A Preliminary Assessment of Size-Fractionated Microplastics in Indoor Aerosol—Kuwait’s Baseline
Abstract
:1. Introduction
2. Sample Collection and Preparation
3. Identification of Microplastics in Aerosol Samples
4. Microplastic in Indoor Air
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dris, R.; Gasperi, J.; Mirande, C.; Mandin, C.; Guerrouache, M.; Langlois, V.; Tassin, B. A first overview of textile fibers, including microplastics, in indoor and outdoor environments. Environ. Pollut. 2017, 221, 453–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dris, R.; Gasperi, J.; Rocher, V.; Mohamed, S.; Tassin, B. Microplastic contamination in an urban area: A case study in Greater Paris. Environ. Chem. 2015, 12, 592–599. [Google Scholar] [CrossRef]
- Dris, R.; Gasperi, J.; Saad, M.; Mirande, C.; Tassin, B. Synthetic fibers in atmospheric fallout: A source of microplastics in the environment? Mar. Pollut. Bull. 2016, 104, 290–293. [Google Scholar] [CrossRef]
- Gasperi, J.; Wright, S.; Dris, R.; Collard, F.; Mandin, C.; Guerrouache, M.; Langlois, V.; Kelly, F.; Tassin, B. Microplastics in air: Are we breathing it in? Curr. Opin. Environ. Sci. Health 2018, 1, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Abbasi, S.; Keshavarzi, B.; Moore, F.; Delshab, H.; Soltani, N.; Sorooshian, A. Investigation of microrubbers, microplastics and heavy metals in street dust: A study in Bushehr city, Iran. Environ. Earth Sci. 2017, 76, 798. [Google Scholar] [CrossRef]
- Abbasi, S.; Keshavarzi, B.; Moore, F.; Turner, A.; Kelly, F.J.; Dominguez, A.O.; Jaafarzadeh, N. Distribution and potential health impacts of microplastics and microrubbers in air and street dusts from Asaluyeh County, Iran. Environ. Pollut. 2019, 244, 153–164. [Google Scholar] [CrossRef] [Green Version]
- Cai, L.; Wang, J.; Tan, Z.; Zhan, Z.; Tan, X.; Chen, Q. Characteristic of microplastics in the atmospheric fallout from Dongguan city, China: Preliminary research and first evidence. Environ. Sci. Pollut. Res. 2017, 24, 24928–24935. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Tian, C.; Luo, Y. Various forms and deposition fluxes of microplastics identified in the coastal urban atmosphere. Chin. Sci. Bull. 2017, 62, 3902–3909. [Google Scholar] [CrossRef] [Green Version]
- Dehghani, S.; Moore, F.; Akhbarizadeh, R. Microplastic pollution in deposited urban dust, Tehran metropolis Iran. Environ. Sci. Pollut. Res. 2017, 24, 20360–20371. [Google Scholar] [CrossRef]
- Kaya, A.; Yurtsever, M.; Bayraktar, S. Ubiquitous exposure to microfiber pollution in the air. Eur. Phys. J. Plus 2018, 133, 488. [Google Scholar] [CrossRef]
- Liu, K.; Wang, X.H.; Wei, N.; Song, Z.Y.; Li, D.J. Accurate quantification and transport estimation of suspended atmospheric microplastics in megacities: Implications for human health. Environ. Int. 2019, 132, 105127. [Google Scholar] [CrossRef]
- Wang, X.; Li, C.; Liu, K.; Zhu, L.; Song, Z.; Li, D. Atmospheric microplastic over the South China Sea and East Indian Ocean: Abundance, distribution and source. J. Hazard. Mater. 2019, 389, 121846. [Google Scholar] [CrossRef]
- Liu, K.; Wang, X.; Fang, T.; Xu, P.; Zhu, L.; Li, D. Source and potential risk assessment of suspended atmospheric microplastics in Shanghai. Sci. Total Environ. 2019, 675, 462. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, J.; Zhang, Y.; Wang, L.; Deng, J.; Gao, Y.; Yu, L.; Zhang, J.; Sun, H. Widespread distribution of PET and PC microplastics in dust in urban China and their estimated human exposure. Environ. Int. 2019, 128, 116–124. [Google Scholar] [CrossRef]
- Klein, M.; Fischer, E.K. Microplastic abundance in atmospheric deposition within the Metropolitan area of Hamburg, Germany. Sci. Total Environ. 2019, 685, 96. [Google Scholar] [CrossRef]
- Wright, S.L.; Levermore, J.M.; Kelly, F.J. Raman Spectral Imaging for the Detection of Inhalable Microplastics in Ambient Particulate Matter Samples. Environ. Sci. Technol. 2019, 53, 8947–8956. [Google Scholar] [CrossRef] [PubMed]
