Effects of Benzene: Hematological and Hypersensitivity Manifestations in Resident Living in Oil Refinery Areas
Abstract
:1. Introduction
1.1. Generalities
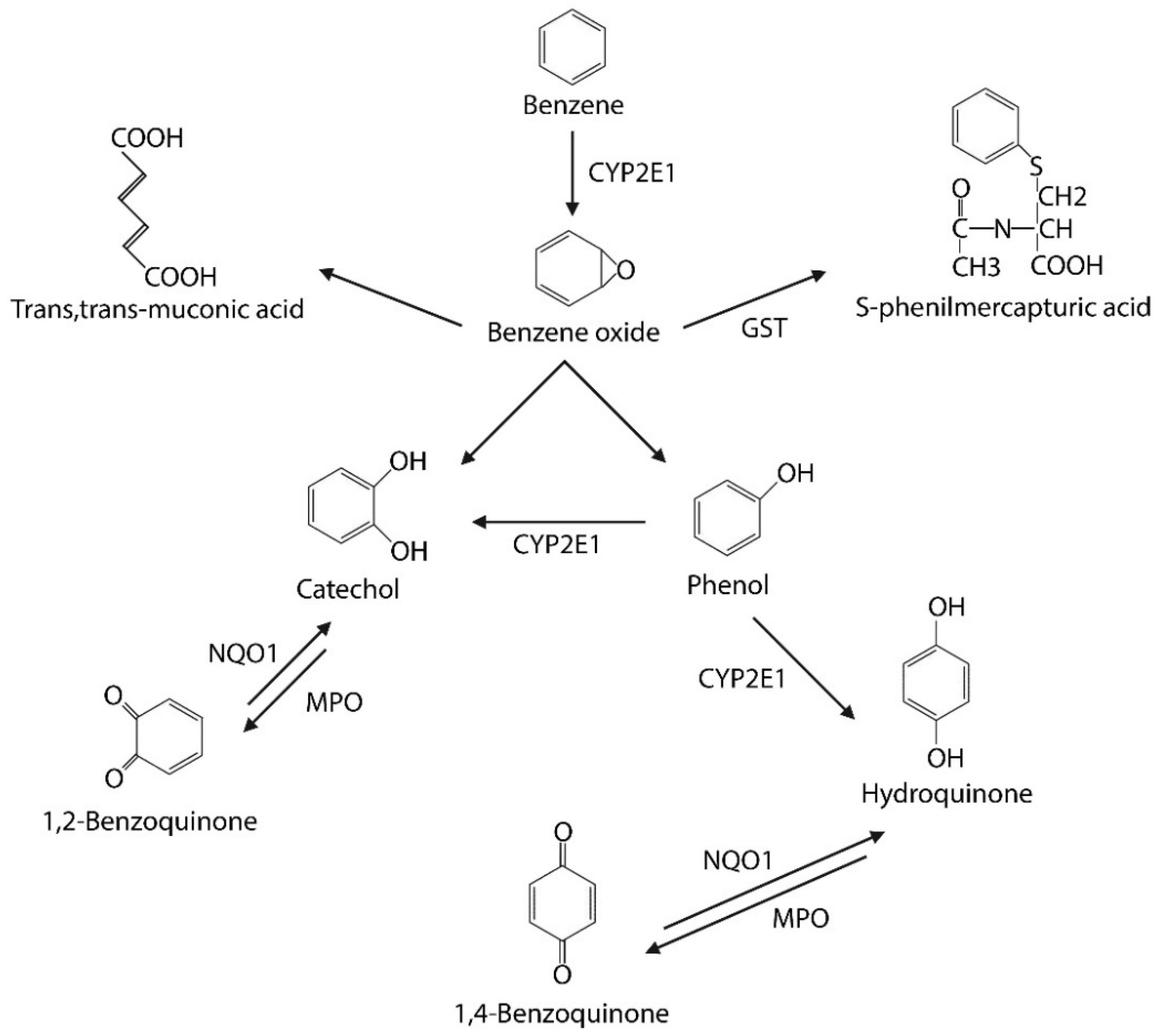
1.2. Metabolism
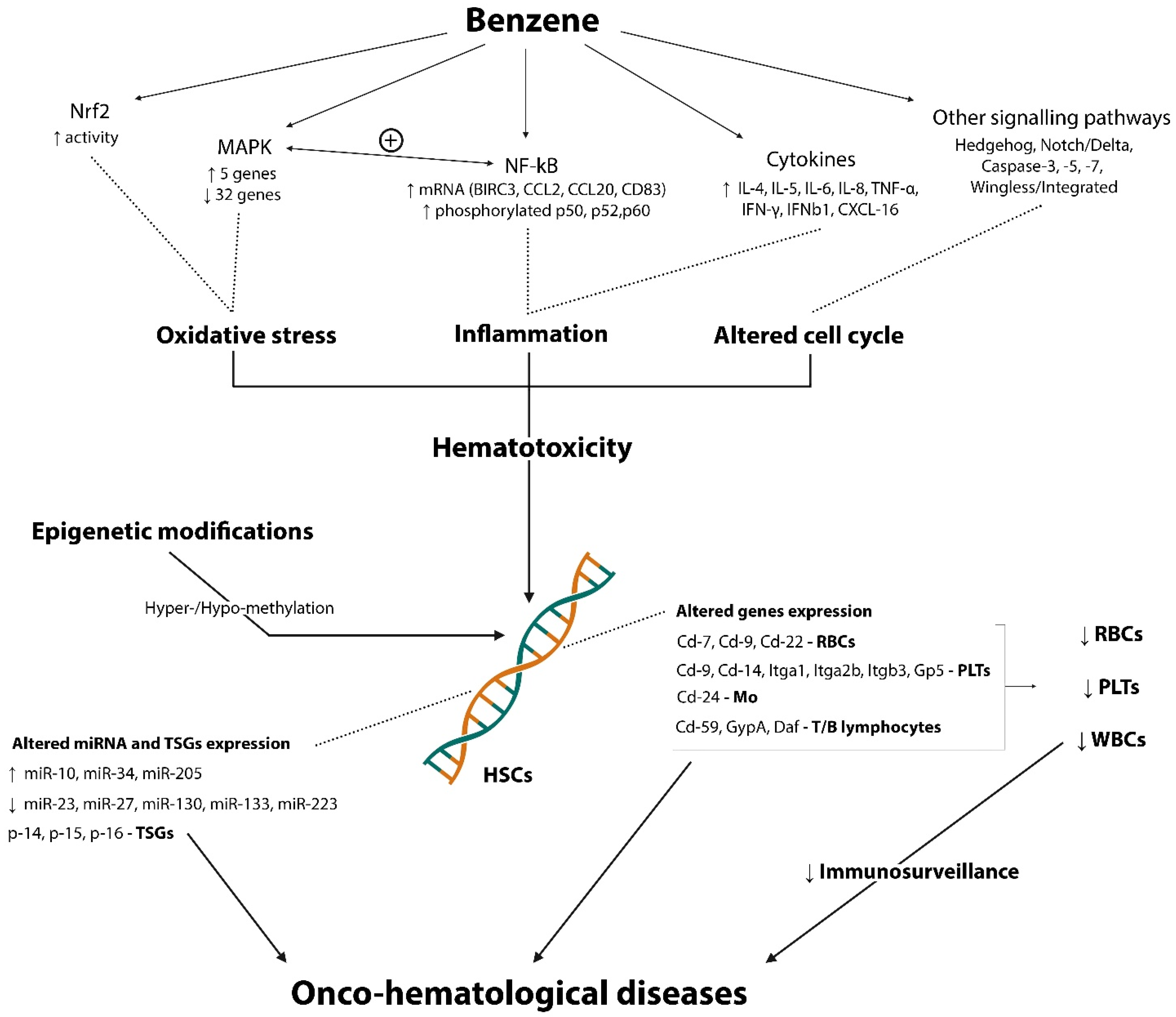
1.3. Mechanisms
1.4. Hematological and Hypersensitivity Occupational Manifestations
2. Materials and Methods
3. Results and Discussion
3.1. Hematological Side
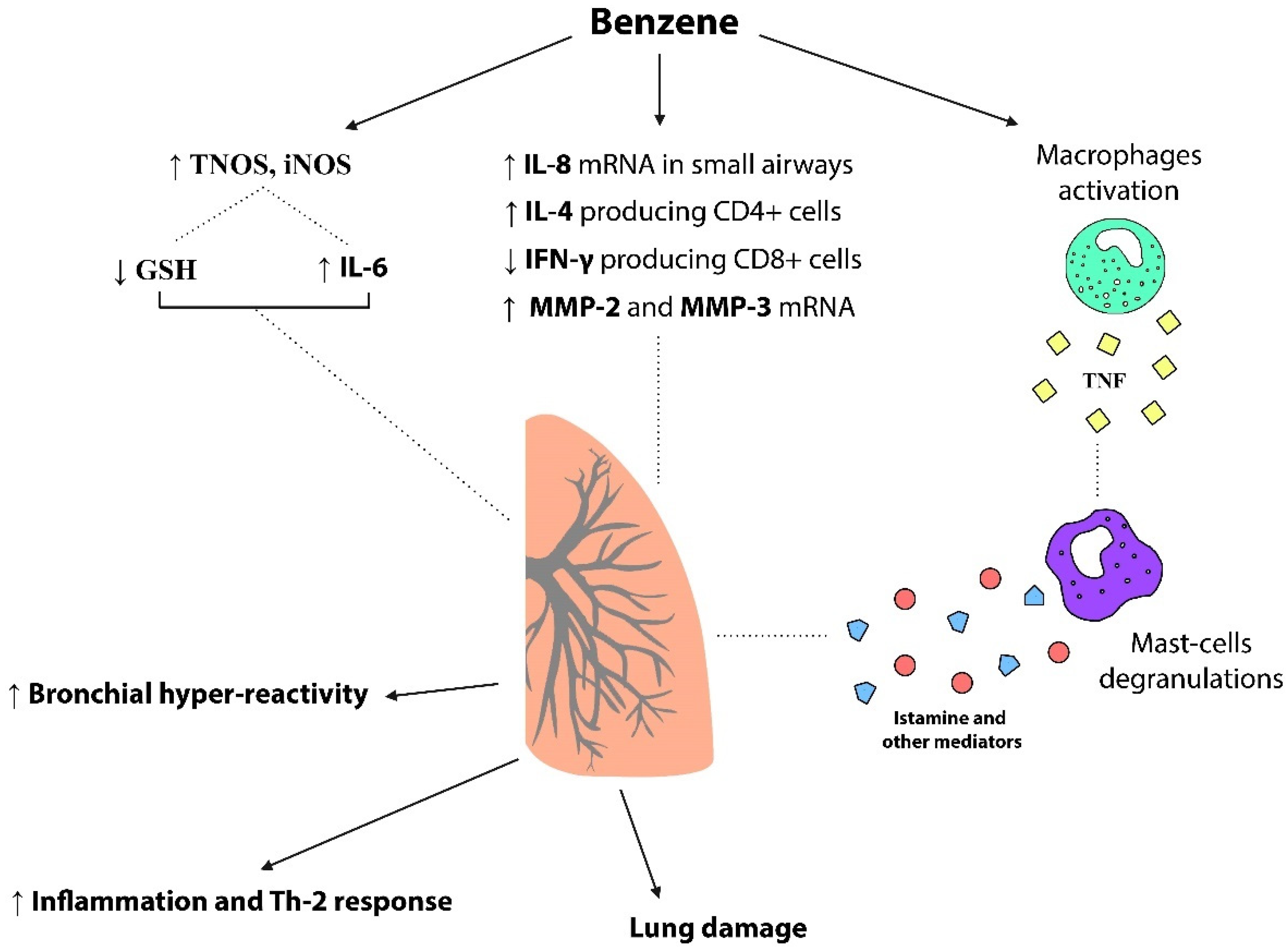
3.2. Hypersensitivity Side
3.3. Immunological Aspects
3.4. Hematological Highlights
- Involvement of many pathways (NF-kB, Nrf2, MAPK, Hedgehog, Notch/Delta, Wingless/Integrated, Caspase-3, -7, -9);
- Augmented pro-inflammatory cytokines (IL-6, IL-8, TNF- α, IFN-γ, and IFNB1) and chemokines (CXCL16);
- Augmented Th2 cytokines (IL-4, IL-5) and controversial role of IL-10;
- Changes in the expression of genes related to the differentiation of T- and B- lymphocytes (Cd7, Cd9, and Cd22) and with the differentiation of myeloid stem cells (Cd24-monocytes; GypA, Daf, and Cd59-RBCs; Itgb3, Cd9, Cd14, Itga2B, Gp5, and Itga1-PLTs);
- Epigenetic modifications impairing TSGs (p14, p15, p16) and affecting miRNA expression (miR-10, miR-23, miR-27, miR-34, miR-130, miR-133, miR-205, miR-233);
- Reduction in WBCs, PLTs and RBCs count in exposed subjects;
- Discordant results about Hb concentration and HCT percentage in acute exposure and reduction in chronic exposure.
3.5. Hypersensitivity Highlights
- Increased production of TNF elicited by HQ and consequent mast-cell degranulation involved in hyperresponsiveness;
- Reduced counts of IFN-γ producing type 1 CD8+ subpopulation of T cells and increased counts of IL-4 producing type 2 CD4+ T cells benzene-induced;
