Legacy Chemical Pollutants in House Dust of Homes of Pregnant African Americans in Atlanta
Abstract
1. Introduction
2. Methods
2.1. Chemicals and Reagents
2.2. Preparation of Standard Calibrants and Labeled Standard Spiking Solutions
2.3. Quality Control and Assurance
2.4. Study Design and Population
2.5. Dust Collection
2.6. Sample Preparation and Analysis
2.7. Validation
2.8. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- EPA. TSCA Chemical Substance Inventory. Available online: https://www.epa.gov/tsca-inventory#:~:text=The%20Toxic%20Substances%20Control%20Act,EPA%20on%20TSCA%20regulatory%20matters. (accessed on 12 October 2022).
- Bigus, P.; Tobiszewski, M.; Namieśnik, J. Historical records of organic pollutants in sediment cores. Mar. Pollut. Bull. 2014, 78, 26–42. [Google Scholar] [CrossRef] [PubMed]
- EPA. Report on the Environment. Available online: https://www.epa.gov/report-environment (accessed on 12 October 2022).
- Guo, W.; Pan, B.; Sakkiah, S.; Yavas, G.; Ge, W.; Zou, W.; Tong, W.; Hong, H. Persistent Organic Pollutants in Food: Contamination Sources, Health Effects and Detection Methods. Int. J. Environ. Res. Public Health 2019, 16, 4361. [Google Scholar] [CrossRef] [PubMed]
- Peshin, S.S.; Lall, S.B.; Gupta, S.K. Potential food contaminants and associated health risks. Acta Pharmacol. Sin. 2002, 23, 193–202. [Google Scholar] [PubMed]
- Rigét, F.; Vorkamp, K.; Bossi, R.; Sonne, C.; Letcher, R.J.; Dietz, R. Twenty years of monitoring of persistent organic pollutants in Greenland biota. A review. Environ. Pollut. 2016, 217, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.M.; Bharat, G.K.; Tayal, S.; Nizzetto, L.; Cupr, P.; Larssen, T. Environment and human exposure to persistent organic pollutants (POPs) in India: A systematic review of recent and historical data. Environ. Int. 2014, 66, 48–64. [Google Scholar] [CrossRef]
- Weber, R.; Gaus, C.; Tysklind, M.; Johnston, P.; Forter, M.; Hollert, H.; Heinisch, E.; Holoubek, I.; Lloyd-Smith, M.; Masunaga, S.; et al. Dioxin- and POP-contaminated sites—Contemporary and future relevance and challenges: Overview on background, aims and scope of the series. Environ. Sci. Pollut. Res. Int. 2008, 15, 363–393. [Google Scholar] [CrossRef]
- Barr, D.B.; Olsson, A.O.; Wong, L.Y.; Udunka, S.; Baker, S.E.; Whitehead, R.D.; Magsumbol, M.S.; Williams, B.L.; Needham, L.L. Urinary concentrations of metabolites of pyrethroid insecticides in the general U.S. population: National Health and Nutrition Examination Survey 1999–2002. Environ. Health Perspect. 2010, 118, 742–748. [Google Scholar] [CrossRef]
- Barr, D.B.; Wong, L.Y.; Bravo, R.; Weerasekera, G.; Odetokun, M.; Restrepo, P.; Kim, D.G.; Fernandez, C.; Whitehead, R.D., Jr.; Perez, J.; et al. Urinary concentrations of dialkylphosphate metabolites of organophosphorus pesticides: National Health and Nutrition Examination Survey 1999–2004. Int. J. Environ. Res. Public Health 2011, 8, 3063–3098. [Google Scholar] [CrossRef]
- Clune, A.L.; Ryan, P.B.; Barr, D.B. Have regulatory efforts to reduce organophosphorus insecticide exposures been effective? Environ. Health Perspect. 2012, 120, 521–525. [Google Scholar] [CrossRef]
- Crinnion, W.J. The CDC fourth national report on human exposure to environmental chemicals: What it tells us about our toxic burden and how it assist environmental medicine physicians. Altern. Med. Rev. 2010, 15, 101–109. [Google Scholar]
