Co-Exposure with an Invasive Seaweed Exudate Increases Toxicity of Polyamide Microplastics in the Marine Mussel Mytilus galloprovincialis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Asparagopsis armata Sampling and Exudate Production
2.2. Mytilus galloprovincialis Sampling and Acclimation
2.3. Microplastic Preparation
2.4. Experimental Setup
2.5. Digestion of Mussel Tissues and Microplastic Quantification
2.6. Biomarker Analysis
2.6.1. Sample Preparation
2.6.2. Oxidative Stress and Neurophysiological Biomarkers
2.6.3. Cellular Energy Allocation (CEA)
2.7. Byssal Thread Production
2.8. Statistical Analysis
3. Results
3.1. Polyamide Microplastics Quantification
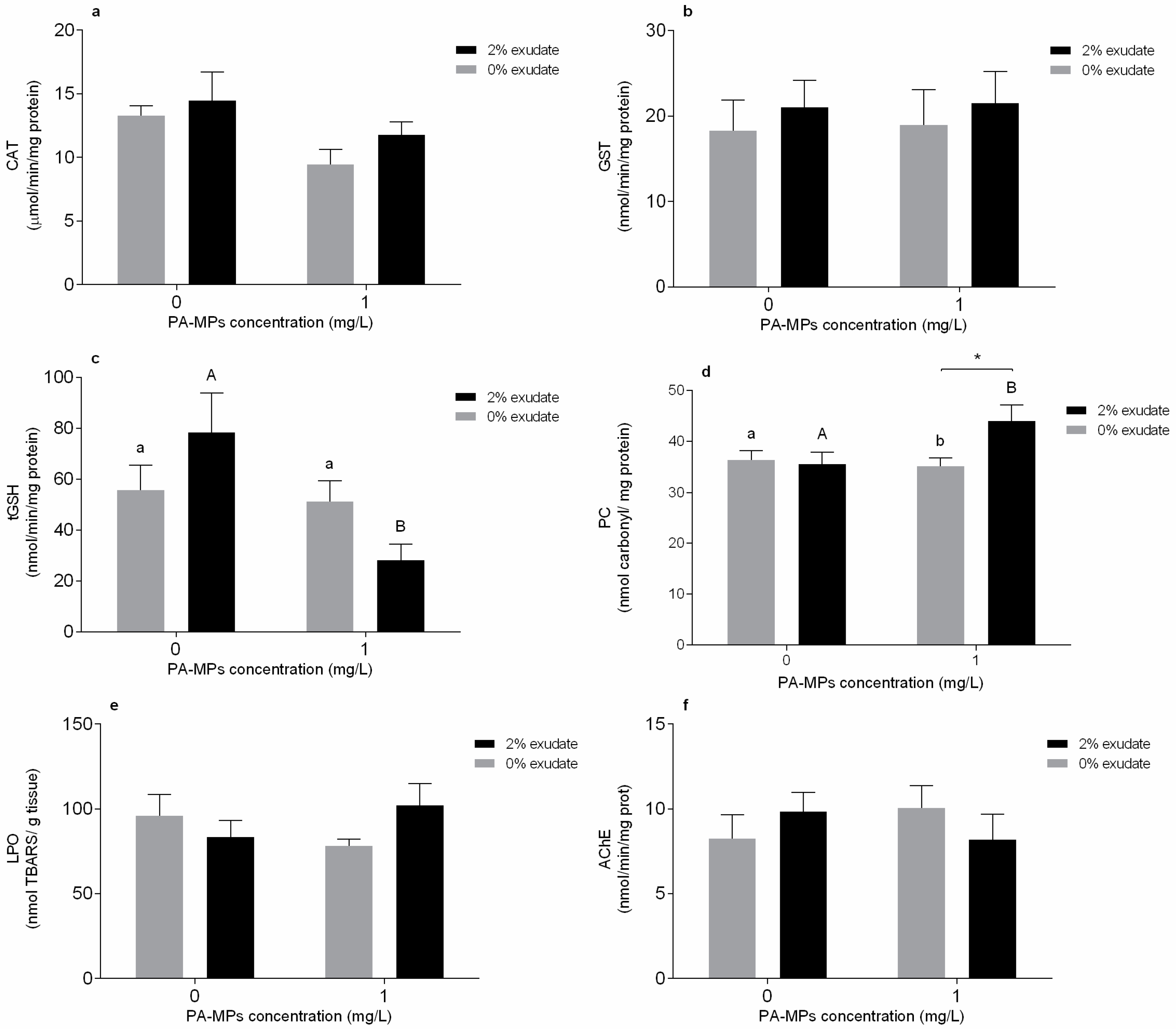
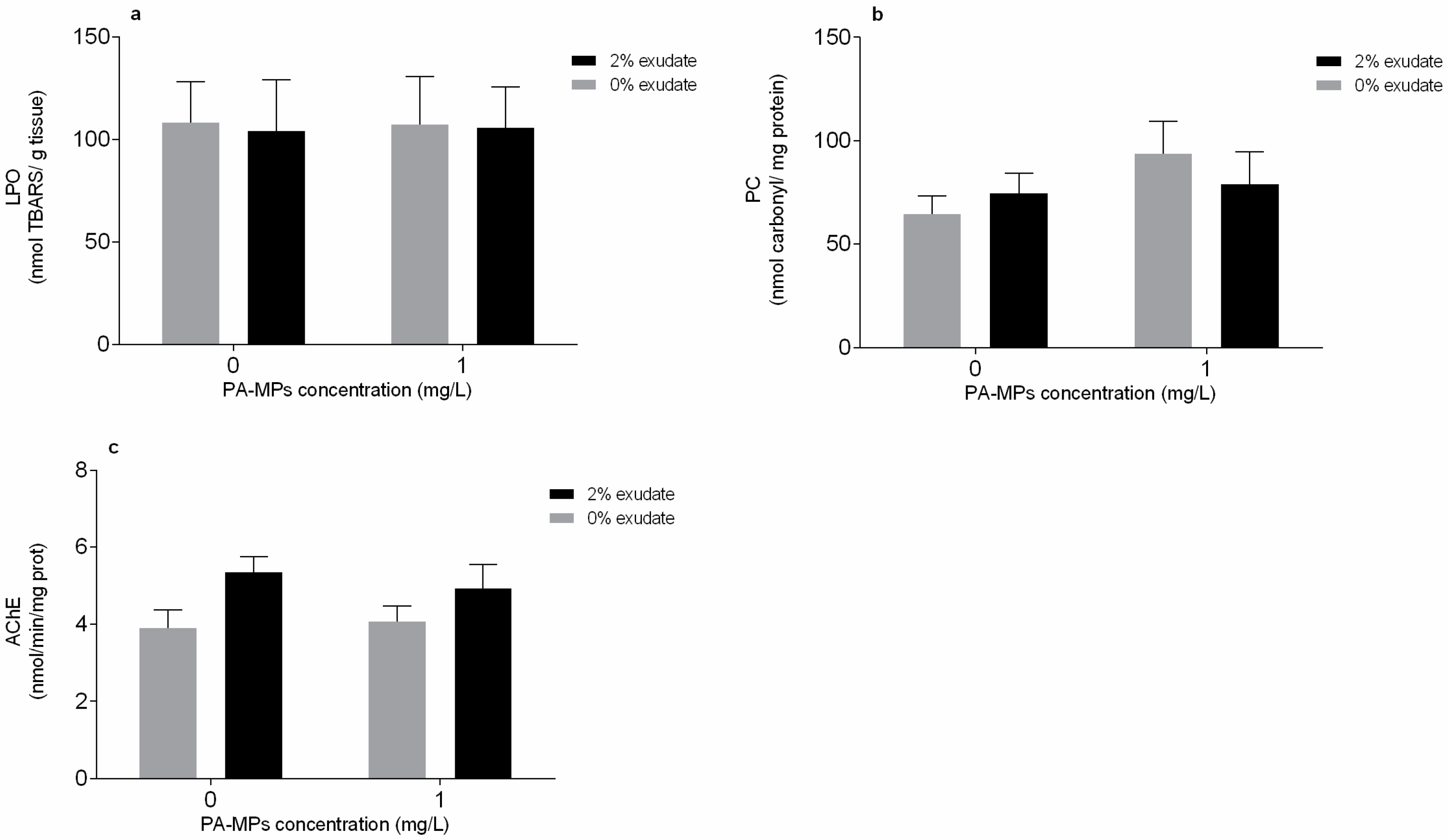
3.2. Oxidative Stress and Neurophysiological Biomarkers
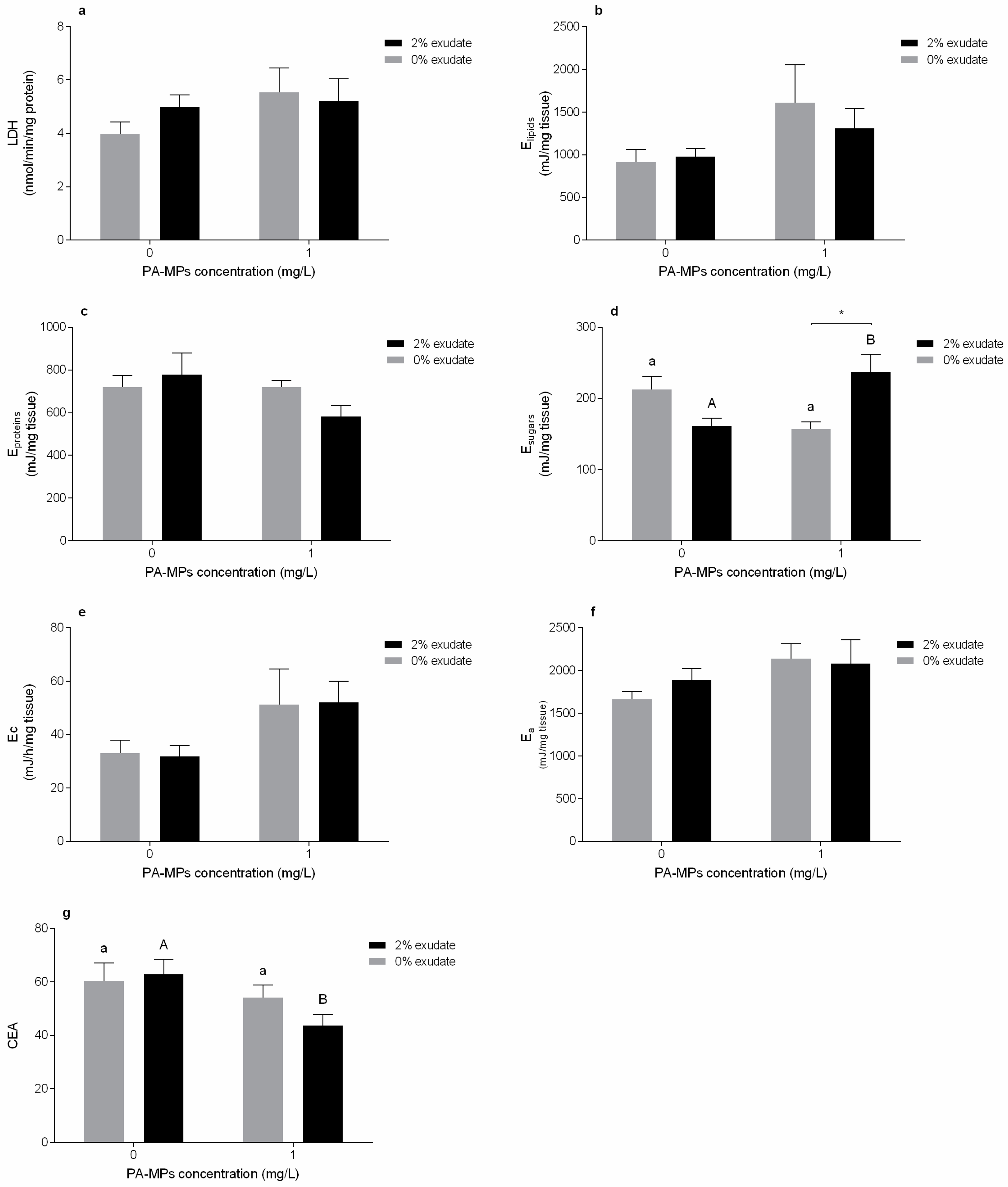
3.3. Energy Metabolism Biomarkers
3.4. Byssal Thread Production
4. Discussion
4.1. Microplastics in the Tissues
4.2. Oxidative Stress and Neurophysiological Biomarkers
4.3. Energy Metabolism Biomarkers
4.4. Byssal Thread Production
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pinteus, S.; Lemos, M.F.L.; Alves, C.; Neugebauer, A.; Silva, J.; Thomas, O.P.; Botana, L.M.; Gaspar, H.; Pedrosa, R. Marine invasive macroalgae: Turning a real threat into a major opportunity—The biotechnological potential of Sargassum muticum and Asparagopsis armata. Algal Res. 2018, 34, 217–234. [Google Scholar] [CrossRef]
- Halpern, B.S.; Walbridge, S.; Selkoe, K.A.; Kappel, C.V.; Micheli, F.; D’Agrosa, C.; Bruno, J.F.; Casey, K.S.; Ebert, C.; Fox, H.E.; et al. A global map of human impact on marine ecosystems. Science 2008, 319, 948–952. [Google Scholar] [CrossRef] [Green Version]
- PlasticsEurope. Plastics–the Facts 2020: An analysis of European plastics production, Demand and Waste Data; PlasticsEurope: Brussels, Belgium, 2020. [Google Scholar]
- Li, J.; Lusher, A.L.; Rotchell, J.M.; Deudero, S.; Turra, A.; Bråte, I.L.N.; Sun, C.; Shahadat Hossain, M.; Li, Q.; Kolandhasamy, P.; et al. Using mussel as a global bioindicator of coastal microplastic pollution. Environ. Pollut. 2019, 244, 522–533. [Google Scholar] [CrossRef] [PubMed]
- Cole, M.; Lindeque, P.; Halsband, C.; Galloway, T.S. Microplastics as contaminants in the marine environment: A review. Mar. Pollut. Bull. 2011, 62, 2588–2597. [Google Scholar] [CrossRef] [PubMed]
- Alprol, A.E.; Gaballah, M.S.; Hassaan, M.A. Micro and Nanoplastics analysis: Focus on their classification, sources, and impacts in marine environment. Reg. Stud. Mar. Sci. 2021, 42, 101625. [Google Scholar] [CrossRef]
- Beiras, R.; Schönemann, A.M. Currently monitored microplastics pose negligible ecological risk to the global ocean. Sci. Rep. 2020, 10, 22281. [Google Scholar] [CrossRef] [PubMed]
- Lindeque, P.K.; Cole, M.; Coppock, R.L.; Lewis, C.N.; Miller, R.Z.; Watts, A.J.R.; Wilson-McNeal, A.; Wright, S.L.; Galloway, T.S. Are we underestimating microplastic abundance in the marine environment? A comparison of microplastic capture with nets of different mesh-size. Environ. Pollut. 2020, 265, 114721. [Google Scholar] [CrossRef]