- Benzene-induced upregulation of IL-8 mRNA levels in small airways epithelial cells;
- DNA damage in airway inflammatory and epithelial cells related to chronic benzene exposure;
- Benzene-related lung damage due to upregulation of matrix metalloproteinases (MMP-2 and MMP-3) and downregulation of their inhibitors (TIMP-1 and TIMP-2);
- Association of GSH reduction and increase in IL-6 as effects of short-term exposure to a high dose of VOCs determine airway inflammation and bronchoconstriction.
4. Conclusions, Recommendations and Future Goals
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sarigiannis, D.A.; Karakitsios, S.P.; Gotti, A.; Liakos, I.L.; Katsoyiannis, A. Exposure to Major Volatile Organic Compounds and Carbonyls in European Indoor Environments and Associated Health Risk. Environ. Int. 2011, 37, 743–765. [Google Scholar] [CrossRef] [PubMed]
- Paciência, I.; Madureira, J.; Rufo, J.; Moreira, A.; Fernandes, E.d.O. A Systematic Review of Evidence and Implications of Spatial and Seasonal Variations of Volatile Organic Compounds (VOC) in Indoor Human Environments. J. Toxicol. Environ. Health B Crit. Rev. 2016, 19, 47–64. [Google Scholar] [CrossRef] [PubMed]
- Arnold, S.M.; Angerer, J.; Boogaard, P.J.; Hughes, M.F.; O’Lone, R.B.; Robison, S.H.; Schnatter, A.R. The Use of Biomonitoring Data in Exposure and Human Health Risk Assessment: Benzene Case Study. Crit. Rev. Toxicol. 2013, 43, 119–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- IARC Benzene. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; WHO: Geneva, Switzerland, 2018; Volume 120.