- Hendryx, M.; Luo, J. Children’s environmental chemical exposures in the USA, NHANES 2003–2012. Environ. Sci. Pollut. Res. Int. 2018, 25, 5336–5343. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.J.; Barr, D.B.; Reidy, J.A.; Malek, N.A.; Hodge, C.C.; Caudill, S.P.; Brock, J.W.; Needham, L.L.; Calafat, A.M. Urinary levels of seven phthalate metabolites in the U.S. population from the National Health and Nutrition Examination Survey (NHANES) 1999–2000. Environ. Health Perspect. 2004, 112, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Sjödin, A.; Wong, L.Y.; Jones, R.S.; Park, A.; Zhang, Y.; Hodge, C.; Dipietro, E.; McClure, C.; Turner, W.; Needham, L.L.; et al. Serum concentrations of polybrominated diphenyl ethers (PBDEs) and polybrominated biphenyl (PBB) in the United States population: 2003–2004. Environ. Sci. Technol. 2008, 42, 1377–1384. [Google Scholar] [CrossRef] [PubMed]
- Needham, L.L.; Calafat, A.M.; Barr, D.B. Assessing developmental toxicant exposures via biomonitoring. Basic Clin. Pharmacol. Toxicol. 2008, 102, 100–108. [Google Scholar] [CrossRef]
- Needham, L.L.; Patterson, D.G., Jr.; Barr, D.B.; Grainger, J.; Calafat, A.M. Uses of speciation techniques in biomonitoring for assessing human exposure to organic environmental chemicals. Anal. Bioanal. Chem. 2005, 381, 397–404. [Google Scholar] [CrossRef]
- Nguyen, V.K.; Kahana, A.; Heidt, J.; Polemi, K.; Kvasnicka, J.; Jolliet, O.; Colacino, J.A. A comprehensive analysis of racial disparities in chemical biomarker concentrations in United States women, 1999–2014. Environ. Int. 2020, 137, 105496. [Google Scholar] [CrossRef]
- Chan, M.; Mita, C.; Bellavia, A.; Parker, M.; James-Todd, T. Racial/Ethnic Disparities in Pregnancy and Prenatal Exposure to Endocrine-Disrupting Chemicals Commonly Used in Personal Care Products. Curr. Environ. Health Rep. 2021, 8, 98–112. [Google Scholar] [CrossRef]
- Nigra, A.E.; Navas-Acien, A. Racial Inequalities in Drinking Water Lead Exposure: A Wake-Up Call to Protect Patients with End Stage Kidney Disease. J. Am. Soc. Nephrol. 2021, 32, 2419–2421. [Google Scholar] [CrossRef]
- Santaliz Casiano, A.; Lee, A.; Teteh, D.; Madak Erdogan, Z.; Treviño, L. Endocrine-Disrupting Chemicals and Breast Cancer: Disparities in Exposure and Importance of Research Inclusivity. Endocrinology 2022, 163, bqac034. [Google Scholar] [CrossRef]
- Zota, A.R.; VanNoy, B.N. Integrating Intersectionality into the Exposome Paradigm: A Novel Approach to Racial Inequities in Uterine Fibroids. Am. J. Public Health 2021, 111, 104–109. [Google Scholar] [CrossRef]
- Chang, C.J.; Barr, D.B.; Ryan, P.B.; Panuwet, P.; Smarr, M.M.; Liu, K.; Kannan, K.; Yakimavets, V.; Tan, Y.; Ly, V.; et al. Per- and polyfluoroalkyl substance (PFAS) exposure, maternal metabolomic perturbation, and fetal growth in African American women: A meet-in-the-middle approach. Environ. Int. 2022, 158, 106964. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.J.; Ryan, P.B.; Smarr, M.M.; Kannan, K.; Panuwet, P.; Dunlop, A.L.; Corwin, E.J.; Barr, D.B. Serum per- and polyfluoroalkyl substance (PFAS) concentrations and predictors of exposure among pregnant African American women in the Atlanta area, Georgia. Environ. Res. 2021, 198, 110445. [Google Scholar] [CrossRef] [PubMed]
- Mutic, A.D.; Barr, D.B.; Hertzberg, V.S.; Brennan, P.A.; Dunlop, A.L.; McCauley, L.A. Polybrominated Diphenyl Ether Serum Concentrations and Depressive Symptomatology in Pregnant African American Women. Int. J. Environ. Res. Public Health 2021, 18, 3614. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Barr, D.B.; Ryan, P.B.; Fedirko, V.; Sarnat, J.A.; Gaskins, A.J.; Chang, C.J.; Tang, Z.; Marsit, C.J.; Corwin, E.J.; et al. High-resolution metabolomics of exposure to tobacco smoke during pregnancy and adverse birth outcomes in the Atlanta African American maternal-child cohort. Environ. Pollut. 2022, 292, 118361. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Barr, D.B.; Dunlop, A.L.; Panuwet, P.; Sarnat, J.A.; Lee, G.E.; Tan, Y.; Corwin, E.J.; Jones, D.P.; Ryan, P.B.; et al. Assessment of metabolic perturbations associated with exposure to phthalates among pregnant African American women. Sci. Total Environ. 2022, 818, 151689. [Google Scholar] [CrossRef]