- Wright, S.L.; Thompson, R.C.; Galloway, T.S. The physical impacts of microplastics on marine organisms: A review. Environ. Pollut. 2013, 178, 483–492. [Google Scholar] [CrossRef]
- Cole, M.; Liddle, C.; Consolandi, G.; Drago, C.; Hird, C.; Lindeque, P.K.; Galloway, T.S. Microplastics, microfibres and nanoplastics cause variable sub-lethal responses in mussels (Mytilus spp.). Mar. Pollut. Bull. 2020, 160, 111552. [Google Scholar] [CrossRef]
- Li, J.; Qu, X.; Su, L.; Zhang, W.; Yang, D.; Kolandhasamy, P.; Li, D.; Shi, H. Microplastics in mussels along the coastal waters of China. Environ. Pollut. 2016, 214, 177–184. [Google Scholar] [CrossRef]
- Kinjo, A.; Mizukawa, K.; Takada, H.; Inoue, K. Size-dependent elimination of ingested microplastics in the Mediterranean mussel Mytilus galloprovincialis. Mar. Pollut. Bull. 2019, 149, 110512. [Google Scholar] [CrossRef]
- Von Moos, N.; Burkhardt-Holm, P.; Köhler, A. Uptake and effects of microplastics on cells and tissue of the blue mussel Mytilus edulis L. after an experimental exposure. Environ. Sci. Technol. 2012, 46, 11327–11335. [Google Scholar] [CrossRef] [PubMed]
- Avio, C.G.; Gorbi, S.; Milan, M.; Benedetti, M.; Fattorini, D.; D’Errico, G.; Pauletto, M.; Bargelloni, L.; Regoli, F. Pollutants bioavailability and toxicological risk from microplastics to marine mussels. Environ. Pollut. 2015, 198, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xu, K.; Zhang, B.; Ye, Y.; Zhang, Q.; Jiang, W. Cellular internalization and release of polystyrene microplastics and nanoplastics. Sci. Total Environ. 2021, 779, 146523. [Google Scholar] [CrossRef] [PubMed]
- Foley, C.J.; Feiner, Z.S.; Malinich, T.D.; Höök, T.O. A meta-analysis of the effects of exposure to microplastics on fish and aquatic invertebrates. Sci. Total Environ. 2018, 631–632, 550–559. [Google Scholar] [CrossRef] [Green Version]
- Avio, C.G.; Gorbi, S.; Regoli, F. Plastics and microplastics in the oceans: From emerging pollutants to emerged threat. Mar. Environ. Res. 2017, 128, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xia, M.; Zhao, J.; Cao, Z.; Zou, W.; Zhou, Q. Photoaging enhanced the adverse effects of polyamide microplastics on the growth, intestinal health, and lipid absorption in developing zebrafish. Environ. Int. 2022, 158, 106922. [Google Scholar] [CrossRef]
- Lusher, A.; Hollman, P.; Mandoza-Hill, J. Microplastics in fisheries and aquaculture: Status of Knowledge on Their Occurrence and Implications for Aquatic Organisms and Food Safety; FAO: Rome, Italy, 2017; Volume 615. [Google Scholar]
- Lusher, A.L.; McHugh, M.; Thompson, R.C. Occurrence of microplastics in the gastrointestinal tract of pelagic and demersal fish from the English Channel. Mar. Pollut. Bull. 2013, 67, 94–99. [Google Scholar] [CrossRef]
- Thushari, G.G.N.; Senevirathna, J.D.M.; Yakupitiyage, A.; Chavanich, S. Effects of microplastics on sessile invertebrates in the eastern coast of Thailand: An approach to coastal zone conservation. Mar. Pollut. Bull. 2017, 124, 349–355. [Google Scholar] [CrossRef]
- Pedrotti, M.L.; Petit, S.; Elineau, A.; Bruzaud, S.; Crebassa, J.C.; Dumontet, B.; Martí, E.; Gorsky, G.; Cózar, A. Changes in the floating plastic pollution of the mediterranean sea in relation to the distance to land. PLoS ONE 2016, 11, e0161581. [Google Scholar] [CrossRef] [Green Version]