- Bahadar, H.; Mostafalou, S.; Abdollahi, M. Current Understandings and Perspectives on Non-Cancer Health Effects of Benzene: A Global Concern. Toxicol. Appl. Pharmacol. 2014, 276, 83–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dere, E.; Ari, F. Effect of Benzene on Liver Functions in Rats (Rattus norvegicus). Environ. Monit. Assess. 2009, 154, 23–27. [Google Scholar] [CrossRef]
- Mandiracioglu, A.; Akgur, S.; Kocabiyik, N.; Sener, U. Evaluation of Neuropsychological Symptoms and Exposure to Benzene, Toluene and Xylene among Two Different Furniture Worker Groups in Izmir. Toxicol. Ind. Health 2011, 27, 802–809. [Google Scholar] [CrossRef]
- Baslo, A.; Aksoy, M. Neurological Abnormalities in Chronic Benzene Poisoning. A Study of Six Patients with Aplastic Anemia and Two with Preleukemia. Environ. Res. 1982, 27, 457–465. [Google Scholar] [CrossRef]
- Kotseva, K.; Popov, T. Study of the Cardiovascular Effects of Occupational Exposure to Organic Solvents. Int. Arch. Occup. Environ. Health 1998, 71, S87–S91. [Google Scholar]
- Snyder, R.; Hedli, C.C. An Overview of Benzene Metabolism. Environ. Health Perspect. 1996, 104 (Suppl. S6), 1165–1171. [Google Scholar] [CrossRef]
- Tsuruta, T.; Tani, K.; Hoshika, A.; Asano, S. Myeloperoxidase Gene Expression and Regulation by Myeloid Cell Growth Factors in Normal and Leukemic Cells. Leuk. Lymphoma 1999, 32, 257–267. [Google Scholar] [CrossRef]
- Carrieri, M.; Bartolucci, G.B.; Scapellato, M.L.; Spatari, G.; Sapienza, D.; Soleo, L.; Lovreglio, P.; Tranfo, G.; Manno, M.; Trevisan, A. Influence of Glutathione S-Transferases Polymorphisms on Biological Monitoring of Exposure to Low Doses of Benzene. Toxicol. Lett. 2012, 213, 63–68. [Google Scholar] [CrossRef]
- Guo, H.; Ahn, S.; Zhang, L. Benzene-Associated Immunosuppression and Chronic Inflammation in Humans: A Systematic Review. Occup. Environ. Med. 2021, 78, 377–384. [Google Scholar] [CrossRef]
- Uzma, N.; Kumar, B.S.; Hazari, M.A.H. Exposure to Benzene Induces Oxidative Stress, Alters the Immune Response and Expression of P53 in Gasoline Filling Workers. Am. J. Ind. Med. 2010, 53, 1264–1270. [Google Scholar] [CrossRef]
- Fenga, C.; Gangemi, S.; Costa, C. Benzene Exposure Is Associated with Epigenetic Changes (Review). Mol. Med. Rep. 2016, 13, 3401–3405. [Google Scholar] [CrossRef] [Green Version]
- Medzhitov, R. Inflammation 2010: New Adventures of an Old Flame. Cell 2010, 140, 771–776. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory Responses and Inflammation-Associated Diseases in Organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef] [Green Version]
- Smith, M.T.; Guyton, K.Z.; Gibbons, C.F.; Fritz, J.M.; Portier, C.J.; Rusyn, I.; DeMarini, D.M.; Caldwell, J.C.; Kavlock, R.J.; Lambert, P.F.; et al. Key Characteristics of Carcinogens as a Basis for Organizing Data on Mechanisms of Carcinogenesis. Environ. Health Perspect. 2016, 124, 713–721. [Google Scholar] [CrossRef] [Green Version]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [Green Version]
- Kuang, H.; Liu, J.; Zeng, Y.; Zhou, W.; Wu, P.; Tan, J.; Li, Y.; Pang, Q.; Jiang, W.; Fan, R. Co-Exposure to Polycyclic Aromatic Hydrocarbons, Benzene and Toluene May Impair Lung Function by Increasing Oxidative Damage and Airway Inflammation in Asthmatic Children. Environ. Pollut. 2020, 266, 115220. [Google Scholar] [CrossRef]
- Barreto, G.; Madureira, D.; Capani, F.; Aon-Bertolino, L.; Saraceno, E.; Alvarez-Giraldez, L.D. The Role of Catechols and Free Radicals in Benzene Toxicity: An Oxidative DNA Damage Pathway. Environ. Mol. Mutagen. 2009, 50, 771–780. [Google Scholar] [CrossRef]
- Yardley-Jones, A.; Anderson, D.; Jenkinson, P.C.; Lovell, D.P.; Blowers, S.D.; Davies, M.J. Genotoxic Effects in Peripheral Blood and Urine of Workers Exposed to Low Level Benzene. Occup. Environ. Med. 1988, 45, 694–700. [Google Scholar] [CrossRef] [PubMed]
- Sze, C.C.; Shi, C.Y.; Ong, C.N. Cytotoxicity and DNA Strand Breaks Induced by Benzene and Its Metabolites in Chinese Hamster Ovary Cells. J. Appl. Toxicol. 1996, 16, 259–264. [Google Scholar] [CrossRef]
- Pénard-Morand, C.; Raherison, C.; Charpin, D.; Kopferschmitt, C.; Lavaud, F.; Caillaud, D.; Annesi-Maesano, I. Long-Term Exposure to Close-Proximity Air Pollution and Asthma and Allergies in Urban Children. Eur. Respir. J. 2010, 36, 33–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nurmatov, U.; Tagieva, N.; Semple, S.; Devereux, G.; Sheikh, A. Volatile Organic Compounds and Risk of Asthma and Allergy: A Systematic Review and Meta-Analysis of Observational and Interventional Studies. Prim. Care Respir. J. 2013, 22, S9–S15. [Google Scholar] [CrossRef] [PubMed]
- Ye, D.; Klein, M.; Chang, H.H.; Sarnat, J.A.; Mulholland, J.A.; Edgerton, E.S.; Winquist, A.; Tolbert, P.E.; Sarnat, S.E. Estimating Acute Cardiorespiratory Effects of Ambient Volatile Organic Compounds. Epidemiology 2017, 28, 197–206. [Google Scholar] [CrossRef]
- Ha, E.K.; Kim, J.H.; Park, D.; Lee, E.; Lee, S.W.; Jee, H.M.; Shin, Y.H.; Han, M.Y. Personal Exposure to Total VOC Is Associated with Symptoms of Atopic Dermatitis in Schoolchildren. J. Korean Med. Sci. 2022, 37, e63. [Google Scholar] [CrossRef]
- Yang, C.Y.; Wang, J.D.; Chan, C.C.; Chen, P.C.; Huang, J.S.; Cheng, M.F. Respiratory and Irritant Health Effects of a Population Living in a Petrochemical-Polluted Area in Taiwan. Environ. Res. 1997, 74, 145–149. [Google Scholar] [CrossRef]
- Brand, A.; McLean, K.E.; Henderson, S.B.; Fournier, M.; Liu, L.; Kosatsky, T.; Smargiassi, A. Respiratory Hospital Admissions in Young Children Living near Metal Smelters, Pulp Mills and Oil Refineries in Two Canadian Provinces. Environ. Int. 2016, 94, 24–32. [Google Scholar] [CrossRef]
- Dunea, D.; Liu, H.-Y.; Iordache, S.; Buruleanu, L.; Pohoata, A. Liaison between Exposure to Sub-Micrometric Particulate Matter and Allergic Response in Children from a Petrochemical Industry City. Sci. Total Environ. 2020, 745, 141170. [Google Scholar] [CrossRef]
- Glass, D.C.; Gray, C.N.; Jolley, D.J.; Gibbons, C.; Sim, M.R.; Fritschi, L.; Adams, G.G.; Bisby, J.A.; Manuell, R. Leukemia Risk Associated with Low-Level Benzene Exposure. Epidemiology 2003, 14, 569–577. [Google Scholar] [CrossRef]
- Rushton, L.; Schnatter, A.R.; Tang, G.; Glass, D.C. Acute Myeloid and Chronic Lymphoid Leukaemias and Exposure to Low-Level Benzene among Petroleum Workers. Br. J. Cancer 2014, 110, 783–787. [Google Scholar] [CrossRef]