- Butte, W.; Heinzow, B. Pollutants in house dust as indicators of indoor contamination. Rev. Environ. Contam. Toxicol. 2002, 175, 1–46. [Google Scholar]
- Darrow, L.A.; Jacobson, M.H.; Preston, E.V.; Lee, G.E.; Panuwet, P.; Hunter, R.E., Jr.; Marder, M.E.; Marcus, M.; Barr, D.B. Predictors of Serum Polybrominated Diphenyl Ether (PBDE) Concentrations among Children Aged 1–5 Years. Environ. Sci. Technol. 2017, 51, 645–654. [Google Scholar] [CrossRef]
- He, H.; Li, Y.; Shen, R.; Shim, H.; Zeng, Y.; Zhao, S.; Lu, Q.; Mai, B.; Wang, S. Environmental occurrence and remediation of emerging organohalides: A review. Environ. Pollut. 2021, 290, 118060. [Google Scholar] [CrossRef]
- Klinčić, D.; Dvoršćak, M.; Jagić, K.; Mendaš, G.; Herceg Romanić, S. Levels and distribution of polybrominated diphenyl ethers in humans and environmental compartments: A comprehensive review of the last five years of research. Environ. Sci. Pollut. Res. Int. 2020, 27, 5744–5758. [Google Scholar] [CrossRef]
- Oomen, A.G.; Janssen, P.; Dusseldorp, A.; Noorlander, C.W. Exposure to chemicals via house dust. In Exposure to Chemicals via House Dust; National Institute for Public Health and the Environment: Bilthoven, The Netherlands, 2008. [Google Scholar]
- Thatcher, T.L.; Layton, D.W. Deposition, resuspension, and penetration of particles within a residence. Atmos. Environ. 1995, 29, 1487–1497. [Google Scholar] [CrossRef]
- Booij, P.; Holoubek, I.; Klánová, J.; Kohoutek, J.; Dvorská, A.; Magulová, K.; Al-Zadjali, S.; Čupr, P. Current implications of past DDT indoor spraying in Oman. Sci. Total Environ. 2016, 550, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Bramwell, L.; Glinianaia, S.V.; Rankin, J.; Rose, M.; Fernandes, A.; Harrad, S.; Pless-Mulolli, T. Associations between human exposure to polybrominated diphenyl ether flame retardants via diet and indoor dust, and internal dose: A systematic review. Environ. Int. 2016, 92–93, 680–694. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, T.P.; Brown, F.R.; Metayer, C.; Park, J.S.; Does, M.; Dhaliwal, J.; Petreas, M.X.; Buffler, P.A.; Rappaport, S.M. Polychlorinated biphenyls in residential dust: Sources of variability. Environ. Sci. Technol. 2014, 48, 157–164. [Google Scholar] [CrossRef]
- Abelsohn, A.; Gibson, B.L.; Sanborn, M.D.; Weir, E. Identifying and managing adverse environmental health effects: 5. Persistent organic pollutants. Cmaj 2002, 166, 1549–1554. [Google Scholar] [PubMed]
- Brunström, B.; Halldin, K. Ecotoxicological risk assessment of environmental pollutants in the Arctic. Toxicol. Lett. 2000, 112–113, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Letcher, R.J.; Bustnes, J.O.; Dietz, R.; Jenssen, B.M.; Jørgensen, E.H.; Sonne, C.; Verreault, J.; Vijayan, M.M.; Gabrielsen, G.W. Exposure and effects assessment of persistent organohalogen contaminants in arctic wildlife and fish. Sci. Total Environ. 2010, 408, 2995–3043. [Google Scholar] [CrossRef] [PubMed]
- Montaño, M.; Gutleb, A.C.; Murk, A.J. Persistent toxic burdens of halogenated phenolic compounds in humans and wildlife. Environ. Sci. Technol. 2013, 47, 6071–6081. [Google Scholar] [CrossRef]