- Cincinelli, A.; Scopetani, C.; Chelazzi, D.; Martellini, T.; Pogojeva, M.; Slobodnik, J. Microplastics in the Black Sea sediments. Sci. Total Environ. 2021, 760, 143898. [Google Scholar] [CrossRef]
- Erni-Cassola, G.; Zadjelovic, V.; Gibson, M.I.; Christie-Oleza, J.A. Distribution of plastic polymer types in the marine environment; A meta-analysis. J. Hazard. Mater. 2019, 369, 691–698. [Google Scholar] [CrossRef]
- Davidson, A.D.; Campbell, M.L.; Hewitt, C.L.; Schaffelke, B. Assessing the impacts of nonindigenous marine macroalgae: An update of current knowledge. Bot. Mar. 2015, 58, 55–79. [Google Scholar] [CrossRef]
- Thomsen, M.S.; Wernberg, T.; South, P.M.; Schiel, D.R. Non-native Seaweeds Drive Changes in Marine Coastal Communities Around the World. In Seaweed Phylogeography; Hu, Z.M., Fraser, C., Eds.; Springer: Dordrecht, The Netherlands, 2016; pp. 147–185. [Google Scholar] [CrossRef]
- Katsanevakis, S.; Wallentinus, I.; Zenetos, A.; Leppäkoski, E.; Çinar, M.E.; Oztürk, B.; Grabowski, M.; Golani, D.; Cardoso, A.C. Impacts of invasive alien marine species on ecosystem services and biodiversity: A pan-European review. Aquat. Invasions 2014, 9, 391–423. [Google Scholar] [CrossRef]
- Williams, S.L.; Smith, J.E. A global review of the distribution, taxonomy, and impacts of introduced seaweeds. Annu. Rev. Ecol. Evol. Syst. 2007, 38, 327–359. [Google Scholar] [CrossRef] [Green Version]
- Molnar, J.L.; Gamboa, R.L.; Revenga, C.; Spalding, M.D. Assessing the global threat of invasive species to marine biodiversity. Front. Ecol. Environ. 2008, 6, 485–492. [Google Scholar] [CrossRef]
- Simberloff, D.; Martin, J.L.; Genovesi, P.; Maris, V.; Wardle, D.A.; Aronson, J.; Courchamp, F.; Galil, B.; García-Berthou, E.; Pascal, M.; et al. Impacts of biological invasions: What’s what and the way forward. Trends Ecol. Evol. 2013, 28, 58–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonin, D.R.; Hawkes, M.W. Systematics and life histories of New Zealand Bonnemaisoniaceae (Bonnemaisoniales, Rhodophyta): I. The genus Asparagopsis. N. Z. J. Bot. 1987, 25, 577–590. [Google Scholar] [CrossRef] [Green Version]
- Boudouresque, C.F.; Verlaque, M. Biological pollution in the Mediterranean Sea: Invasive versus introduced macrophytes. Mar. Pollut. Bull. 2002, 44, 32–38. [Google Scholar] [CrossRef]
- Ní Chualáin, F.; Maggs, C.A.; Saunders, G.W.; Guiry, M.D. The invasive genus Asparagopsis (Bonnemaisoniaceae, Rhodophyta): Molecular systematics, morphology, and ecophysiology of Falkenbergia isolates. J. Phycol. 2004, 40, 1112–1126. [Google Scholar] [CrossRef]
- Andreakis, N.; Procaccini, G.; Maggs, C.; Kooistra, W.H.C.F. Phylogeography of the invasive seaweed Asparagopsis (Bonnemaisoniales, Rhodophyta) reveals cryptic diversity. Mol. Ecol. 2007, 16, 2285–2299. [Google Scholar] [CrossRef]
- Martins, G.M.; Cacabelos, E.; Faria, J.; Álvaro, N.; Prestes, A.C.L.; Neto, A.I. Patterns of distribution of the invasive alga Asparagopsis armata Harvey: A multi-scaled approach. Aquat. Invasions 2019, 14, 582–593. [Google Scholar] [CrossRef]