- Bonzini, M.; Grillo, P.; Consonni, D.; Cacace, R.; Ancona, C.; Forastiere, F.; Cocco, P.L.; Satta, G.; Boldori, L.; Carugno, M.; et al. Cancer Risk in Oil Refinery Workers: A Pooled Mortality Study in Italy. Med. Lav. 2019, 110, 3–10. [Google Scholar] [CrossRef]
- Järvholm, B.; Mellblom, B.; Norrman, R.; Nilsson, R.; Nordlinder, R. Cancer Incidence of Workers in the Swedish Petroleum Industry. Occup. Environ. Med. 1997, 54, 686–691. [Google Scholar] [CrossRef] [Green Version]
- Paz-y-Miño, C.; López-Cortés, A.; Arévalo, M.; Sánchez, M.E. Monitoring of DNA Damage in Individuals Exposed to Petroleum Hydrocarbons in Ecuador. Ann. N. Y. Acad. Sci. 2008, 1140, 121–128. [Google Scholar] [CrossRef]
- Koh, D.-H.; Jeon, H.-K.; Lee, S.-G.; Ryu, H.-W. The Relationship between Low-Level Benzene Exposure and Blood Cell Counts in Korean Workers. Occup. Environ. Med. 2015, 72, 421–427. [Google Scholar] [CrossRef] [Green Version]
- Monyarch, G.; de Castro Reis, F.; Zock, J.-P.; Giraldo, J.; Pozo-Rodríguez, F.; Espinosa, A.; Rodríguez-Trigo, G.; Verea, H.; Castaño-Vinyals, G.; Gómez, F.P.; et al. Chromosomal Bands Affected by Acute Oil Exposure and DNA Repair Errors. PLoS ONE 2013, 8, e81276. [Google Scholar] [CrossRef] [Green Version]
- Farris, G.M.; Robinson, S.N.; Gaido, K.W.; Wong, B.A.; Wong, V.A.; Hahn, W.P.; Shah, R.S. Benzene-Induced Hematotoxicity and Bone Marrow Compensation in B6C3F1 Mice. Fundam. Appl. Toxicol. 1997, 36, 119–129. [Google Scholar] [CrossRef]
- Zhang, L.; Han, L.; Liu, Z.; Jing, J.; Wang, J.; Zhang, W.; Gao, A. Early Hematopoietic Injury Triggered by Benzene Characterized with Inhibition of Erythrocyte Differentiation Involving the Mollicutes_RF39-Derived Citrulline. Chemosphere 2022, 303, 135009. [Google Scholar] [CrossRef]
- Lan, Q.; Zhang, L.; Li, G.; Vermeulen, R.; Weinberg, R.S.; Dosemeci, M.; Rappaport, S.M.; Shen, M.; Alter, B.P.; Wu, Y.; et al. Hematotoxicity in Workers Exposed to Low Levels of Benzene. Science 2004, 306, 1774–1776. [Google Scholar] [CrossRef] [Green Version]
- Qu, Q.; Shore, R.; Li, G.; Jin, X.; Chen, L.C.; Cohen, B.; Melikian, A.A.; Eastmond, D.; Rappaport, S.M.; Yin, S.; et al. Hematological Changes among Chinese Workers with a Broad Range of Benzene Exposures. Am. J. Ind. Med. 2002, 42, 275–285. [Google Scholar] [CrossRef]
- Farris, G.M.; Robinson, S.N.; Wong, B.A.; Wong, V.A.; Hahn, W.P.; Shah, R. Effects of Benzene on Splenic, Thymic, and Femoral Lymphocytes in Mice. Toxicology 1997, 118, 137–148. [Google Scholar] [CrossRef]
- Bogadi-Sare, A.; Zavalic, M.; Trosić, I.; Turk, R.; Kontosić, I.; Jelcić, I. Study of Some Immunological Parameters in Workers Occupationally Exposed to Benzene. Int. Arch. Occup. Environ. Health 2000, 73, 397–400. [Google Scholar] [CrossRef] [PubMed]
- Kirkeleit, J.; Ulvestad, E.; Riise, T.; Bråtveit, M.; Moen, B.E. Acute Suppression of Serum IgM and IgA in Tank Workers Exposed to Benzene. Scand. J. Immunol. 2006, 64, 690–698. [Google Scholar] [CrossRef] [PubMed]
- Baïz, N.; Slama, R.; Béné, M.-C.; Charles, M.-A.; Kolopp-Sarda, M.-N.; Magnan, A.; Thiebaugeorges, O.; Faure, G.; Annesi-Maesano, I. Maternal Exposure to Air Pollution before and during Pregnancy Related to Changes in Newborn’s Cord Blood Lymphocyte Subpopulations. The EDEN Study Cohort. BMC Pregnancy Childbirth 2011, 11, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Li, H.; Peng, Z.; Cao, J.; Bao, J.; Li, L.; Wang, X.; Ji, Y.; Chen, Z. Meta-Analysis of the Effect of Low-Level Occupational Benzene Exposure on Human Peripheral Blood Leukocyte Counts in China. J. Environ. Sci. 2022, 114, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Busse, W.W.; Viswanathan, R. What Has Been Learned by Cytokine Targeting of Asthma? J. Allergy Clin. Immunol. 2022, 150, 235–249. [Google Scholar] [CrossRef] [PubMed]
- Kuang, H.; Li, Z.; Lv, X.; Wu, P.; Tan, J.; Wu, Q.; Li, Y.; Jiang, W.; Pang, Q.; Wang, Y.; et al. Exposure to Volatile Organic Compounds May Be Associated with Oxidative DNA Damage-Mediated Childhood Asthma. Ecotoxicol. Environ. Saf. 2021, 210, 111864. [Google Scholar] [CrossRef] [PubMed]
- Minov, J.; Karadzinska-Bislimovska, J.; Vasilevska, K.; Trajceva, L.; Risteska-Kuc, S.; Stoleski, S.; Mijakoski, D. Respiratory and Nasal Symptoms, Immunological Changes and Lung Function among Petroleum Refinery Workers. Med. Lav. 2010, 101, 364–374. [Google Scholar]
- Meo, S.A.; Alrashed, A.H.; Almana, A.A.; Altheiban, Y.I.; Aldosari, M.S.; Almudarra, N.F.; Alwabel, S.A. Lung Function and Fractional Exhaled Nitric Oxide among Petroleum Refinery Workers. J. Occup. Med. Toxicol. 2015, 10, 37. [Google Scholar] [CrossRef] [Green Version]
- Liao, Q.; Du, R.; Ma, R.; Liu, X.; Zhang, Y.; Zhang, Z.; Ji, P.; Xiao, M.; Cui, Y.; Xing, X.; et al. Association between Exposure to a Mixture of Benzene, Toluene, Ethylbenzene, Xylene, and Styrene (BTEXS) and Small Airways Function: A Cross-Sectional Study. Environ. Res. 2022, 212, 113488. [Google Scholar] [CrossRef]
- Lawrence, K.G.; Niehoff, N.M.; Keil, A.P.; Braxton Jackson, W., 2nd; Christenbury, K.; Stewart, P.A.; Stenzel, M.R.; Huynh, T.B.; Groth, C.P.; Ramachandran, G.; et al. Associations between Airborne Crude Oil Chemicals and Symptom-Based Asthma. Environ. Int. 2022, 167, 107433. [Google Scholar] [CrossRef]
- Rosenquist, N.A.; Metcalf, W.J.; Ryu, S.Y.; Rutledge, A.; Coppes, M.J.; Grzymski, J.J.; Strickland, M.J.; Darrow, L.A. Acute Associations between PM2.5 and Ozone Concentrations and Asthma Exacerbations among Patients with and without Allergic Comorbidities. J. Expo. Sci. Environ. Epidemiol. 2020, 30, 795–804. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, X.; Guo, C.; Zhang, X.; Zhang, Y.; Deng, N.; Lai, G.; Yang, A.; Huang, Y.; Dang, S.; et al. Hematological Effects and Benchmark Doses of Long-Term Co-Exposure to Benzene, Toluene, and Xylenes in a Follow-up Study on Petrochemical Workers. Toxics 2022, 10, 502. [Google Scholar] [CrossRef]
- D’Andrea, M.A.; Singh, O.; Reddy, G.K. Health Consequences of Involuntary Exposure to Benzene Following a Flaring Incident at British Petroleum Refinery in Texas City. Am. J. Disaster Med. 2013, 8, 169–179. [Google Scholar] [CrossRef]
- Carrieri, M.; Spatari, G.; Tranfo, G.; Sapienza, D.; Scapellato, M.L.; Bartolucci, G.B.; Manno, M. Biological Monitoring of Low Level Exposure to Benzene in an Oil Refinery: Effect of Modulating Factors. Toxicol. Lett. 2018, 298, 70–75. [Google Scholar] [CrossRef]