- Vasseur, P.; Cossu-Leguille, C. Linking molecular interactions to consequent effects of persistent organic pollutants (POPs) upon populations. Chemosphere 2006, 62, 1033–1042. [Google Scholar] [CrossRef]
- Vested, A.; Giwercman, A.; Bonde, J.P.; Toft, G. Persistent organic pollutants and male reproductive health. Asian J. Androl. 2014, 16, 71–80. [Google Scholar] [CrossRef]
- Donaldson, S.G.; Van Oostdam, J.; Tikhonov, C.; Feeley, M.; Armstrong, B.; Ayotte, P.; Boucher, O.; Bowers, W.; Chan, L.; Dallaire, F.; et al. Environmental contaminants and human health in the Canadian Arctic. Sci. Total Environ. 2010, 408, 5165–5234. [Google Scholar] [CrossRef]
- Latchney, S.E.; Majewska, A.K. Persistent organic pollutants at the synapse: Shared phenotypes and converging mechanisms of developmental neurotoxicity. Dev. Neurobiol. 2021, 81, 623–652. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.; Kim, S.; Barr, D.B.; Buck Louis, G.M. Maternal and paternal serum concentrations of persistent organic pollutants and the secondary sex ratio: A population-based preconception cohort study. Environ. Res. 2018, 161, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Gross, R.S.; Ghassabian, A.; Vandyousefi, S.; Messito, M.J.; Gao, C.; Kannan, K.; Trasande, L. Persistent organic pollutants exposure in newborn dried blood spots and infant weight status: A case-control study of low-income Hispanic mother-infant pairs. Environ. Pollut. 2020, 267, 115427. [Google Scholar] [CrossRef]
- Hjermitslev, M.H.; Long, M.; Wielsøe, M.; Bonefeld-Jørgensen, E.C. Persistent organic pollutants in Greenlandic pregnant women and indices of foetal growth: The ACCEPT study. Sci. Total Environ. 2020, 698, 134118. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, T.; Fréour, T.; Ploteau, S.; Le Bizec, B.; Antignac, J.P.; Cano-Sancho, G. Associations between human internal chemical exposure to Persistent Organic Pollutants (POPs) and In Vitro Fertilization (IVF) outcomes: Systematic review and evidence map of human epidemiological evidence. Reprod. Toxicol. 2021, 105, 184–197. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.S.; Lu, A.X.; Cao, L.L.; Ran, X.F.; Wang, Y.Q.; Liu, C.; Yan, C.H. Effects of prenatal exposure to persistent organic pollutants on neonatal Outcomes:A mother-child cohort (Shanghai, China). Environ. Res. 2022, 203, 111767. [Google Scholar] [CrossRef] [PubMed]
- Barr, D.B.; Weihe, P.; Davis, M.D.; Needham, L.L.; Grandjean, P. Serum polychlorinated biphenyl and organochlorine insecticide concentrations in a Faroese birth cohort. Chemosphere 2006, 62, 1167–1182. [Google Scholar] [CrossRef]
- Corwin, E.J.; Hogue, C.J.; Pearce, B.; Hill, C.C.; Read, T.D.; Mulle, J.; Dunlop, A.L. Protocol for the Emory University African American Vaginal, Oral, and Gut Microbiome in Pregnancy Cohort Study. BMC Pregnancy Childbirth 2017, 17, 161. [Google Scholar] [CrossRef]
- Finkelstein, M.M.; Verma, D.K. Exposure estimation in the presence of nondetectable values: Another look. Aihaj 2001, 62, 195–198. [Google Scholar] [CrossRef]
- Stapleton, H.M. Instrumental methods and challenges in quantifying polybrominated diphenyl ethers in environmental extracts: A review. Anal. Bioanal. Chem. 2006, 386, 807–817. [Google Scholar] [CrossRef]
- FDA. Bioanalytical Method Validation Guidance for Industry Biopharmaceutics Bioanalytical Method Validation Guidance for Industry Biopharmaceutics Contains Nonbinding Recommendations. Available online: https://www.fda.gov/media/70858/download (accessed on 22 September 2022).