- Rubal, M.; Costa-Garcia, R.; Besteiro, C.; Sousa-Pinto, I.; Veiga, P. Mollusc diversity associated with the non-indigenous macroalga Asparagopsis armata Harvey, 1855 along the Atlantic coast of the Iberian Peninsula. Mar. Environ. Res. 2018, 136, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Maggs, C.A.; Stegenga, H. Red algal exotics on North Sea coasts. Helgol. Meeresunters. 1999, 52, 243–258. [Google Scholar] [CrossRef] [Green Version]
- Paul, N.A.; De Nys, R.; Steinberg, P.D. Seaweed-herbivore interactions at a small scale: Direct tests of feeding deterrence by filamentous algae. Mar. Ecol. Prog. Ser. 2006, 323, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Sala, E.; Boudouresque, C.F. The role of fishes in the organization of a Mediterranean sublittoral community. I: Algal communities. J. Exp. Mar. Bio. Ecol. 1997, 212, 25–44. [Google Scholar] [CrossRef]
- Genovese, G.; Tedone, L.; Hamann, M.T.; Morabito, M. The Mediterranean red alga Asparagopsis: A source of compounds against Leishmania. Mar. Drugs 2009, 7, 361–366. [Google Scholar] [CrossRef] [Green Version]
- Bansemir, A.; Blume, M.; Schröder, S.; Lindequist, U. Screening of cultivated seaweeds for antibacterial activity against fish pathogenic bacteria. Aquaculture 2006, 252, 79–84. [Google Scholar] [CrossRef]
- Guerra-García, J.M.; Ros, M.; Izquierdo, D.; Soler-Hurtado, M.M. The invasive Asparagopsis armata versus the native Corallina elongata: Differences in associated peracarid assemblages. J. Exp. Mar. Bio. Ecol. 2012, 416–417, 121–128. [Google Scholar] [CrossRef]
- Silva, C.O.; Lemos, M.F.L.; Gaspar, R.; Gonçalves, C.; Neto, J.M.; Silva, C.O. The effects of the invasive seaweed Asparagopsis armata on native rock pool communities: Evidences from experimental exclusion. Ecol. Indic. 2021, 125, 107463. [Google Scholar] [CrossRef]
- Silva, C.O.; Novais, S.C.; Soares, A.M.V.M.; Barata, C.; Lemos, M.F.L. Impacts of the Invasive Seaweed Asparagopsis armata Exudate on Energetic Metabolism of Rock Pool Invertebrates. Toxins 2021, 13, 15. [Google Scholar] [CrossRef] [PubMed]
- Coelho, S.D.; Vieira, H.C.; Oliveira, J.M.M.; Pires, S.F.S.; Rocha, R.J.M.; Rodrigues, A.C.M.; Soares, A.M.V.M.; Bordalo, M.D. How Does Mytilus galloprovincialis Respond When Exposed to the Gametophyte Phase of the Invasive Red Macroalga Asparagopsis armata Exudate? Water 2021, 13, 460. [Google Scholar] [CrossRef]
- Vieira, H.C.; Rodrigues, A.C.M.; Pires, S.F.S.; Oliveira, J.M.M.; Rocha, R.J.M.; Soares, A.M.V.M.; Bordalo, M.D. Ocean warming may enhance biochemical alterations induced by an invasive seaweed exudate in the mussel Mytilus galloprovincialis. Toxics 2021, 9, 121. [Google Scholar] [CrossRef] [PubMed]
- Beyer, J.; Green, N.W.; Brooks, S.; Allan, I.J.; Ruus, A.; Gomes, T.; Bråte, I.L.N.; Schøyen, M. Blue mussels (Mytilus edulis spp.) as sentinel organisms in coastal pollution monitoring: A review. Mar. Environ. Res. 2017, 130, 338–365. [Google Scholar] [CrossRef] [PubMed]
- Abidli, S.; Pinheiro, M.; Lahbib, Y.; Neuparth, T.; Santos, M.M.; Trigui El Menif, N. Effects of environmentally relevant levels of polyethylene microplastic on Mytilus galloprovincialis (Mollusca: Bivalvia): Filtration rate and oxidative stress. Environ. Sci. Pollut. Res. 2021, 28, 26643–26652. [Google Scholar] [CrossRef]