- Allen, S.; Allen, D.; Phoenix, V.R.; Le Roux, G.; Duranteza, P.; Simonneau, A.; Stéphane, B.; Galop, D. Atmospheric transport and deposition of microplastics in a remote mountain catchment. Nat. Geosci. 2019, 12, 339–344. [Google Scholar] [CrossRef]
- Stanton, T.; Johnson, M.; Nathanail, P.; MacNaughtan, W.; Gomes, R.L. Freshwater and airborne textile fibre populations are dominated by ‘natural’, not microplastic, fibres. Sci. Total Environ. 2019, 666, 377–389. [Google Scholar] [CrossRef] [PubMed]
- Syafei, A.; Nurasrin, N.; Assomadi, A.; Boedisantoso, R. Microplastic Pollution in the Ambient Air of Surabaya, Indonesia. Curr. World Environ. 2019, 14, 290–298. [Google Scholar] [CrossRef] [Green Version]
- Bergmann, M.; Mützel, S.; Primpke, S.; Tekman, M.B.; Trachsel, J.; Gerdts, G. White and wonderful? Microplastics prevail in snow from the Alps to the Arctic. Sci. Adv. 2019, 5, eaax1157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scopetani, C.; Chelazzi, D.; Cincinelli, A.; Esterhuizen-Londt, M. Assessment of microplastic pollution: Occurrence and characterisation in Vesijärvi lake and Pikku Vesijärvi pond, Finland. Environ. Monit. Assess. 2019, 191, 652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Shao, L.; Wang, W.; Zhang, M.; Feng, X.; Li, W.; Zhang, D. Airborne fiber particles: Types, size and concentration observed in Beijing. Sci. Total Environ. 2020, 705, 135967. [Google Scholar] [CrossRef]
- Vianello, A.; Jensen, R.L.; Liu, L.; Vollertsen, J. Simulating human exposure to indoor airborne microplastics using a Breathing Thermal Manikin. Sci. Rep. 2019, 9, 8670. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, L.; Kannan, K. Microplastics in house dust from 12 countries and associated human exposure. Environ. Int. 2020, 134, 105314. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhao, Y.; Du, F.; Cai, H.; Wang, G.; Shi, H. Microplastic Fallout in Different Indoor Environments. Environ. Sci. Technol. 2020, 54, 6530–6539. [Google Scholar] [CrossRef]
- Soltani, N.S.; Taylor, M.P.; Wilson, S.P. Quantification and exposure assessment of microplastics in Australian indoor house dust. Environ. Pollut. 2021, 283, 117064. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Ji, X.; Ma, Y.; Lv, B.; Huang, W.; Zhu, X.; Fang, M.; Wang, Q.; Wang, X.; Dahlgren, R.; et al. Airborne microplastics in indoor and outdoor environments of a coastal city in Eastern China. J. Hazard. Mater. 2021, 417, 126007. [Google Scholar] [CrossRef]
- Prata, J.C.; Castro, J.L.; da Costa, J.P.; Duarte, A.C.; Rocha-Santos, T.; Cerqueira, M. The importance of contamination control in airborne fibers and microplastic sampling: Experiences from indoor and outdoor air sampling in Aveiro, Portugal. Mar. Pollut. Bull. 2020, 159, 111522. [Google Scholar] [CrossRef]
- Dris, R.; Gasperi, J.; Tassin, B. Sources and Fate of Microplastics in Urban Areas: A Focus on Paris Megacity. In Freshwater Microplastics: Emerging Environmental Contaminants? Wagner, M., Lambert, S., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 69–83. [Google Scholar] [CrossRef] [Green Version]
- Habibi, N.; Uddin, S.; Fowler, S.W.; Behbehani, M. Microplastics in the atmosphere: A review. J. Environ. Expo. Assess. 2022, 1, 6. [Google Scholar] [CrossRef]
- Uddin, S.; Fowler, S.W.; Habibi, N.; Behbehani, B. Micro-Nano plastic in the aquatic environment: Methodological problem and challenges. Animals 2022, 12, 297. [Google Scholar] [CrossRef]
- Wright, S.L.; Ulke, J.; Font, A.; Chan, K.L.A.; Kelly, F.J. Atmospheric microplastic deposition in an urban environment and an evaluation of transport. Environ. Int. 2020, 136, 105411. [Google Scholar] [CrossRef] [PubMed]
- Napper, I.E.; Thompson, R.C. Release of synthetic microplastic plastic fibres from domestic washing machines: Effects of fabric type and washing conditions. Mar. Pollut. Bull. 2016, 112, 39–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wright, S.L.; Gouin, T.; Koelmans, A.A.; Scheuermann, L. Development of screening criteria for microplastic particles in air and atmospheric deposition: Critical review and applicability towards assessing human exposure. Microplast. Nanoplast. 2021, 1, 6. [Google Scholar] [CrossRef]