- Guenzi, W.D.; Beard, W.E. Anaerobic biodegradation of DDT to DDD in soil. Science 1967, 156, 1116–1117. [Google Scholar] [CrossRef] [PubMed]
- ATSDR. Toxicological Profile for DDT, DDE and DDD; DHHS: Atlanta, GA, USA, 2022.
- Becker, S.; Halsall, C.J.; Tych, W.; Kallenborn, R.; Schlabach, M.; Manø, S. Changing sources and environmental factors reduce the rates of decline of organochlorine pesticides in the Arctic atmosphere. Atmos. Chem. Phys. 2012, 12, 4033–4044. [Google Scholar] [CrossRef]
- EPA. DDT: A Brief History and Status. Available online: https://www.epa.gov/ingredients-used-pesticide-products/ddt-brief-history-and-status#:~:text=DDT%20(dichloro%2Ddiphenyl%2Dtrichloroethane,both%20military%20and%20civilian%20populations (accessed on 12 October 2022).
- Whitehead, T.P.; Brown, F.R.; Metayer, C.; Park, J.S.; Does, M.; Petreas, M.X.; Buffler, P.A.; Rappaport, S.M. Polybrominated diphenyl ethers in residential dust: Sources of variability. Environ. Int. 2013, 57–58, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, T.P.; Crispo Smith, S.; Park, J.S.; Petreas, M.X.; Rappaport, S.M.; Metayer, C. Concentrations of persistent organic pollutants in California women’s serum and residential dust. Environ. Res. 2015, 136, 57–66. [Google Scholar] [CrossRef]
- Johnson, P.I.; Stapleton, H.M.; Sjodin, A.; Meeker, J.D. Relationships between polybrominated diphenyl ether concentrations in house dust and serum. Environ. Sci. Technol. 2010, 44, 5627–5632. [Google Scholar] [CrossRef]
- Tan, J.; Cheng, S.M.; Loganath, A.; Chong, Y.S.; Obbard, J.P. Selected organochlorine pesticide and polychlorinated biphenyl residues in house dust in Singapore. Chemosphere 2007, 68, 1675–1682. [Google Scholar] [CrossRef]
- Meng, G.; Nie, Z.; Feng, Y.; Wu, X.; Yin, Y.; Wang, Y. Typical halogenated persistent organic pollutants in indoor dust and the associations with childhood asthma in Shanghai, China. Environ. Pollut. 2016, 211, 389–398. [Google Scholar] [CrossRef]