- Arribas, L.P.; Donnarumma, L.; Palomo, M.G.; Scrosati, R.A. Intertidal mussels as ecosystem engineers: Their associated invertebrate biodiversity under contrasting wave exposures. Mar. Biodivers. 2014, 44, 203–211. [Google Scholar] [CrossRef]
- Oliveira, J.; Castilho, F.; Cunha, Â.; Pereira, M.J. Bivalve harvesting and production in Portugal: An overview. J. Shellfish Res. 2013, 32, 911–924. [Google Scholar] [CrossRef]
- Pittura, L.; Avio, C.G.; Giuliani, M.E.; d’Errico, G.; Keiter, S.H.; Cormier, B.; Gorbi, S.; Regoli, F. Microplastics as vehicles of environmental PAHs to marine organisms: Combined chemical and physical hazards to the mediterranean mussels, Mytilus galloprovincialis. Front. Mar. Sci. 2018, 5, 103. [Google Scholar] [CrossRef] [Green Version]
- ASTM Standard E729-96; Standard Guide for Conducting Acute Toxicity Tests on Test Materials with Fishes, Macroinvertebrates, and Amphibians. ASTM International: West Conshohocken, PA, USA, 2014; Voulume 11, pp. 1–22. [CrossRef]
- Prata, J.C.; Sequeira, I.F.; Monteiro, S.S.; Silva, A.L.P.; da Costa, J.P.; Dias-Pereira, P.; Fernandes, A.J.S.; da Costa, F.M.; Duarte, A.C.; Rocha-Santos, T. Preparation of biological samples for microplastic identification by Nile Red. Sci. Total Environ. 2021, 783, 147065. [Google Scholar] [CrossRef]
- Campbell, S.H.; Williamson, P.R.; Hall, B.D. Microplastics in the gastrointestinal tracts of fish and the water from an urban prairie creek. Facets 2017, 2, 395–409. [Google Scholar] [CrossRef] [Green Version]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Domingues, I.; Gravato, C. Oxidative stress assessment in zebrafish larvae. Methods Mol. Biol. 2018, 1797, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Claiborne, A. Catalase activity. In CRC Handbook of Methods in Oxygen Radical Research; Greenwald, R.A., Ed.; CRC Press: Boca Raton, FL, USA, 1985; pp. 283–284. [Google Scholar]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S transferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef]
- Baker, M.A.; Cerniglia, G.J.; Zaman, A. Microtiter plate assay for the measurement of glutathione and glutathione disulfide in large numbers of biological samples. Anal. Biochem. 1990, 190, 360–365. [Google Scholar] [CrossRef]
- Tietze, F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: Applications to mammalian blood and other tissues. Anal. Biochem. 1969, 27, 502–522. [Google Scholar] [CrossRef]
- Rodrigues, A.C.M.; Gravato, C.; Quintaneiro, C.; Bordalo, M.D.; Barata, C.; Soares, A.M.V.M.; Pestana, J.L.T. Energetic costs and biochemical biomarkers associated with esfenvalerate exposure in Sericostoma vittatum. Chemosphere 2017, 189, 445–453. [Google Scholar] [CrossRef]
- Bird, R.P.; Draper, H.H. Comparative Studies on Different Methods of Malonaldehyde Determination. Methods Enzym. 1984, 105, 299–305. [Google Scholar] [CrossRef]
- Mesquita, C.S.; Oliveira, R.; Bento, F.; Geraldo, D.; Rodrigues, J.V.; Marcos, J.C. Simplified 2,4-dinitrophenylhydrazine spectrophotometric assay for quantification of carbonyls in oxidized proteins. Anal. Biochem. 2014, 458, 69–71. [Google Scholar] [CrossRef]