- Enyoh, C.E.; Verla, A.W.; Verla, E.N.; Ibe, F.C.; Amaobi, C.E. Airborne microplastics: A review study on method for analysis, occurrence, movement and risks. Environ. Monit. Assess. 2019, 191, 668. [Google Scholar] [CrossRef]
- Prata, J. Airborne microplastics: Consequences to human health? Environ. Pollut. 2018, 234, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Behbehani, M.; Uddin, S.; Baskaran, M. 210Po concentration in different size fractions of aerosol likely contribution from Industrial Sources. J. Environ. Radioact. 2020, 222, 106323. [Google Scholar] [CrossRef]
- Amato-Lourenço, L.F.; Dos Santos Galvão, L.; de Weger, L.A.; Hiemstra, P.S.; Vijver, M.G.; Mauad, T. An emerging class of air pollutants: Potential effects of microplastics to respiratory human health? Sci. Total Environ. 2020, 749, 141676. [Google Scholar] [CrossRef]
- Sridharan, S.; Kumar, M.; Singh, L.; Bolan, N.S.; Saha, M. Microplastics as an emerging source of particulate air pollution: A critical review. J. Hazard. Mater. 2021, 418, 126245. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.L.; Kelly, F.J. Plastic and Human Health: A Micro Issue? Environ. Sci. Technol. 2017, 51, 6634–6647. [Google Scholar] [CrossRef]
- Bianco, A.; Passananti, M. Atmospheric Micro and Nanoplastics: An Enormous Microscopic Problem. Sustainability 2020, 12, 7327. [Google Scholar] [CrossRef]
- Hartmann, N.B.; Hüffer, T.; Thompson, R.C.; Hassellöv, M.; Verschoor, A.; Daugaard, A.E.; Rist, S.; Karlsson, T.; Brennholt, N.; Cole, M.; et al. Are We Speaking the Same Language? Recommendations for a Definition and Categorization Framework for Plastic Debris. Environ. Sci. Technol. 2019, 53, 1039–1047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schulze, F.; Gao, X.; Virzonis, D.; Damiati, S.; Schneider, M.R.; Kodzius, R. Air Quality Effects on Human Health and Approaches for Its Assessment through Microfluidic Chips. Genes 2017, 8, 244. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.-F.; Xu, Y.-H.; Shi, M.-H.; Lian, Y.-X. The impact of PM2.5 on the human respiratory system. J. Thorac. Dis. 2016, 8, E69–E74. [Google Scholar]
- Pauly, J.L.; Stegmeier, S.J.; Allaart, H.A.; Cheney, R.T.; Zhang, P.J.; Mayer, A.G.; Streck, R.J. Inhaled cellulosic and plastic fibers found in human lung tissue. Cancer Epidemiol. Biomark. Prev. 1998, 7, 419. [Google Scholar]
- Boag, A.H.; Colby, T.V.; Fraire, A.E.; Kuhn, C.; Roggli, V.L.; Travis, W.D.; Vallyathan, V. The pathology of interstitial lung disease in nylon flock workers. Am. J. Surg. Pathol. 1999, 23, 1539. [Google Scholar] [CrossRef] [PubMed]
- Eschenbacher, W.L.; Kreiss, K.; Lougheed, M.D.; Pransky, G.S.; Day, B.; Castellan, R.M. Nylon flock associated interstitial lung disease. Am. J. Respir. Crit. Care Med. 1999, 159, 2003. [Google Scholar] [CrossRef] [PubMed]
- Kremer, A.M.; Pal, T.M.; Boleij, J.S.; Schouten, J.P.; Rijcken, B. Airway hyper-responsiveness and the prevalence of work-related symptoms in workers exposed to irritants. Am. J. Ind. Med. 1994, 26, 655. [Google Scholar] [CrossRef]
- Marzec, A. The Effect of Dyes, Pigments and Ionic Liquids on the Properties of Elastomer Composites. Ph.D. Thesis, Université Claude Bernard—Lyon I and Uniwersytet lódzki, Lodz, Porland, 2014. [Google Scholar]
- Campanale, C.; Massarelli, C.; Savino, I.; Locaputo, V.; Uricchio, V.F. A Detailed Review Study on Potential Effects of Microplastics and Additives of Concern on Human Health. Int. J. Environ. Res. Public Health 2020, 17, 1212. [Google Scholar] [CrossRef] [Green Version]
- Zimmermann, L.; Dierkes, G.; Ternes, T.A.; Völker, C.; Wagner, M. Benchmarking the in Vitro Toxicity and Chemical Composition of Plastic Consumer Products. Environ. Sci. Technol. 2019, 53, 11467–11477. [Google Scholar] [CrossRef] [Green Version]
- Alimba, C.G.; Faggio, C. Microplastics in the marine environment: Current trends in environmental pollution and mechanisms of toxicological profile. Environ. Toxicol. Pharmacol. 2019, 68, 61–74. [Google Scholar] [CrossRef]