- D’Andrea, M.A.; Reddy, G.K. Detrimental Health Effects of Benzene Exposure in Adults after a Flaring Disaster at the BP Refinery Plant in Texas City. Disaster Med. Public Health Prep. 2016, 10, 233–239. [Google Scholar] [CrossRef]
- D’Andrea, M.A.; Reddy, G.K. Hematological and Hepatic Alterations in Nonsmoking Residents Exposed to Benzene Following a Flaring Incident at the British Petroleum Plant in Texas City. Environ. Health 2014, 13, 115. [Google Scholar] [CrossRef] [Green Version]
- D’Andrea, M.A.; Reddy, G.K. Benzene Exposure from the BP Refinery Flaring Incident Alters Hematological and Hepatic Functions among Smoking Subjects. Int. J. Occup. Med. Environ. Health 2017, 30, 849–860. [Google Scholar] [CrossRef] [Green Version]
- D’Andrea, M.A.; Reddy, G.K. Health Effects of Benzene Exposure among Children Following a Flaring Incident at the British Petroleum Refinery in Texas City. Pediatr. Hematol. Oncol. 2014, 31, 1–10. [Google Scholar] [CrossRef]
- D’Andrea, M.A.; Reddy, G.K. Adverse Health Effects of Benzene Exposure among Children Following a Flaring Incident at the British Petroleum Refinery in Texas City. Clin. Pediatr. 2016, 55, 219–227. [Google Scholar] [CrossRef]
- Midzenski, M.A.; McDiarmid, M.A.; Rothman, N.; Kolodner, K. Acute High Dose Exposure to Benzene in Shipyard Workers. Am. J. Ind. Med. 1992, 22, 553–565. [Google Scholar] [CrossRef] [PubMed]
- Travis, L.B.; Li, C.Y.; Zhang, Z.N.; Li, D.G.; Yin, S.N.; Chow, W.H.; Li, G.L.; Dosemeci, M.; Blot, W.; Fraumeni, J.F., Jr. Hematopoietic Malignancies and Related Disorders among Benzene-Exposed Workers in China. Leuk. Lymphoma 1994, 14, 91–102. [Google Scholar] [CrossRef]
- Rothman, N.; Smith, M.T.; Hayes, R.B.; Li, G.L.; Irons, R.D.; Dosemeci, M.; Haas, R.; Stillman, W.S.; Linet, M.; Xi, L.Q.; et al. An Epidemiologic Study of Early Biologic Effects of Benzene in Chinese Workers. Environ. Health Perspect. 1996, 104 (Suppl. S6), 1365–1370. [Google Scholar] [CrossRef] [PubMed]
- Wallace, L. Environmental Exposure to Benzene: An Update. Environ. Health Perspect. 1996, 104, 1129–1136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ray, M.R.; Roychoudhury, S.; Mukherjee, S.; Lahiri, T. Occupational Benzene Exposure from Vehicular Sources in India and Its Effect on Hematology, Lymphocyte Subsets and Platelet P-Selectin Expression. Toxicol. Ind. Health 2007, 23, 167–175. [Google Scholar] [CrossRef]
- Huang, J.; Zhao, M.; Wang, P.; Li, X.; Ma, L.; Zhang, J.; Zhou, Y. Effects of Low Concentrations of Benzene Exposure on Levels of Platelet-Associated Antibodies and Platelet Parameters. J. Occup. Environ. Med. 2014, 56, e92–e97. [Google Scholar] [CrossRef]
- Ahmadi, Z.; Moradabadi, A.; Abdollahdokht, D.; Mehrabani, M.; Nematollahi, M.H. Association of Environmental Exposure with Hematological and Oxidative Stress Alteration in Gasoline Station Attendants. Environ. Sci. Pollut. Res. Int. 2019, 26, 20411–20417. [Google Scholar] [CrossRef]
- Lee, C.R.; Yoo, C.I.; Lee, J.H.; Kim, S.-R.; Kim, Y. Hematological Changes of Children Exposed to Volatile Organic Compounds Containing Low Levels of Benzene. Sci. Total Environ. 2002, 299, 237–245. [Google Scholar] [CrossRef]
- Pelallo-Martínez, N.A.; Batres-Esquivel, L.; Carrizales-Yáñez, L.; Díaz-Barriga, F.M. Genotoxic and Hematological Effects in Children Exposed to a Chemical Mixture in a Petrochemical Area in Mexico. Arch. Environ. Contam. Toxicol. 2014, 67, 1–8. [Google Scholar] [CrossRef]
- Goldman, L.R. Children--Unique and Vulnerable. Environmental Risks Facing Children and Recommendations for Response. Environ. Health Perspect. 1995, 103 (Suppl. S6), 13–18. [Google Scholar] [CrossRef]
- Chen, Q.; Sun, H.; Zhang, J.; Xu, Y.; Ding, Z. The Hematologic Effects of BTEX Exposure among Elderly Residents in Nanjing: A Cross-Sectional Study. Environ. Sci. Pollut. Res. Int. 2019, 26, 10552–10561. [Google Scholar] [CrossRef]
- Li, X.; Guo, Y.; Song, X.; He, Y.; Zhang, H.; Bao, H.; Li, X.; Liu, Y.; Zhai, Y.; Wang, J.; et al. A Cross-Sectional Survey Based on Blood VOCs, Hematological Parameters and Urine Indicators in a Population in Jilin, Northeast China. Environ. Geochem. Health 2019, 41, 1599–1615. [Google Scholar] [CrossRef]
- Silva, C.B.; Mota, C.d.L.; Almeida, Y.R.; Emídio, V.; Fonseca, A.S.A.; Mitri, S.; Moreira, J.C. Environmental Exposure to Benzene: Evaluation of Urinary S-PMA and Polymorphism (CYP2E1-1293G>C and NQO1 609C>T) in Campos Elíseos Residents, Duque de Caxias, Rio de Janeiro State, Brazil. Cad. Saude Publica 2019, 35, e00198618. [Google Scholar] [CrossRef] [Green Version]
- Dougherty, D.; Garte, S.; Barchowsky, A.; Zmuda, J.; Taioli, E. NQO1, MPO, CYP2E1, GSTT1 and GSTM1 Polymorphisms and Biological Effects of Benzene Exposure—A Literature Review. Toxicol. Lett. 2008, 182, 7–17. [Google Scholar] [CrossRef]
- Wang, L.; He, X.; Bi, Y.; Ma, Q. Stem Cell and Benzene-Induced Malignancy and Hematotoxicity. Chem. Res. Toxicol. 2012, 25, 1303–1315. [Google Scholar] [CrossRef]
- Mitri, S.; Fonseca, A.S.A.; Otero, U.B.; Tabalipa, M.M.; Moreira, J.C.; Sarcinelli, P. de N. Metabolic Polymorphisms and Clinical Findings Related to Benzene Poisoning Detected in Exposed Brazilian Gas-Station Workers. Int. J. Environ. Res. Public Health 2015, 12, 8434–8447. [Google Scholar] [CrossRef] [Green Version]
- Seaton, M.J.; Schlosser, P.M.; Bond, J.A.; Medinsky, M.A. Benzene Metabolism by Human Liver Microsomes in Relation to Cytochrome P450 2E1 Activity. Carcinogenesis 1994, 15, 1799–1806. [Google Scholar] [CrossRef]
- Nebert, D.W.; Roe, A.L.; Vandale, S.E.; Bingham, E.; Oakley, G.G. NAD(P)H:Quinone Oxidoreductase (NQO1) Polymorphism, Exposure to Benzene, and Predisposition to Disease: A HuGE Review. Genet. Med. 2002, 4, 62–70. [Google Scholar] [CrossRef] [Green Version]
- Samadi, M.T.; Shakerkhatibi, M.; Poorolajal, J.; Rahmani, A.; Rafieemehr, H.; Hesam, M. Association of Long Term Exposure to Outdoor Volatile Organic Compounds (BTXS) with pro-Inflammatory Biomarkers and Hematologic Parameters in Urban Adults: A Cross-Sectional Study in Tabriz, Iran. Ecotoxicol. Environ. Saf. 2019, 180, 152–159. [Google Scholar] [CrossRef]
- Karaulov, A.V.; Mikhaylova, I.V.; Smolyagin, A.I.; Boev, V.M.; Kalogeraki, A.; Tsatsakis, A.M.; Engin, A.B. The Immunotoxicological Pattern of Subchronic and Chronic Benzene Exposure in Rats. Toxicol. Lett. 2017, 275, 1–5. [Google Scholar] [CrossRef]