- Vassault, A. Lactate dehydrogenase. In Methods of Enzymatic Analysis—Enzymes: Oxireductases, Transferase; Bergmyer, M.O., Ed.; Academic Press: New York, NY, USA, 1983; pp. 118–126. [Google Scholar]
- Diamantino, T.C.; Almeida, E.; Soares, A.M.V.M.; Guilhermino, L. Lactate dehydrogenase activity as an effect criterion in toxicity tests with Daphnia magna straus. Chemosphere 2001, 45, 553–560. [Google Scholar] [CrossRef] [Green Version]
- De Coen, W.; Janssen, C.R. The use of biomarkers in Daphnia magna toxicity testing. IV.Cellular Energy Allocation: A new methodology to assess the energy budget of toxicant-stressed Daphnia populations. J. Aquat. Ecosyst. Stress Recover 1997, 6, 43–45. [Google Scholar] [CrossRef]
- Rodrigues, A.C.M.; Gravato, C.; Quintaneiro, C.; Golovko, O.; Žlábek, V.; Barata, C.; Soares, A.M.V.M.; Pestana, J.L.T. Life history and biochemical effects of chlorantraniliprole on Chironomus riparius. Sci. Total Environ. 2015, 508, 506–513. [Google Scholar] [CrossRef]
- Gnaiger, E. Calculation of Energetic and Biochemical Equivalents of Respiratory Oxygen Consumption. In Polarographic Oxygen Sensors; Springer: Berlin/Heidelberg, Germany, 1983; pp. 337–375. [Google Scholar]
- Browne, M.A.; Dissanayake, A.; Galloway, T.S.; Lowe, D.M.; Thompson, R.C. Ingested microscopic plastic translocates to the circulatory system of the mussel, Mytilus edulis (L.). Environ. Sci. Technol. 2008, 42, 5026–5031. [Google Scholar] [CrossRef]
- Rist, S.E.; Assidqi, K.; Zamani, N.P.; Appel, D.; Perschke, M.; Huhn, M.; Lenz, M. Suspended micro-sized PVC particles impair the performance and decrease survival in the Asian green mussel Perna viridis. Mar. Pollut. Bull. 2016, 111, 213–220. [Google Scholar] [CrossRef]
- Ribeiro, F.; Garcia, A.R.; Pereira, B.P.; Fonseca, M.; Mestre, N.C.; Fonseca, T.G.; Ilharco, L.M.; Bebianno, M.J. Microplastics effects in Scrobicularia plana. Mar. Pollut. Bull. 2017, 122, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Paul-Pont, I.; Lacroix, C.; González Fernández, C.; Hégaret, H.; Lambert, C.; Le Goïc, N.; Frère, L.; Cassone, A.L.; Sussarellu, R.; Fabioux, C.; et al. Exposure of marine mussels Mytilus spp. to polystyrene microplastics: Toxicity and influence on fluoranthene bioaccumulation. Environ. Pollut. 2016, 216, 724–737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guilhermino, L.; Vieira, L.R.; Ribeiro, D.; Tavares, A.S.; Cardoso, V.; Alves, A.; Almeida, J.M. Uptake and effects of the antimicrobial florfenicol, microplastics and their mixtures on freshwater exotic invasive bivalve Corbicula fluminea. Sci. Total Environ. 2018, 622–623, 1131–1142. [Google Scholar] [CrossRef] [PubMed]
- Thapa, H.R.; Lin, Z.; Yi, D.; Smith, J.E.; Schmidt, E.W.; Agarwal, V. Genetic and Biochemical Reconstitution of Bromoform Biosynthesis in Asparagopsis Lends Insights into Seaweed Reactive Oxygen Species Enzymology. ACS Chem. Biol. 2020, 15, 1662–1670. [Google Scholar] [CrossRef]
- Prokić, M.D.; Radovanović, T.B.; Gavrić, J.P.; Faggio, C. Ecotoxicological effects of microplastics: Examination of biomarkers, current state and future perspectives. TrAC-Trends Anal. Chem. 2019, 111, 37–46. [Google Scholar] [CrossRef]