- Andrady, A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef] [PubMed]
- Andrady, A.L. The plastic in microplastics: A review. Mar. Pollut. Bull. 2017, 119, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Auta, H.S.; Emenike, C.U.; Fauziah, S.H. Distribution and importance of microplastics in the marine environment: A review of the sources, fate, effects, and potential solutions. Environ. Int. 2017, 102, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Barboza, L.G.A.; Frias, J.P.G.L.; Booth, A.M.; Vieira, L.R.; Masura, J.; Baker, J.; Foster, G.; Guilhermino, L. Microplastics Pollution in the Marine Environment. In World Seas: An Environmental Evaluation. Volume III: Ecological Issues and Environmental Impacts; Sheppard, C., Ed.; Academic Press (Elsevier): London, UK, 2019; Volume 3, pp. 329–351. [Google Scholar]
- Brennecke, D.; Duarte, B.; Paiva, F.; Caçador, I.; Canning-Clode, J. Microplastics as vector for heavy metal contamination from the marine environment. Estuar. Coast. Shelf Sci. 2016, 178, 189–195. [Google Scholar] [CrossRef]
- De Sa, L.C.; Oliveira, M.; Ribeiro, F.; Rocha, T.L.; Futter, M.N. Studies of the effects of microplastics on aquatic organisms: What do we know and where should we focus our efforts in the future? Sci. Total Environ. 2018, 645, 1029–1039. [Google Scholar] [CrossRef] [PubMed]
- Esmaili, Z.; Naji, A. Comparison of the frequency, type and shape of microplastics in the low and high tidal of the coastline of Bandar Abbas. J. Oceanogr. 2018, 8, 53–61. [Google Scholar] [CrossRef] [Green Version]
- Fahrenfeld, N.L.; Arbuckle-Keil, G.; Naderi Beni, N.; Bartelt-Hunt, S.L. Source tracking microplastics in the freshwater environment. TrAC Trends Anal. Chem. 2019, 112, 248–254. [Google Scholar] [CrossRef]
- Hidalgo-Ruz, V.; Gutow, L.; Thompson, R.C.; Thiel, M. Microplastics in the marine environment: A review of the methods used for identification and quantification. Environ. Sci. Technol. 2012, 46, 3060–3075. [Google Scholar] [CrossRef] [PubMed]
- Lusher, A. Microplastics in the Marine Environment: Distribution, Interactions and Effects. In Marine Anthropogenic Litter; Bergmann, M., Gutow, L., Klages, M., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 245–307. [Google Scholar] [CrossRef] [Green Version]
- Maes, T.; Jessop, R.; Wellner, N.; Haupt, K.; Mayes, A.G. A rapid-screening approach to detect and quantify microplastics based on fluorescent tagging with Nile Red. Sci. Rep. 2017, 7, 44501. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, J.P.; Duarte, A.C.; Santos-Echeandía, J.; Rocha-Santos, T. Significance of interactions between microplastics and POPs in the marine environment: A critical overview. TrAC Trends Anal. Chem. 2019, 111, 252–260. [Google Scholar] [CrossRef]
- Schymanski, D.; Goldbeck, C.; Humpf, H.U.; Furst, P. Analysis of microplastics in water by micro-Raman spectroscopy: Release of plastic particles from different packaging into mineral water. Water Res. 2018, 129, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Gevao, B.; Uddin, S.; Al-Bahloul, M.; Al-Mutairi, A. Persistent organic pollutants on human and sheep hair and comparison with POPs in indoor and outdoor air. J. Environ. Expos. Assess. 2022, 1, 5. [Google Scholar] [CrossRef]
- Vroom, R.J.E.; Koelmans, A.A.; Besseling, E.; Halsband, C. Aging of microplastics promotes their ingestion by marine zooplankton. Environ. Pollut. 2017, 231, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Wagner, M.; Scherer, C.; Alvarez-Munor, D.; Brennholt, N.; Bourrain, X.; Buchinger, S.; Fries, E.; Grosbois, C.; Klasmeier, J.; Marti, T.; et al. Microplastics in freshwater ecosystems: What we now and what we need to know. Environ. Sci. Eur. 2014, 26, 12–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, J.K.; Chu, W.-L.; Kok, Y.; Lee, C. Distribution of Microplastics and Nanoplastics in Aquatic Ecosystems and Their Impacts on Aquatic Organisms, with Emphasis on Microalgae. Rev. Environ. Contam. Toxicol. 2018, 246, 133–158. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, J.; Liu, X.; Qu, F.; Wang, X.; Wang, X.; Li, Y.; Sun, Y. Microplastics in the environment: A review of analytical methods, distribution, and biological effects. TrAC Trends Anal. Chem. 2019, 111, 62–72. [Google Scholar] [CrossRef]