- Robert Schnatter, A.; Kerzic, P.J.; Zhou, Y.; Chen, M.; Nicolich, M.J.; Lavelle, K.; Armstrong, T.W.; Bird, M.G.; Lin, L.; Fu, H.; et al. Peripheral Blood Effects in Benzene-Exposed Workers. Chem. Biol. Interact. 2010, 184, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Barregard, L.; Holmberg, E.; Sallsten, G. Leukaemia Incidence in People Living Close to an Oil Refinery. Environ. Res. 2009, 109, 985–990. [Google Scholar] [CrossRef] [PubMed]
- Axelsson, G.; Barregard, L.; Holmberg, E.; Sallsten, G. Cancer Incidence in a Petrochemical Industry Area in Sweden. Sci. Total Environ. 2010, 408, 4482–4487. [Google Scholar] [CrossRef] [PubMed]
- Beale, L.; Hodgson, S.; Abellan, J.J.; Lefevre, S.; Jarup, L. Evaluation of Spatial Relationships between Health and the Environment: The Rapid Inquiry Facility. Environ. Health Perspect. 2010, 118, 1306–1312. [Google Scholar] [CrossRef] [PubMed]
- Hurtig, A.-K.; San Sebastián, M. Incidence of Childhood Leukemia and Oil Exploitation in the Amazon Basin of Ecuador. Int. J. Occup. Environ. Health 2004, 10, 245–250. [Google Scholar] [CrossRef]
- Weng, H.-H.; Tsai, S.-S.; Chiu, H.-F.; Wu, T.-N.; Yang, C.-Y. Association of Childhood Leukemia with Residential Exposure to Petrochemical Air Pollution in Taiwan. Inhal. Toxicol. 2008, 20, 31–36. [Google Scholar] [CrossRef]
- Yu, C.-L.; Wang, S.-F.; Pan, P.-C.; Wu, M.-T.; Ho, C.-K.; Smith, T.J.; Li, Y.; Pothier, L.; Christiani, D.C.; Group, K.L.R. Residential Exposure to Petrochemicals and the Risk of Leukemia: Using Geographic Information System Tools to Estimate Individual-Level Residential Exposure. Am. J. Epidemiol. 2006, 164, 200–207. [Google Scholar] [CrossRef] [Green Version]
- Lyons, R.A.; Monaghan, S.P.; Heaven, M.; Littlepage, B.N.; Vincent, T.J.; Draper, G.J. Incidence of Leukaemia and Lymphoma in Young People in the Vicinity of the Petrochemical Plant at Baglan Bay, South Wales, 1974 to 1991. Occup. Environ. Med. 1995, 52, 225–228. [Google Scholar] [CrossRef]
- Sans, S.; Elliott, P.; Kleinschmidt, I.; Shaddick, G.; Pattenden, S.; Walls, P.; Grundy, C.; Dolk, H. Cancer Incidence and Mortality near the Baglan Bay Petrochemical Works, South Wales. Occup. Environ. Med. 1995, 52, 217–224. [Google Scholar] [CrossRef]
- Micheli, A.; Meneghini, E.; Mariottini, M.; Baldini, M.; Baili, P.; di Salvo, F.; Sant, M. Risk of Death for Hematological Malignancies for Residents Close to an Italian Petrochemical Refinery: A Population-Based Case-Control Study. Cancer Causes Control 2014, 25, 1635–1644. [Google Scholar] [CrossRef] [Green Version]
- Salerno, C.; Berchialla, P.; Palin, L.A.; Vanhaecht, K.; Panella, M. Cancer Morbidity of Residents Living near an Oil Refinery Plant in North-West Italy. Int. J. Environ. Health Res. 2013, 23, 342–351. [Google Scholar] [CrossRef]
- Dahlgren, J.; Klein, J.; Takhar, H. Cluster of Hodgkin’s Lymphoma in Residents near a Non-Operational Petroleum Refinery. Toxicol. Ind. Health 2008, 24, 683–692. [Google Scholar] [CrossRef]
- Ramis, R.; Diggle, P.; Boldo, E.; Garcia-Perez, J.; Fernandez-Navarro, P.; Lopez-Abente, G. Analysis of Matched Geographical Areas to Study Potential Links between Environmental Exposure to Oil Refineries and Non-Hodgkin Lymphoma Mortality in Spain. Int. J. Health Geogr. 2012, 11, 4. [Google Scholar] [CrossRef] [Green Version]
- Ventura, M.T.; Scichilone, N.; Paganelli, R.; Minciullo, P.L.; Patella, V.; Bonini, M.; Passalacqua, G.; Lombardi, C.; Simioni, L.; Ridolo, E.; et al. Allergic Diseases in the Elderly: Biological Characteristics and Main Immunological and Non-Immunological Mechanisms. Clin. Mol. Allergy 2017, 15, 2. [Google Scholar] [CrossRef] [Green Version]
- Martins-Dos-Santos, G.; Araújo, M.; Prates, S.; Leiria-Pinto, P. Immunoallergic Disorders in the Elderly. Eur. Ann. Allergy Clin. Immunol. 2022, 54, 175–182. [Google Scholar] [CrossRef]
- Smargiassi, A.; Goldberg, M.S.; Wheeler, A.J.; Plante, C.; Valois, M.-F.; Mallach, G.; Kauri, L.M.; Shutt, R.; Bartlett, S.; Raphoz, M.; et al. Associations between Personal Exposure to Air Pollutants and Lung Function Tests and Cardiovascular Indices among Children with Asthma Living near an Industrial Complex and Petroleum Refineries. Environ. Res. 2014, 132, 38–45. [Google Scholar] [CrossRef]
- Wichmann, F.A.; Müller, A.; Busi, L.E.; Cianni, N.; Massolo, L.; Schlink, U.; Porta, A.; Sly, P.D. Increased Asthma and Respiratory Symptoms in Children Exposed to Petrochemical Pollution. J. Allergy Clin. Immunol. 2009, 123, 632–638. [Google Scholar] [CrossRef]
- De Moraes, A.C.L.; Ignotti, E.; Netto, P.A.; Jacobson, L.d.S.V.; Castro, H.; Hacon, S.d.S. Wheezing in Children and Adolescents Living next to a Petrochemical Plant in Rio Grande Do Norte, Brazil. J. Pediatr. 2010, 86, 337–344. [Google Scholar] [CrossRef]
- Rusconi, F.; Catelan, D.; Accetta, G.; Peluso, M.; Pistelli, R.; Barbone, F.; di Felice, E.; Munnia, A.; Murgia, P.; Paladini, L.; et al. Asthma Symptoms, Lung Function, and Markers of Oxidative Stress and Inflammation in Children Exposed to Oil Refinery Pollution. J. Asthma 2011, 48, 84–90. [Google Scholar] [CrossRef]
- Idavain, J.; Julge, K.; Rebane, T.; Lang, A.; Orru, H. Respiratory Symptoms, Asthma and Levels of Fractional Exhaled Nitric Oxide in Schoolchildren in the Industrial Areas of Estonia. Sci. Total Environ. 2018, 650, 65–72. [Google Scholar] [CrossRef]
- Orru, H.; Idavain, J.; Pindus, M.; Orru, K.; Kesanurm, K.; Lang, A.; Tomasova, J. Residents’ Self-Reported Health Effects and Annoyance in Relation to Air Pollution Exposure in an Industrial Area in Eastern-Estonia. Int. J. Environ. Res. Public Health 2018, 15, 252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- The International Study of Asthma and Allergies in Childhood (ISAAC) Steering Committee. Worldwide Variations in the Prevalence of Asthma Symptoms: The International Study of Asthma and Allergies in Childhood (ISAAC). Eur. Respir. J. 1998, 12, 315–335. [Google Scholar] [CrossRef] [PubMed]
- Galassi, C.; Forastiere, F.; Biggeri, A.; Gabellini, C.; de Sario, M.; Ciccone, G.; Biocca, M.; Bisanti, L.; Gruppo Collaborativo SIDRIA-2. SIDRIA Second Phase: Objectives, Study Design and Methods. Epidemiol. Prev. 2005, 29, 9–13. [Google Scholar] [PubMed]
- Tanyanont, W.; Vichit-Vadakan, N. Exposure to Volatile Organic Compounds and Health Risks among Residents in an Area Affected by a Petrochemical Complex in Rayong, Thailand. Southeast Asian J. Trop. Med. Public Health 2012, 43, 201–211. [Google Scholar] [PubMed]