- Regoli, F.; Giuliani, M.E. Oxidative pathways of chemical toxicity and oxidative stress biomarkers in marine organisms. Mar. Environ. Res. 2014, 93, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Gosling, E. Marine Bivalve Molluscs, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- Webb, S.; Gaw, S.; Marsden, I.D.; McRae, N.K. Biomarker responses in New Zealand green-lipped mussels Perna canaliculus exposed to microplastics and triclosan. Ecotoxicol. Environ. Saf. 2020, 201, 110871. [Google Scholar] [CrossRef]
- Magara, G.; Elia, A.C.; Syberg, K.; Khan, F.R. Single contaminant and combined exposures of polyethylene microplastics and fluoranthene: Accumulation and oxidative stress response in the blue mussel, Mytilus edulis. J. Toxicol. Environ. Health-Part A Curr. Issues 2018, 81, 761–773. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.J.; Carini, M.; Butterfield, D.A. Protein carbonylation. Antioxid. Redox Signal. 2010, 12, 323–325. [Google Scholar] [CrossRef]
- Lacroix, C.; Richard, G.; Seguineau, C.; Guyomarch, J.; Moraga, D.; Auffret, M. Active and passive biomonitoring suggest metabolic adaptation in blue mussels (Mytilus spp.) chronically exposed to a moderate contamination in Brest harbor (France). Aquat. Toxicol. 2015, 162, 126–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dobal, V.; Suárez, P.; Ruiz, Y.; García-Martín, O.; Juan, F.S. Activity of antioxidant enzymes in Mytilus galloprovincialis exposed to tar: Integrated response of different organs as pollution biomarker in aquaculture areas. Aquaculture 2022, 548, 737638. [Google Scholar] [CrossRef]
- Silva, C.J.M.; Patrício Silva, A.L.; Campos, D.; Machado, A.L.; Pestana, J.L.T.; Gravato, C. Oxidative damage and decreased aerobic energy production due to ingestion of polyethylene microplastics by Chironomus riparius (Diptera) larvae. J. Hazard. Mater. 2021, 402, 123775. [Google Scholar] [CrossRef]
- Zhang, X.J.; Yang, L.; Zhao, Q.; Caen, J.P.; He, H.Y.; Jin, Q.H.; Guo, L.H.; Alemany, M.; Zhang, L.Y.; Shi, Y.F. Induction of acetylcholinesterase expression during apoptosis in various cell types. Cell Death Differ. 2002, 9, 790–800. [Google Scholar] [CrossRef] [Green Version]
- Fonte, E.; Ferreira, P.; Guilhermino, L. Temperature rise and microplastics interact with the toxicity of the antibiotic cefalexin to juveniles of the common goby (Pomatoschistus microps): Post-exposure predatory behaviour, acetylcholinesterase activity and lipid peroxidation. Aquat. Toxicol. 2016, 180, 173–185. [Google Scholar] [CrossRef]
- Silva, C.O.; Simões, T.; Félix, R.; Soares, A.M.V.M.; Barata, C.; Novais, S.C.; Lemos, M.F.L. Asparagopsis armata Exudate Cocktail: The Quest for the Mechanisms of Toxic Action of an Invasive Seaweed on Marine Invertebrates. Biology 2021, 10, 223. [Google Scholar] [CrossRef]
- Espinoza, B.; Silman, I.; Arnon, R.; Tarrab-Hazdai, R. Phosphatidylinositol-specific phospholipase C induces biosynthesis of acetylcholinesterase via diacylglycerol in Schistosoma mansoni. Eur. J. Biochem. 1991, 195, 863–870. [Google Scholar] [CrossRef]