- Klepeis, N.E.; Nelson, W.C.; Ott, W.R.; Robinson, J.P.; Tsang, A.M.; Switzer, P.; Behar, J.V.; Hern, S.C.; Engelmann, W.H. The National Human Activity Pattern Survey (NHAPS): A resource for assessing exposure to environmental pollutants. J. Expo. Sci. Environ. Epidemiol. 2001, 11, 231–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Löder, M.G.L.; Gerdts, G. Methodology Used for the Detection and Identification of Microplastics—A Critical Appraisal. In Marine Anthropogenic Litter; Bargeman, M., Gutow, G., Klages, M., Eds.; Springer Open: Heidelbderg, Germany, 2015; pp. 201–227. [Google Scholar] [CrossRef] [Green Version]
- Van Cauwenberghe, L.; Devriese, L.; Galgani, F.; Robbens, J.; Janssen, C.R. Microplastics in sediments: A review of techniques, occurrence and effects. Mar. Environ. Res. 2015, 111, 5–17. [Google Scholar] [CrossRef] [Green Version]
- De Witte, B.; Devriese, L.; Bekaert, K.; Hoffman, S.; Vandermeersch, G.; Cooreman, K.; Robbens, J. Quality assessment of the blue mussel (Mytilus edulis): Comparison between commercial and wild types. Mar. Pollut. Bull. 2014, 85, 146. [Google Scholar] [CrossRef]
- Alzona, J.; Cohen, B.L.; Rudolph, H.; Jow, H.N.; Frohliger, J.O. Indoor-outdoor relationships for airborne particulate matter of outdoor origin. Atmos. Environ. 1979, 13, 55–60. [Google Scholar] [CrossRef]
- Uddin, S.; Fowler, S.W.; Uddin, M.F.; Behbehani, M.; Naji, A. A review of microplastic distribution in sediment profiles. Mar. Poll. Bull. 2021, 163, 111973. [Google Scholar] [CrossRef] [PubMed]
- Andrade, J.M.; Ferreiro, B.; López-Mahía, P.; Muniategui-Lorenzo, S. Standardization of the minimum information for publication of infrared-related data when microplastics are characterized. Mar. Pollut. Bull. 2020, 154, 111035. [Google Scholar] [CrossRef] [PubMed]
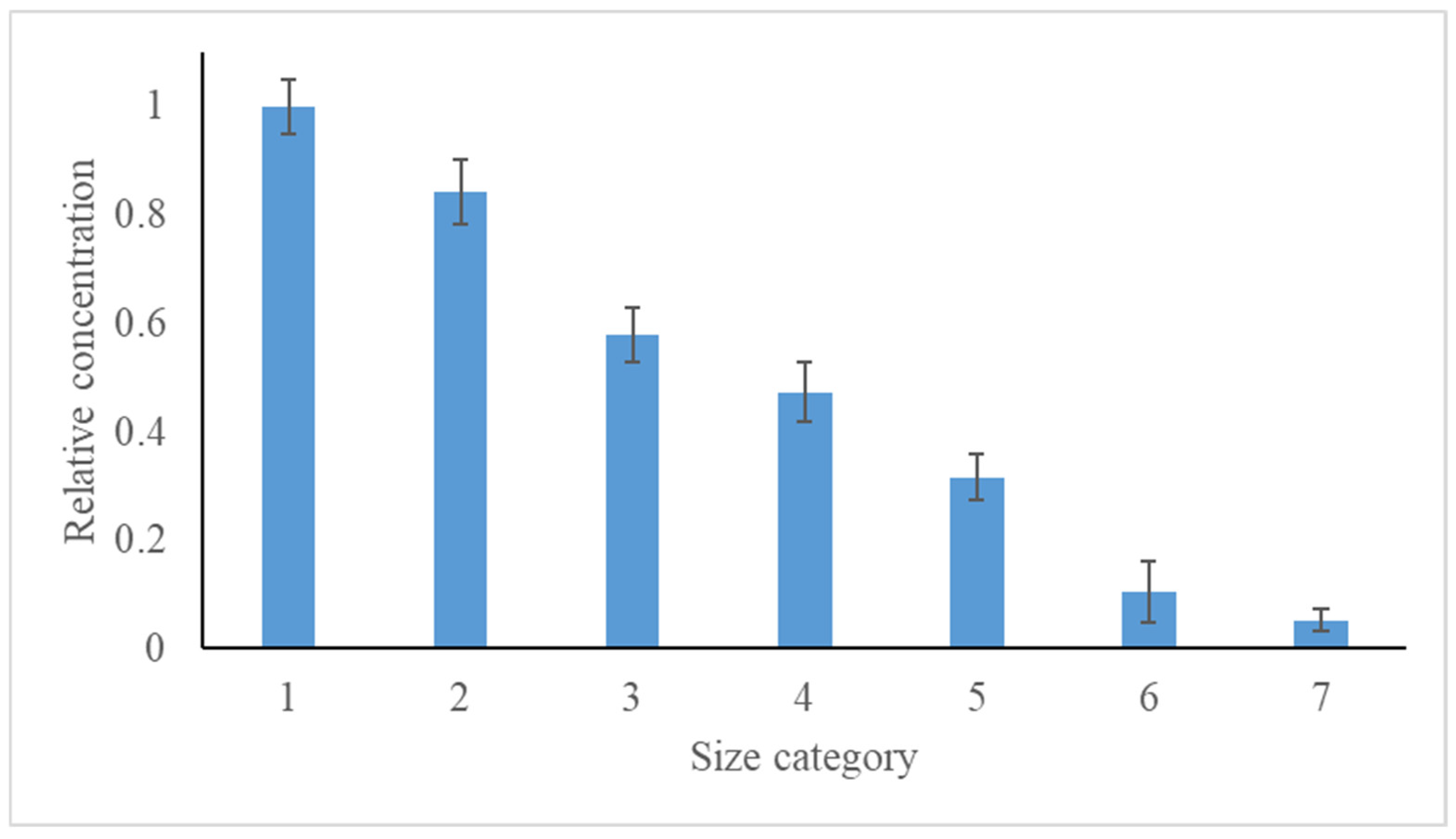




| Stage of Impactor | Orifice Diameter (mm) | Particle Size Range Captures (µm) |
|---|---|---|
| 1 | 1.18 | >7.0 |
| 2 | 0.91 | 4.7–7.0 |
| 3 | 0.71 | 3.3–4.7 |
| 4 | 0.53 | 2.1–3.3 |
| 5 | 0.34 | 1.1–2.1 |
| 6 | 0.25 | 0.65–1.1 |
| Base plate 7 | 0.10 | <0.65 |
| Building Type | Date | Location | 1 | 2 | 3 | 4 | 5 | 6 | 7 | Total | MP m−3 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Government/Public Buildings | 6 January 2021 | KISR Building No. 44 corridor (Laboratory 1501) | 19 | 16 | 11 | 9 | 6 | 2 | 1 | 64 | 5.9 |
| 5 April 2021 | KISR main entrance * (~100 employees) | 5 | 11 | 7 | 9 | 4 | 4 | 0 | 40 | 3.7 | |
| 21 April 2021 | KISR Lobby * (~100 employees) | 6 | 7 | 5 | 9 | 4 | 3 | 1 | 35 | 3.2 | |
| 4 May 2021 | KISR corridor building No. 44 (eastside) | 7 | 16 | 18 | 19 | 9 | 21 | 9 | 99 | 9.2 | |
| 14 March 2021 | KISR office (Carpeted) Room 2205 | 12 | 14 | 16 | 19 | 26 | 31 | 0 | 119 | 11.0 | |
| 25 July 2021 | KISR office (Carpeted) Room 2200 | 29 | 16 | 9 | 6 | 16 | 12 | 1 | 89 | 8.2 | |
| 17 May 2021 | KISR main entrance (~300 employees) | 20 | 27 | 19 | 20 | 19 | 8 | 0 | 113 | 10.5 | |
| 14 April 2021 | Government building with about ~l000 visitors | 25 | 39 | 38 | 21 | 15 | 8 | 1 | 147 | 13.6 | |
| 27 April 2021 | Attendance section office building with ~500 employees | 48 | 44 | 22 | 21 | 19 | 8 | 3 | 165 | 15.3 | |
| 6 July 2021 | Reception of a government building ~500 visitors | 16 | 18 | 26 | 21 | 6 | 4 | 3 | 94 | 8.7 | |
| 5 October 2021 | Attendance section of office building ~300 employees | 32 | 9 | 18 | 17 | 11 | 8 | 2 | 97 | 9.0 | |
| Private housing, low and high density | 14 March 2021 | High density residential (carpeted flat) Hawally | 59 | 36 | 28 | 18 | 22 | 18 | 10 | 191 | 17.7 |
| 14 June 2021 | High density residential (carpeted flat) Abu Haleefa—9th floor | 75 | 62 | 59 | 49 | 28 | 11 | 9 | 293 | 27.1 | |
| 15 June 2021 | High density residential (carpeted flat) Jaleeb—8th floor | 49 | 19 | 20 | 13 | 9 | 5 | 2 | 117 | 10.8 | |