- Rovira, E.; Cuadras, A.; Aguilar, X.; Esteban, L.; Borràs-Santos, A.; Zock, J.-P.; Sunyer, J. Asthma, Respiratory Symptoms and Lung Function in Children Living near a Petrochemical Site. Environ. Res. 2014, 133, 156–163. [Google Scholar] [CrossRef] [Green Version]
- Gillis, B.; Gavin, I.M.; Arbieva, Z.; King, S.T.; Jayaraman, S.; Prabhakar, B.S. Identification of Human Cell Responses to Benzene and Benzene Metabolites. Genomics 2007, 90, 324–333. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Guo, X.; Chen, Y.; Zhang, W.; Ren, J.; Gao, A. Association between Benzene Exposure, Serum Levels of Cytokines and Hematological Measures in Chinese Workers: A Cross-Sectional Study. Ecotoxicol. Environ. Saf. 2021, 207, 111562. [Google Scholar] [CrossRef]
- Lu, P.C.W.; Shahbaz, S.; Winn, L.M. Benzene and Its Effects on Cell Signaling Pathways Related to Hematopoiesis and Leukemia. J. Appl. Toxicol. 2020, 40, 1018–1032. [Google Scholar] [CrossRef]
- Yang, X.; Lu, Y.; He, F.; Hou, F.; Xing, C.; Xu, P.; Wang, Q.-F. Benzene Metabolite Hydroquinone Promotes DNA Homologous Recombination Repair via the NF-ΚB Pathway. Carcinogenesis 2019, 40, 1021–1030. [Google Scholar] [CrossRef]
- Sun, R.; Xu, K.; Ji, S.; Pu, Y.; Yu, L.; Yin, L.; Zhang, J.; Pu, Y. Toxicity in Hematopoietic Stem Cells from Bone Marrow and Peripheral Blood in Mice after Benzene Exposure: Single-Cell Transcriptome Sequencing Analysis. Ecotoxicol. Environ. Saf. 2021, 207, 111490. [Google Scholar] [CrossRef]
- Stokes, S.E.; Winn, L.M. NF-ΚB Signaling Is Increased in HD3 Cells Following Exposure to 1,4-Benzoquinone: Role of Reactive Oxygen Species and P38-MAPK. Toxicol. Sci. 2014, 137, 303–310. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Li, D.; He, Z.; Fan, J.; Li, Q.; Liu, X.; Guo, P.; Zhang, H.; Chen, S.; Li, Q.; et al. The Effects of Nrf2 Knockout on Regulation of Benzene-Induced Mouse Hematotoxicity. Toxicol. Appl. Pharmacol. 2018, 358, 56–67. [Google Scholar] [CrossRef]
- Sun, R.; Liu, M.; Xu, K.; Pu, Y.; Huang, J.; Liu, J.; Zhang, J.; Yin, L.; Pu, Y. Ferroptosis Is Involved in the Benzene-Induced Hematotoxicity in Mice via Iron Metabolism, Oxidative Stress and NRF2 Signaling Pathway. Chem. Biol. Interact. 2022, 362, 110004. [Google Scholar] [CrossRef]
- Malovichko, M.V.; Abplanalp, W.T.; McFall, S.A.; Taylor, B.S.; Wickramasinghe, N.S.; Sithu, I.D.; Zelko, I.N.; Uchida, S.; Hill, B.G.; Sutaria, S.R.; et al. Subclinical Markers of Cardiovascular Toxicity of Benzene Inhalation in Mice. Toxicol. Appl. Pharmacol. 2021, 431, 115742. [Google Scholar] [CrossRef]
- Spatari, G.; Allegra, A.; Carrieri, M.; Pioggia, G.; Gangemi, S. Epigenetic Effects of Benzene in Hematologic Neoplasms: The Altered Gene Expression. Cancers 2021, 13, 2392. [Google Scholar] [CrossRef]
- Triggiani, M.; Loffredo, S.; Granata, F.; Staiano, R.I.; Marone, G. Modulation of Mast Cell and Basophil Functions by Benzene Metabolites. Curr. Pharm. Des. 2011, 17, 3830–3835. [Google Scholar] [CrossRef]
- Shimada, A.L.B.; Lino-Dos-Santos-Franco, A.; Bolonheis, S.M.; Nakasato, A.; Damazo, A.S.; Tavares-de-Lima, W.; Farsky, S.H.P. In Vivo Hydroquinone Exposure Causes Tracheal Hyperresponsiveness Due to TNF Secretion by Epithelial Cells. Toxicol. Lett. 2012, 211, 10–17. [Google Scholar] [CrossRef]
- Kawasaki, S.; Takizawa, H.; Takami, K.; Desaki, M.; Okazaki, H.; Kasama, T.; Kobayashi, K.; Yamamoto, K.; Nakahara, K.; Tanaka, M.; et al. Benzene-Extracted Components Are Important for the Major Activity of Diesel Exhaust Particles: Effect on Interleukin-8 Gene Expression in Human Bronchial Epithelial Cells. Am. J. Respir. Cell Mol. Biol. 2001, 24, 419–426. [Google Scholar] [CrossRef] [Green Version]
- Lehmann, I.; Rehwagen, M.; Diez, U.; Seiffart, A.; Rolle-Kampczyk, U.; Richter, M.; Wetzig, H.; Borte, M.; Herbarth, O.; Leipzig Allergy Risk Children Study. Enhanced in Vivo IgE Production and T Cell Polarization toward the Type 2 Phenotype in Association with Indoor Exposure to VOC: Results of the LARS Study. Int. J. Hyg. Environ. Health 2001, 204, 211–221. [Google Scholar] [CrossRef]
- Shute, J.K.; Vrugt, B.; Lindley, I.J.; Holgate, S.T.; Bron, A.; Aalbers, R.; Djukanović, R. Free and Complexed Interleukin-8 in Blood and Bronchial Mucosa in Asthma. Am. J. Respir. Crit. Care Med. 1997, 155, 1877–1883. [Google Scholar] [CrossRef]
- Jatakanon, A.; Lalloo, U.G.; Lim, S.; Chung, K.F.; Barnes, P.J. Increased Neutrophils and Cytokines, TNF-Alpha and IL-8, in Induced Sputum of Non-Asthmatic Patients with Chronic Dry Cough. Thorax 1999, 54, 234–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukherjee, B.; Dutta, A.; Roychoudhury, S.; Ray, M.R. Chronic Inhalation of Biomass Smoke Is Associated with DNA Damage in Airway Cells: Involvement of Particulate Pollutants and Benzene. J. Appl. Toxicol. 2013, 33, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, M.; Stellavato, A.; Cammarota, M.; Lamberti, M.; Miraglia, N.; Sannolo, N.; de Rosa, M. Effects of Low Concentrations of Benzene on Human Lung Cells in vitro. Toxicol. Lett. 2009, 188, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Li, C.; Liu, W.; Jin, Y. Effect of Exposure to Volatile Organic Compounds (VOCs) on Airway Inflammatory Response in Mice. J. Toxicol. Sci. 2012, 37, 739–748. [Google Scholar] [CrossRef] [Green Version]
- Spatari, G.; Saitta, S.; Cimino, F.; Sapienza, D.; Quattrocchi, P.; Carrieri, M.; Barbaro, M.; Saija, A.; Gangemi, S. Increased Serum Levels of Advanced Oxidation Protein Products and Glycation End Products in Subjects Exposed to Low-Dose Benzene. Int. J. Hyg. Environ. Health 2012, 215, 389–392. [Google Scholar] [CrossRef]
| Author | Date | Refineries Site | Cohort | Age | Distance from Refineries | Exposure Time | Observational Time | Outcome in Exposed Group |
|---|---|---|---|---|---|---|---|---|
| Data studies | ||||||||
| D’andrea et al. [55] | 2013 | Texas City, USA | 200 (100 exposed) | All | <48 km | 40 days | 2010–2012 | ↑ WBCs, PLTs, ALP and AST; ↓BUN and creatinine. Adults vs. Children (exposed): ↑ Hb, HCT, ALT, creatinine, beta2-microglobulin and urinary phenol; ↓ PLTs and ALP |
| D’andrea et al. [57] | April-16 | Texas City, USA | 2213 (1826 exposed) | Adults | <48 km | 40 days | 2010–2012 | ↑ WBCs, PLTs, creatinine, ALT, AST and ALP |
| D’andrea et al. [58] | December-14 | Texas City, USA | 1422 non-smokers (1093 exposed) | Adults | <48 km | 40 days | 2010–2012 | ↑ WBCs, PLTs, Hb, HCT, BUN, ALP, AST and ALT |
| D’andrea et al. [59] | October-17 | Texas City, USA | 791 smokers (733 exposed) | Adults | <48 km | 40 days | 2010–2012 | ↑WBCs, PLTs, ALP, AST and ALT |