- Chandra Yadav, I.; Devi, N.L.; Li, J.; Zhang, G. Examining the role of total organic carbon and black carbon in the fate of legacy persistent organic pollutants (POPs) in indoor dust from Nepal: Implication on human health. Ecotoxicol. Environ. Saf. 2019, 175, 225–235. [Google Scholar] [CrossRef]
- Shoeib, M.; Harner, T.; Webster, G.M.; Sverko, E.; Cheng, Y. Legacy and current-use flame retardants in house dust from Vancouver, Canada. Environ. Pollut. 2012, 169, 175–182. [Google Scholar] [CrossRef]

| Compound | Ion Type | Precursor Ion (m/z) | Product Ion (m/z) | Dwell Time (ms) | Collision Energy (V) | Retention Window | Approx. RT (min) |
|---|---|---|---|---|---|---|---|
| TNC | Q | 406.7 | 299.8 | 75 | 25 | 1 | |
| TNC | C | 408.6 | 299.9 | 75 | 25 | 1 | 6.79 |
| p,p’-DDE | L | 257.9 | 188.2 | 75 | 35 | 1 | |
| p,p’-DDE | Q | 245.9 | 176 | 75 | 35 | 1 | 6.87 |
| p,p’-DDE | C | 247.9 | 176 | 75 | 35 | 1 | |
| PCB 118 | Q | 323.7 | 254 | 50 | 20 | 2 | |
| PCB 118 | C | 325.6 | 256 | 50 | 40 | 2 | 7.30 |
| p,p’-DDT | L | 247 | 177.2 | 50 | 25 | 2 | 7.87 |
| p,p’-DDT | Q | 234.9 | 165 | 50 | 25 | 2 | |
| p,p’-DDT | C | 236.9 | 165 | 50 | 25 | 3 | |
| PCB 153 | L | 373.7 | 301.9 | 50 | 30 | 2 | |
| PCB 153 | Q | 359.7 | 289.9 | 50 | 40 | 2 | |
| PCB 153 | C | 361.6 | 289.9 | 50 | 40 | 2 | 7.57 |
| PCB 138 | L | 373.7 | 301.9 | 50 | 30 | 3 | |
| PCB 138 | Q | 359.7 | 289.9 | 50 | 40 | 3 | |
| PCB 138 | C | 361.6 | 289.9 | 50 | 40 | 3 | 7.96 |
| PCB 180 | L | 405.8 | 335.7 | 75 | 30 | 4 | |
| PCB 180 | Q | 393.6 | 324 | 75 | 30 | 4 | |
| PCB 180 | C | 395.6 | 323.8 | 75 | 30 | 4 | 9.01 |
| PBDE 47 | L | 497.8 | 338 | 75 | 30 | 4 | |
| PBDE 47 | Q | 485.6 | 326 | 75 | 30 | 4 | |
| PBDE 47 | C | 325.7 | 217 | 75 | 30 | 4 | 9.10 |
| PBDE 85 | Q | 565.5 | 406 | 75 | 20 | 6 | s |
| PBDE 85 | C | 563.6 | 404 | 75 | 20 | 6 | 13.48 |
| PBDE 99 | L | 577.7 | 417.7 | 75 | 25 | 5 | |
| PBDE 99 | Q | 563.5 | 404 | 75 | 25 | 5 | |
| PBDE 99 | C | 565.5 | 406 | 75 | 25 | 5 | 11.80 |
| PBDE 100 | L | 577.7 | 418 | 75 | 25 | 5 | |
| PBDE 100 | Q | 563.5 | 404 | 75 | 25 | 5 | |