| 19 June 2021 | High density residential (carpeted flat) Regga—1st floor | 39 | 41 | 24 | 14 | 8 | 7 | 5 | 138 | 12.8 | |
| 25 June 2021 | House with low occupancy (rugs) Daiya Area | 42 | 39 | 28 | 16 | 8 | 6 | 1 | 140 | 13.0 | |
| 3 August 2021 | House with low occupancy (rugs) Shuwaikh Area | 20 | 11 | 13 | 10 | 8 | 5 | 1 | 68 | 6.3 | |
| Hospital | 8 January 2021 | Causality ward Sheikh Jaber Hospital | 12 | 14 | 9 | 4 | 1 | 2 | 0 | 42 | 3.9 |
| 29 June 2021 | COVID ward of Sheikh Jaber Hospital | 10 | 9 | 11 | 7 | 6 | 4 | 0 | 47 | 4.4 | |
| Mosque | 1 September2021 | Mosque carpeted Eagila | 45 | 39 | 32 | 16 | 11 | 7 | 4 | 154 | 14.3 |
| Control (used for identification only) | 5 | 5 | 5 | 10 | 10 | 10 | 10 | 55 | |||
| Blank 1 (exposed to indoor air—passive) | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | |||
| Blank 2 (exposed to indoor air—passive) | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | |||
| Blank 3 (exposed—passive) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||
| Blank 4 (exposed—passive) | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| City/Country | Sample Matrix | Sampling Technique | Sampling Extraction/Treatment | Reference |
|---|---|---|---|---|
| Kuwait | Aerosol | Active Sampling: Sampling using 6-stage compactor, sample drawn at a rate of 30 L min−1. A total of 10.8 m3 of aerosol was drawn. | This Study | |
| Paris, France | Aerosol | Active Sampling: A pump for drawing indoor air (8 L/min) and quartz fiber GF/A Whatman filters (1.6 mm, 47 mm) for sample collection (2–5 m3) from two apartments and an office. | Samples passed through a 2.5 mm mesh size sieve and the retained fraction (>2.5 mm) visually inspected to verify plastics presence. Mass of 5.5 mg introduced in a separation funnel with 50 mL of ZnCl2 for density separation. Floating fraction homogenized, then a 1 ml subsample filtered on quartz filters (1.6 mm, 47 mm). | [1] |
| 39 cities, China | Atmospheric fallout (Dust) | Passive Sampling: Hog bristle brushes and pre-cleaned, sealed paper bag with aluminum foil lining for dust collection and storage, respectively, from 39 cities’ bedroom and living room (4 m2 each). | Dust sample weighed and placed in a beaker, and 50 mL of ZnCl2 solution added for MP density separation. Upper fraction was separated into another tube with a steel spoon and homogenized in 20 mL of the ZnCl2 solution. After oscillating, 100 μL aliquots of the solution were added to a grid counter and counted under a light microscope at 100× magnification. Process repeated twice to calculate MP per unit mass of dust. | [14] |
| Aarhus, Denmark | Aerosol | Active Sampling: Breathing thermal manikin made of aluminum and glass fiber and simulating human presence, and connected to mechanical artificial lung system, consisting of two pneumatic cylinders moved by an electric motor, producing an airflow to simulate breathing with respiration volume of 0.82 L min−1. | Filter sonicated for 5 min in pre-cleaned small beaker filled with just enough ethanol (99.9%, HPLC grade) to cover the filter. Membrane then flushed using additional ethanol, after which all the liquid containing the sample was deposited on a pre-heated (55 °C) zinc selenide (ZnSe) window held in a compression cell (PIKE technologies, Fitchburg, WI, USA) using a capillary glass pipette. Enriched ZnSe window dried at 55 °C for 48 h for final sample deposition for determination. | [23] |
| 12 countries; China, Colombia, Greece, India, Japan, Kuwait, Pakistan, Romania, Saudi Arabia, South Korea, USA, and Vietnam | House Dust | Passive Sampling: Nylon brush (China and India) or vacuum cleaner (other 10 countries); indoor pooled floor dust samples were collected from bedrooms and living rooms. | All samples sieved through a 150 μm sieve, and ones <150 µm were collected, homogenized, and stored at 4 °C until analysis. Dust samples (50 mg; spiked with 500 ng D4-TPA and 200 ng 13C12-BPA) weighed, placed in 100 mL round-bottom flask, then both 0.1 g of KOH and 20 mL of 1-pentanol added. The mixture was digested by stirring in heating mantle at 135 °C for 30 