin sensitization, nausea, vomiting, etc.; neurological: hyperexcitability or sedation. |
| Phthalamides | Causes inhibition of Succinate Dehydrogenase Inhibitor (SDHI), interfering with mitochondrial function. | ||
| Triazoles | Activation of the key to oxidative stress and lipid peroxidation. |
| INCLUSION | EXCLUSION |
|---|---|
| Incidence or prevalence of cancer and congenital malformations to contact with pesticides. | Absence of cancer and congenital malformation occurrence. |
| The full paper was not found. | |
| Relationship of environment contamination and cancer and congenital malformation. | The paper is not in English. |
| Animal research. |
| Reference | Country | Study Period | Study Type | Sample Characteristics | Study Aim | Main Findings |
|---|---|---|---|---|---|---|
| Teufel et al. [58] | Germany | 1985–1988 | Case–control | Healthy children (n = 183) Children with malignant tumors (n = 46) Children with congenital malformations or benign tumors (n = 33) | To measure de chlorinated hydrocarbons in fat tissue of German pediatric patients. | The same chlorinated hydrocarbons (mainly, β-HCH and γ-HCH, dieldrin, p,p′-DDE, total PCB) were detected in all groups of pediatric patients, and no significant difference was observed in the increased concentrations of HCC in pediatric patients with malformations and tumors. However, the number of cases analyzed is still too small to rule out any possible risk. |
| Revich et al. [59] | Russia | 1997–1998 | Cross-sectional | Chapaevsk’s population | To measure the dioxin levels in milk and blood and to analyze data about cancer and congenital malformation of Chapaevsk’s population. | High levels of dioxin were detected in soil and water, as well as in women’s blood and milk. The rate of CMGC per child ranged from 0 to 10. The average number of CMGC per child was 4.5 for boys and 4.4 for girls. |
| Nordby et al. [31] | Norway | 1925–1991 | Cohort study | Parental generation Male (n = 131,243) Female (n = 105,403) Filial generation Male (n = 146,934) Female (n = 139,541) | To investigate the association of mancozeb exposure with cancer and congenital malformation in farmers’ families. | The study identified 131 cases of neural tube defect, of which 118 cases used pesticides in potato farming and 319 cases of thyroid cancer, of which 58 cases also used the same pesticide. The study concluded that neural tube defects were associated with potato farming, but thyroid cancer was not related to exposure to mancozeb. |
| Embrandiri et al. [60] | India | 2008–2009 | Cross-sectional | 0–14 years old Male (n = 104) Female (n = 166) 15–30 years old Male (n = 209) Female (n = 210) 31–45 years old Male (n = 189) Female (n = 100) >46 years old Male (n = 96) Female (n = 77) | To verify the prevalence of common health problems associated with endosulfan in the Kasaragod district, India, seven years after the banning of this pesticide use in cashew plantation. | 0–14 years old 46.0% of men and 42.5% of women had congenital abnormalities. 15–30 years old 30.4% of men and 31.7% of women had congenital abnormalities. 31–45 years old High prevalence rate of skin problems and infertility. >46 years old High prevalence rate of cancer and respiratory problems. |
| Rigotto et al. [61] | Brazil | 2000–2010 | Case–control | Case population habitants from three Brazilian cities known for their intensive use of pesticides (n = 145,509). Control Population Habitants from 11 other cities that do not use pesticides extensively (n = 494,939). | To compare morbidity and mortality related to pesticides between the case population and control population. | Increase in hospitalization rate due to cancer: case population, being 1.76 times higher than the control population. Annual increase in mortality rates due to neoplasia: 1.38 times higher in the case municipalities, compared to control. Fetal deaths: an increasing trend in case population and stability trend in the control population. Congenital malformation: there are no differences in rates between the case and control groups. |
| Ruckart et al. [27] | USA | 1968–1985 | Case–control | Children with cancer and/or congenital malformation (n = 58) Children without cancer and/or congenital malformation (n = 526) | To determine if children born during 1968–1985 to mothers with residential exposure to contaminated drinking water during pregnancy were more likely to have cancer and/or congenital malformation. | There was association between 1st trimester exposure to TCE and benzene and NTDs, and there is an association of response to monotonic exposure for TCE; results suggested weaker associations between 1st trimester exposure to PCE, vinyl chloride, and DCE and hematopoietic cancers in childhood. |
| Paoli et al. [62] | Italy | - | Case–control | Testicular cancer patients (n = 125) Control group (n = 103) | To investigate the possible role of occupational and environmental exposure to endocrine disruptors (polychlorinated biphenyls and hexachlorobenzene). | Semen analysis: sperm number and total motility were lower in cancer patients than in control patients and there were more malformations in cases samples than in controls. Organochlorine analysis: PCB congeners were found in 16 patients and no controls. HCB was detected in five cases and one control. The study also found a high association between higher levels of reproductive tract birth defects and an increased risk of testicular cancer. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melanda, V.S.; Galiciolli, M.E.A.; Lima, L.S.; Figueiredo, B.C.; Oliveira, C.S. Impact of Pesticides on Cancer and Congenital Malformation: A Systematic Review. Toxics 2022, 10, 676. https://doi.org/10.3390/toxics10110676
Melanda VS, Galiciolli MEA, Lima LS, Figueiredo BC, Oliveira CS. Impact of Pesticides on Cancer and Congenital Malformation: A Systematic Review. Toxics. 2022; 10(11):676. https://doi.org/10.3390/toxics10110676
Chicago/Turabian StyleMelanda, Viviane Serra, Maria Eduarda A. Galiciolli, Luíza S. Lima, Bonald C. Figueiredo, and Cláudia S. Oliveira. 2022. "Impact of Pesticides on Cancer and Congenital Malformation: A Systematic Review" Toxics 10, no. 11: 676. https://doi.org/10.3390/toxics10110676