| PBDE 100 | C | 565.5 | 406 | 75 | 25 | 5 | 11.09 |
| PBDE 153 | L | 655.5 | 495.5 | 100 | 20 | 7 | |
| PBDE 153 | Q | 643.5 | 484 | 100 | 20 | 7 | |
| PBDE 153 | C | 483.5 | 324 | 100 | 20 | 7 | 14.98 |
| PBDE 154 | L | 655.5 | 495.5 | 100 | 20 | 6 | |
| PBDE 154 | Q | 643.5 | 484 | 100 | 20 | 6 | |
| PBDE 154 | C | 641.5 | 482 | 100 | 40 | 6 | 14.36 |
| Analyte | LOQ (ng/g Dust) | Accuracy (%) * | Recovery (%) ** | RSD (%) | |
|---|---|---|---|---|---|
| Low QC | High QC | ||||
| TNC | 2.00 | 81.8 | 88 ± 6 | 15.6 | 3.0 |
| p,p’-DDE | 0.40 | 92.2 | 92 ± 4 | 10.2 | 1.3 |
| p,p’-DDT | 1.00 | 96.8 | 88 ± 10 | 9.4 | 3.3 |
| PCB118 | 1.00 | 85.9 | 91 ± 5 | 11.2 | 5.6 |
| PCB138 | 1.00 | 92.9 | 86 ± 11 | 9.9 | 4.7 |
| PCB153 | 1.00 | 96.7 | 101 ± 6 | 6.1 | 3.4 |
| PCB180 | 2.00 | 100.2 | 93 ± 4 | 12.0 | 2.6 |
| PBDE47 | 0.10 | 89.2 | 102 ± 4 | 10.2 | 5.9 |
| PBDE85 | 0.25 | 107.6 | 81 ± 11 | 11.6 | 10.2 |
| PBDE99 | 0.50 | 94.5 | 97 ± 5 | 9.5 | 6.7 |
| PBDE100 | 2.50 | 97.4 | 95 ± 7 | 9.1 | 4.8 |
| PBDE153 | 2.50 | 90.5 | 98 ± 5 | 13.1 | 11.9 |
| PBDE154 | 2.50 | 84.8 | 105 ± 9 | 12.2 | 10.7 |
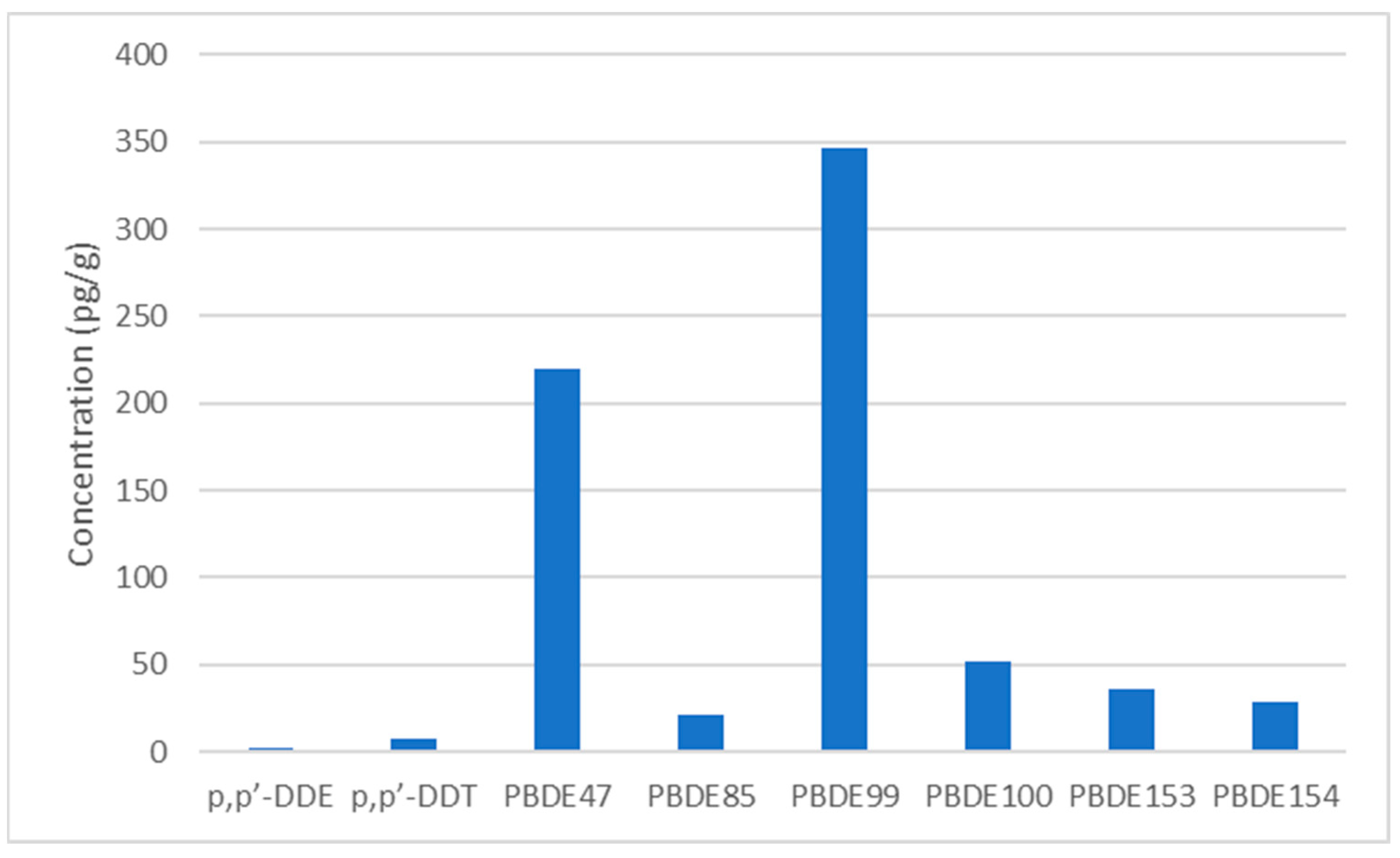
| Analyte | FOD (%) | GM | Median | 95th%ile | Maximum |
|---|---|---|---|---|---|
| TNC | 85.3 | 4.90 | 6.54 | 71.5 | 356 |
| p,p’-DDE | 100 | 3.45 | 2.46 | 57.6 | 356 |
| p,p’-DDT | 85.3 | 5.59 | 7.08 | 227 | 880 |
| PCB118 | 23.5 | <1 | <1 | 3.07 | 11.2 |
| PCB138 | 41.2 | <1 | <1 | 3.04 | 95 |
| PCB153 | 17.6 | 8.8 | 85.3 | 7.84 | 239 |
| PCB180 | 8.8 | <0.1 | <0.1 | 1.98 | 4.62 |
| ΣPCB4 | 44 | 3.59 | <2.17 | 15.6 | 246 |
| PBDE47 | 100 | 252 | 220 | 3078 | 6741 |
| PBDE85 | 100 | 24.4 | 20.8 | 465 | 519 |
| PBDE99 | 100 | 380 | 347 | 6779 | 8167 |
| PBDE100 | 100 | 64.5 | 51.9 | 1216 | 1720 |
| PBDE153 | 100 | 44.5 | 35.8 | 774 | 888 |
| PBDE154 | 100 | 34.2 | 28.5 | 603 | 801 |
| ΣPBDE6 | 100 | 2187 | 1628 | 18,400 | 23,150 |
| Citation | Year | Location | N | Median/GM PCB 153 ng/g | Median/GM p,p-DDE ng/g | Median/GMPBDE 47 ng/g |
|---|---|---|---|---|---|---|