min, then allowed to cool down at room temperature while pentanol solution was transferred into a 50 mL PP tube. Flask rinsed twice with 10 mL of HPLC-grade water and rinsate transferred into PP tube. Depolymerized products of PET/PC-based MPs extracted from pentanol by shaking PP tube at 180 strokes per minute for 5 min in orbital shaker (Eberbach Corp., Ann Arbor, MI, USA), followed by centrifugation at 1620× g for 5 min (Eppendorf Centrifuge 5804× g, Hamburg, Germany). Upper organic phase of pentanol transferred to another tube with 20 mL of HPLC-grade water added and extraction repeated. Aqueous layer (water solution) containing TPA and BPA combined to total volume of 50 mL with HPLC-grade water. A 10 mL aliquot of the solution purified by passing through a SPE cartridge. Dust samples analyzed separately to determine concentrations of freely available TPA and BPA. Briefly, 50 mg of dust sample weighed and transferred into 15 mL PP conical tube. After spiking with 250 ng of D4-TPA and 50 ng of 13C12-BPA, samples extracted with 5 mL of methanol by shaking in orbital shaker for 30 min. Mixture centrifuged at 2880× g for 5 min, and supernatant transferred into new PP tube. Extraction repeated twice with 5 mL of methanol, and extracts combined and concentrated to approximately 1 mL under gentle nitrogen stream. Solution diluted to 5 mL with solvent mixture of methanol and HPLC-grade water at 2:8 ratio (v/v). Finally, 1 mL diluted solution centrifuged at 9030× g for 5 min, then transferred into amber glass vial for HPLC-MS/MS analysis. | [24] |
| East China Normal University, Shanghai, China | Dust Fallout | Passive Sampling: Fallout into a stainless steel sink; pooled samples collected over 24 h. Samples were collected from dormitory space (25 m2, occupied by 2 people), office space (40 m2, occupied by 12 people), and spot samples collected in corridor. | Samples collected on filters, carefully removed, and quickly transferred to marked, clean-air sampling cassettes using stainless steel tweezers. Filters examined with stereomicroscope for suspected microplastics and photographed. | [25] |
| Sydney, Australia | House Dust | Passive Sampling: Fallout was collected into 12 cm glass Petri dishes, placed at 1.2 m height for 30 days. A total of 32 sampling locations in 22 local government areas of metropolitan Sydney. | Sample collected in pre-cleaned, pre weighed Petri dish. Post-collection, the Petri dish was weighed again. The samples were washed from the Petri dish using Milli Q water and filtered under vacuum in a laminar flow unit on a 90 mm diameter glass fiber filter paper of 0.6 µm pore size. Filter paper was marked with 1 cm2 grids for ease of navigation under microscope. No sample processing step, i.e., digestion and density separation, was applied. | [26] |
| Wenzhou, China | Indoor Aerosol | Active Sampling: Sample was collected on 90 mm glass fiber filter with 0.7 µm filter using a LB-120F sampler with flow rate of 100 L min−1, a combined 1 m3 sample was collected from 39 indoor locations. | Samples collected on 90 mm glass fiber filter with 0.7 µm pore size. Sample was digested in glass beaker using approximately 30 mL 30% H2O2, heated at 70 °C for 1 h to remove organic matter. The sample was re-filtered on 47 mm diameter PTFE filter membrane with 0.45 µm pore size for Nile red staining. Three drops of 5 mg mL−1 Nile red were used for 30 min at room temperature. Samples were digitally photographed using florescence stereo microscope. | [27] |
| Aveiro, Portugal | Indoor aerosol | Active Sampling: Sample collected using PM10 collector @ 5 L min−1 for 48 h on glass fiber filter of 2.2 µm pore size. Sample was collected from from the living room of a two-story house with five residents. | Samples were collected on quartz filter with 2.2 µm pore size at rate of 5 L min−1 for 48 h (14.4 m3). Sample washed with 15 mL ultrapure water, to which 15 mL of 30% H2O2 was added and left at room temperature for 8 days to remove organic matter. This digested sample was filtered on glass fiber filter with 1.2 µm pore size, this filter was washed with 1.6 g cm−3 NaI solution for density separation. Solution was shaken in vortex for 1 min and left to settle for 90 min, followed by filtration of the supernatant and washing with ultrapure water in glass fiber filters and observed under stereo microscope. | [28] |