- Gambardella, C.; Morgana, S.; Ferrando, S.; Bramini, M.; Piazza, V.; Costa, E.; Garaventa, F.; Faimali, M. Effects of polystyrene microbeads in marine planktonic crustaceans. Ecotoxicol. Environ. Saf. 2017, 145, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Shahriari, A.; Dawson, N.J.; Bell, R.A.V.; Storey, K.B. Stable suppression of lactate dehydrogenase activity during anoxia in the foot muscle of Littorina littorea and the potential role of acetylation as a novel posttranslational regulatory mechanism. Enzyme Res. 2013, 2013, 461374. [Google Scholar] [CrossRef] [Green Version]
- Van Cauwenberghe, L.; Claessens, M.; Vandegehuchte, M.B.; Janssen, C.R. Microplastics are taken up by mussels (Mytilus edulis) and lugworms (Arenicola marina) living in natural habitats. Environ. Pollut. 2015, 199, 10–17. [Google Scholar] [CrossRef]
- Erk, M.; Ivanković, D.; Strižak, Ž. Cellular energy allocation in mussels (Mytilus galloprovincialis) from the stratified estuary as a physiological biomarker. Mar. Pollut. Bull. 2011, 62, 1124–1129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shang, Y.; Wang, X.; Chang, X.; Sokolova, I.M.; Wei, S.; Liu, W.; Fang, J.K.H.; Hu, M.; Huang, W.; Wang, Y. The Effect of Microplastics on the Bioenergetics of the Mussel Mytilus coruscus Assessed by Cellular Energy Allocation Approach. Front. Mar. Sci. 2021, 8, 1–8. [Google Scholar] [CrossRef]
- Bell, E.C.; Gosline, J.M. Mechanical design of mussel byssus: Material yield enhances attachment strength. J. Exp. Biol. 1996, 199, 1005–1017. [Google Scholar] [CrossRef]


| Tissue | Number of Particles per Gram Tissue (ww) | |
|---|---|---|
| PA-MP | PA-MP + Exudate | |
| Gills | 6.97 ± 3.08 | 11.95 ± 4.83 |
| Digestive gland | 35.04 ± 16.09 | 62.25 ± 25.98 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, F.G.; Vieira, H.C.; Campos, D.; Pires, S.F.S.; Rodrigues, A.C.M.; Silva, A.L.P.; Soares, A.M.V.M.; Oliveira, J.M.M.; Bordalo, M.D. Co-Exposure with an Invasive Seaweed Exudate Increases Toxicity of Polyamide Microplastics in the Marine Mussel Mytilus galloprovincialis. Toxics 2022, 10, 43. https://doi.org/10.3390/toxics10020043
Rodrigues FG, Vieira HC, Campos D, Pires SFS, Rodrigues ACM, Silva ALP, Soares AMVM, Oliveira JMM, Bordalo MD. Co-Exposure with an Invasive Seaweed Exudate Increases Toxicity of Polyamide Microplastics in the Marine Mussel Mytilus galloprovincialis. Toxics. 2022; 10(2):43. https://doi.org/10.3390/toxics10020043
Chicago/Turabian StyleRodrigues, Filipa G., Hugo C. Vieira, Diana Campos, Sílvia F. S. Pires, Andreia C. M. Rodrigues, Ana L. P. Silva, Amadeu M. V. M. Soares, Jacinta M. M. Oliveira, and Maria D. Bordalo. 2022. "Co-Exposure with an Invasive Seaweed Exudate Increases Toxicity of Polyamide Microplastics in the Marine Mussel Mytilus galloprovincialis" Toxics 10, no. 2: 43. https://doi.org/10.3390/toxics10020043
APA StyleRodrigues, F. G., Vieira, H. C., Campos, D., Pires, S. F. S., Rodrigues, A. C. M., Silva, A. L. P., Soares, A. M. V. M., Oliveira, J. M. M., & Bordalo, M. D. (2022). Co-Exposure with an Invasive Seaweed Exudate Increases Toxicity of Polyamide Microplastics in the Marine Mussel Mytilus galloprovincialis. Toxics, 10(2), 43. https://doi.org/10.3390/toxics10020043