| This study | 2016–2020 | Atlanta, GA, USA | 34 | 240 | 18 | 720 |
| Darrow et al., 2017 [29] | 2011–2012 | Atlanta, GA, USA | 15 | NQ | NQ | 404 |
| Whitehead et al., 2013, 2014, 2015 [36,59,60] | 2001–2007, 2010 | CA | 292, 203 | NQ | NQ | 1500, 1300 |
| Johnson et al., 2010 [61] | 2002–2008 | MA | 50 | NQ | NQ | 390 |
| Tan et al., 2007 [62] | NP | Singapore | 31 | 0.5 (ΣPCB) | 3.3 | NQ |
| Meng et al., 2016 [63] | 2014 | Shanghai, China | 60 | Case: 0.09 Control: 0.08 | Case: 32 Control: 16.36 | Case: 3.02 Control: 1 |
| Chandra Yadav et al., 2019 [64] | NP | Nepal | 200 | 1–2.89 (ΣPCB) | 91–371 (∑DDT) | NQ |
| Shoeib et al., 2012 [65] | 2007–2008 | Vancouver, Canada | 116 | NQ | NQ | 280 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barr, K.J.; Johnson, C.L.; Cohen, J.; D’Souza, P.; Gallegos, E.I.; Tsai, C.-C.; Dunlop, A.L.; Corwin, E.J.; Barr, D.B.; Ryan, P.B.; et al. Legacy Chemical Pollutants in House Dust of Homes of Pregnant African Americans in Atlanta. Toxics 2022, 10, 755. https://doi.org/10.3390/toxics10120755
Barr KJ, Johnson CL, Cohen J, D’Souza P, Gallegos EI, Tsai C-C, Dunlop AL, Corwin EJ, Barr DB, Ryan PB, et al. Legacy Chemical Pollutants in House Dust of Homes of Pregnant African Americans in Atlanta. Toxics. 2022; 10(12):755. https://doi.org/10.3390/toxics10120755
Chicago/Turabian StyleBarr, Kathryn J., Cierra L. Johnson, Jordan Cohen, Priya D’Souza, Estefani Ignacio Gallegos, Chia-Chen Tsai, Anne L. Dunlop, Elizabeth J. Corwin, Dana Boyd Barr, P. Barry Ryan, and et al. 2022. "Legacy Chemical Pollutants in House Dust of Homes of Pregnant African Americans in Atlanta" Toxics 10, no. 12: 755. https://doi.org/10.3390/toxics10120755
APA StyleBarr, K. J., Johnson, C. L., Cohen, J., D’Souza, P., Gallegos, E. I., Tsai, C.-C., Dunlop, A. L., Corwin, E. J., Barr, D. B., Ryan, P. B., & Panuwet, P. (2022). Legacy Chemical Pollutants in House Dust of Homes of Pregnant African Americans in Atlanta. Toxics, 10(12), 755. https://doi.org/10.3390/toxics10120755