| D’andrea et al. [60] | February-14 | Texas City, USA | 312 (157 exposed) | Children (<17) | <48 km | 40 days | 2010–2012 | ↓ WBCs; ↑ PLTs, ALP, AST, ALT and creatinine; Neurological, respiratory and dermatological symptoms |
| D’andrea et al. [61] | March-16 | Texas City, USA | 899 (641 exposed) | Children (<17) | <48 km | 40 days | 2010–2012 | ↓WBCs, HCT, Hb and BUN; ↑ PLTs, ALP, AST and ALT |
| Lee et al. [69] | November-02 | Ulsan, Korea | 192 (97 exposed) | Children (8–11) | <16 km | 4–11 years | 2000 | In April measurement: ↓ WBCs, absolute neutrophil and lymphocyte count, RBCs, PTLs and Hb. In July measurement: no differences. In October measurement: ↓ RBCs and Hb. |
| Pelallo-Martinez et al. [70] | July-14 | Coatzacoalcos, Mexico | 102 | Children (6–12) | ~7 km | 6–12 years | NS | Negative correlation between urinary t,t-MA and WBCs and RBCs count |
| Chen et al. [72] | April-19 | Nanjing, China | 421 (240 exposed) | Adults (50–71) | ~3 km | 5–71 years | 2016 | ↓ Neutrophil counts, RBC counts, Hb concentration, HCT percentage, MCHC levels and PLT counts; ↑ monocyte and basophil counts |
| Li et al. [73] | June-19 | Jilin, China | 499 | Adults | NS | >5 years | 2016 | ↑ Detection rate of blood benzene; changes in urinary WBC related to benzene |
| Silva et al. [74] | August-19 | Campos Elíseos district, Rio de Janeiro State, Brazil | 190 | Adults | <1 km | >3 months | 2016–2017 | Association between detectable S-PMA and variant allele in NQO1 with hematological abnormalities |
| Epidemiological studies | ||||||||
| Barregard et al. [83] | November-09 | Lysekil, Sweden | NS | NS | <5 km | NS | 1975–2004 | ↑ Incidence of leukemia |
| Axelsson et al. [84] | September-10 | Stenungsund, Sweden | NS | NS | NS | NS | 1974–2005 | No evidence of increased risk of leukemia and lymphoma |
| Beale et al. [85] | September-10 | Utah, USA | NS | NS | <5 km | NS | 1973–2006 | Increased risk of NHL; no evidence of increased risk of HL, MM and leukemia |
| Hurtig et al. [86] | 2004 | Ecuador | 357,000 ca | Children (0–14) | NS | NS | 1985–2000 | ↑ Risk of childhood leukemia |
| Weng et al. [87] | January-08 | Taiwan | NS | Children (0–19) | NS | NS | 1995–2005 | ↑ Incidence of childhood leukemia |
| Yu et al. [88] | August-06 | Taiwan | NS | All (0–19; 20–29) | 657.1 km2 | NS | 1997–2003 | ↑ Risk leukemia risk in 20–29 y.o. subjects |
| Lyons et al. [89] | April-95 | Baglan Bay, South Wales | NS | 0–24 | 1.5–3 km | NS | 1974–1991 | No augmented incidence of leukemias and lymphomas |
| Sans et al. [90] | April-95 | Baglan Bay, South Wales | 115,000 ca | All | 7.5 km | NS | 1974–1984 (incidence) 1981–1991 (mortality) | Augmented incidence of all tumors |
| Micheli et al. [91] | December-14 | Falconara, Italy | 526 (177 HM related deaths) | NS | 65 km2 | NS | 1994–2003 | ↑ Risk of death from leukemia or NHL in females and retired-homemaker-unemployed category |
| Salerno et al. [92] | 2013 | Cerano, Italy | NS | NS | <3 km | NS | 2003–2009 | ↑ Incidence of MM in women and leukemic lymphoid neoplasm in women and men |
| Dahlgren et al. [93] | November-08 | Sugar Creek, Missouri | 4500 ca | NS | 1.6 km | NS | NS | ↑Prevalence for Hodgkin’s disease |
| Ramis et al. [94] | February-12 | 10 site in Spain | 1,744,988 | NS | 10 km | NS | 1997–2006 | Possible increased risk of NHL mortality, no gradient with increasing distance |
| Author | Date | Refineries Sites | Cohort | Age | Distance from Refineries | Exposure Time | Observational Time | Outcome in Exposed Groups |
|---|---|---|---|---|---|---|---|---|
| Smargiassi et al. [97] | July-14 | Montreal, Quebec | 72 recruited | Children (8–12) | NS | NS | 10 days | Suggestion for a small ↓ in respiratory function with total concentrations of PAHs |
| Wichmann et al. [98] | March-09 | La Plata, Argentina | 1212 recruited | Children (6–12) | ~10 km | NS | 2005–2006 | ↑ Prevalence of asthma, ↑ asthma exacerbations, ↑ respiratory symptoms |
| De Moraes et al. [99] | August-10 | Rio Grande do Norte, Brazil | 209 recruited | Children (0–14) | within a 5-km radius | at least one year | 1 year (2006) | ↑ Frequency of respiratory symptoms |
| Rusconi et al. [100] | February-11 | Sarroch, Sardinia | 300 recruited | Children (6–14) | NS | NS | January–June 2007 | ↓ Lung function, ↑ markers of bronchial inflammation and oxidative DNA damage, ↑ MDA–dG adduct values |
| Idavain et al. [101] | February-19 | Ida-Viru county, Estonia | 1326 recruited | Children (8–12) | NS | NS | NS | ↑ Prevalence of respiratory symptoms and of high fractional exhaled NO (FeNO) values (≥30 ppb) |
| Orru et al. [102] | February-18 | Ida-Viru County, Estonia | 5250 recruited | Adults (18–70) | NS | NS | 2001–2013 | ↑ Frequency of wheezing, chest tightness, shortness of breath, asthma attack, long-term cough, cardiovascular diseases and diabetes in oil shale workers compared with exposed residents |
| Tanyanont et al. [105] | January-12 | Map Ta Phut Municipality, Thailand | 24.980 recruited | All (>13) | 10 km radius | at least 1 year | September 2006–August 2007 | ↑ Frequency of all acute respiratory symptoms among residents living < 5 km, ↑ risk of dyspnea and wheezing in residents longer than 5 years near the industrial complex |
| Rovira et al. [106] | August-14 | Tarragona county, Catalonia, Spain | 5.196 recruited | Children (6–7; 13–14) | 10 km | NS | 2010 | Not found higher prevalence of asthma, allergic symptoms and lower lung function. Significantly ↑ prevalence of respiratory hospitalizations and nocturnal cough |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cordiano, R.; Papa, V.; Cicero, N.; Spatari, G.; Allegra, A.; Gangemi, S. Effects of Benzene: Hematological and Hypersensitivity Manifestations in Resident Living in Oil Refinery Areas. Toxics 2022, 10, 678. https://doi.org/10.3390/toxics10110678
Cordiano R, Papa V, Cicero N, Spatari G, Allegra A, Gangemi S. Effects of Benzene: Hematological and Hypersensitivity Manifestations in Resident Living in Oil Refinery Areas. Toxics. 2022; 10(11):678. https://doi.org/10.3390/toxics10110678
Chicago/Turabian StyleCordiano, Raffaele, Vincenzo Papa, Nicola Cicero, Giovanna Spatari, Alessandro Allegra, and Sebastiano Gangemi. 2022. "Effects of Benzene: Hematological and Hypersensitivity Manifestations in Resident Living in Oil Refinery Areas" Toxics 10, no. 11: 678. https://doi.org/10.3390/toxics10110678