| Country | Sample Matrix | MP Concentrations | Size | Shape | Polymer | Color | Reference |
|---|---|---|---|---|---|---|---|
| Kuwait | Indoor aerosol | 3.24 to 27.13 MP m−3 | 0.45–2800 µm | Fibers (91%), fragments (9%) | Polyester, nylon, polyamide | Black, transparent, blue, red, grey | This Study |
| Paris, France | Indoor aerosol | 0.4 to 59.4 fibers m−3 Avg. 5.4 fibers m−3 | 50–3250 μm | Fibers | PA, PP | N/A | [1] |
| 39 major cities in China | Fallout (indoor) | PET: 1550–120,000 mg kg−1 (average 26,800 mg kg−1), geomean (GM) conc. 23,000 mg kg−1; PC: (74.4%) <LOQ-107 mg kg−1 (average 4.6 mg kg−1), GM 1.8 mg kg−1. | 50 μm–2 mm | Fibres (88%): 17–620 fibers/mg (Average 342 fibers/mg); granules: 6–184 particles/mg | Polyester, polyacrylonitrile, nylon, polyethylene, polypropylene, poly(ethylene:propylene), acrylic, polyurethane, polyethylenimine, alkyd | N/A | [76] |
| Aarhus, Denmark | Indoor air | 1.7−16.2 particles m−3 (average 9.3 ± 5.8 NMP m−3) | 4−398 µm (Average 177 µm) | Fragments (87%), fibers (13%) | Polyester (81%), polyethylene (6%), nylon (5%), polypropylene (2%), other polymers (6%) | N/A | [23] |
| Sydney, Australia | Fallout (indoor) | 22–6169 fibers m−2 d−1 (Avg. 3095 fibers m−2 d−1) | 50–2000 µm | 7401 fibers, 64 fragments, 18 films. | Fibers were predominantly polyethylene (25%), polyester and PET (17%), polyamide (16%), polyvinyl (15%) | Black, green, blue, red, grey, brown, and transparent. | [26] |
| Wenzhou, China | Indoor air | 1583 ± 1181 MP m−3 | 5–30 µm (60.4 ± 2.7%) 30–100 µm (28.5 ± 2.3%) >100 µm (11%) | Fragments 89.6%, fibers 10.4% | Polyester (28.4%), polyamide (20.54%), polyethylene (16.3%), polystyrene. | NA | [27] |
| Aveiro, Portugal | Indoor air | 6 fibers m−3 and smaller 5 MP m−3 (6% fibers were synthetic) | 17–3669 | Fibers | NA | Light color | [28] |
| 12 Countries | Indoor dust | PET—concentrations ranged between 29–120,000 µg/g. Concentrations are arranged in decreasing order of concentration as: South Korea (25,000 µg/g), Japan (23,000 µg/g), Saudi Arabia (13,000 µg/g) Greece (9700 µg/g), Romania (9100 µg/g), United States (8900 µg/g), Kuwait (8600 µg/g), Vietnam (3900 µg/g), China (3700 µg/g) (one sample had 120,000 µg/g, which was 12% of the total mass of dust), Pakistan (1900 µg/g), India (1600 µg/g), Colombia (1000 µg/g). Free TPA median concentrations in dust samples ranged from 2.0 μg/g (Pakistan) to 34 μg/g (Japan). The highest TPA concentration was found in the sample from India (200 μg/g). PC concentration range of <0.11–1700 μg/g. Saudi Arabia (2.5–190 μg/g, median: 45 μg/g) and South Korea (6.7–140 μg/g, median: 38 μg/g) contained the highest concentrations of PC. Free BPA concentrations ranged from <0.05–36 μg/g. | NA | NA | PET, PC | [24] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uddin, S.; Fowler, S.W.; Habibi, N.; Sajid, S.; Dupont, S.; Behbehani, M. A Preliminary Assessment of Size-Fractionated Microplastics in Indoor Aerosol—Kuwait’s Baseline. Toxics 2022, 10, 71. https://doi.org/10.3390/toxics10020071
Uddin S, Fowler SW, Habibi N, Sajid S, Dupont S, Behbehani M. A Preliminary Assessment of Size-Fractionated Microplastics in Indoor Aerosol—Kuwait’s Baseline. Toxics. 2022; 10(2):71. https://doi.org/10.3390/toxics10020071
Chicago/Turabian StyleUddin, Saif, Scott W. Fowler, Nazima Habibi, Sufiya Sajid, Sam Dupont, and Montaha Behbehani. 2022. "A Preliminary Assessment of Size-Fractionated Microplastics in Indoor Aerosol—Kuwait’s Baseline" Toxics 10, no. 2: 71. https://doi.org/10.3390/toxics10020071
APA StyleUddin, S., Fowler, S. W., Habibi, N., Sajid, S., Dupont, S., & Behbehani, M. (2022). A Preliminary Assessment of Size-Fractionated Microplastics in Indoor Aerosol—Kuwait’s Baseline. Toxics, 10(2), 71. https://doi.org/10.3390/toxics10020071









