In Situ Passive Sampling to Monitor Long Term Cap Effectiveness at a Tidally Influenced Shoreline
Abstract
:1. Introduction
2. Materials and Methods
2.1. PDMS SPME Fibers, Sampling Devices, and PRCs
2.2. Site and Sampling Design
2.3. Chemical Analysis
2.4. Determination of the Freely Dissolved Concentration
2.5. CapSim Modeling
3. Results
3.1. Remedy Effectiveness
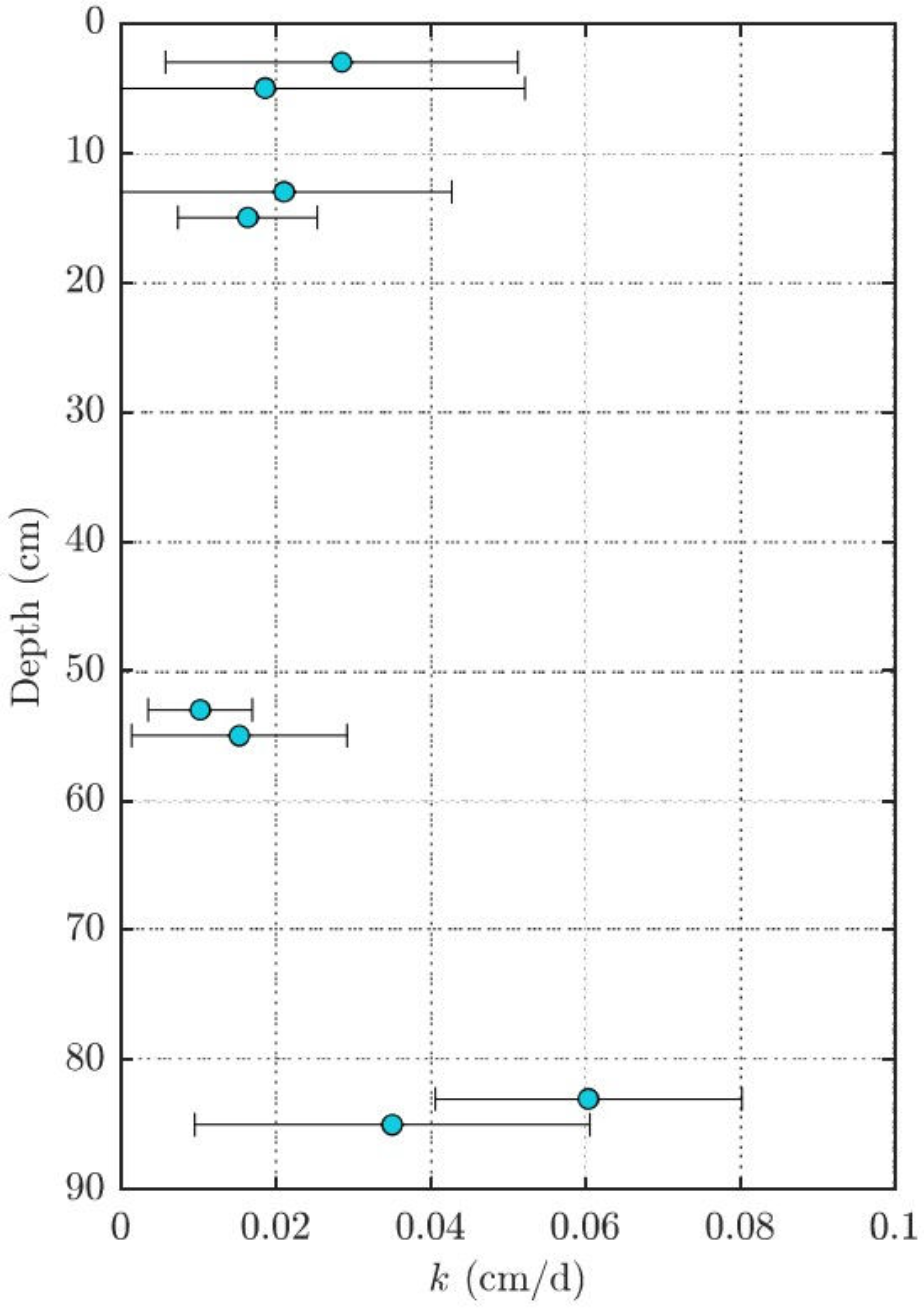
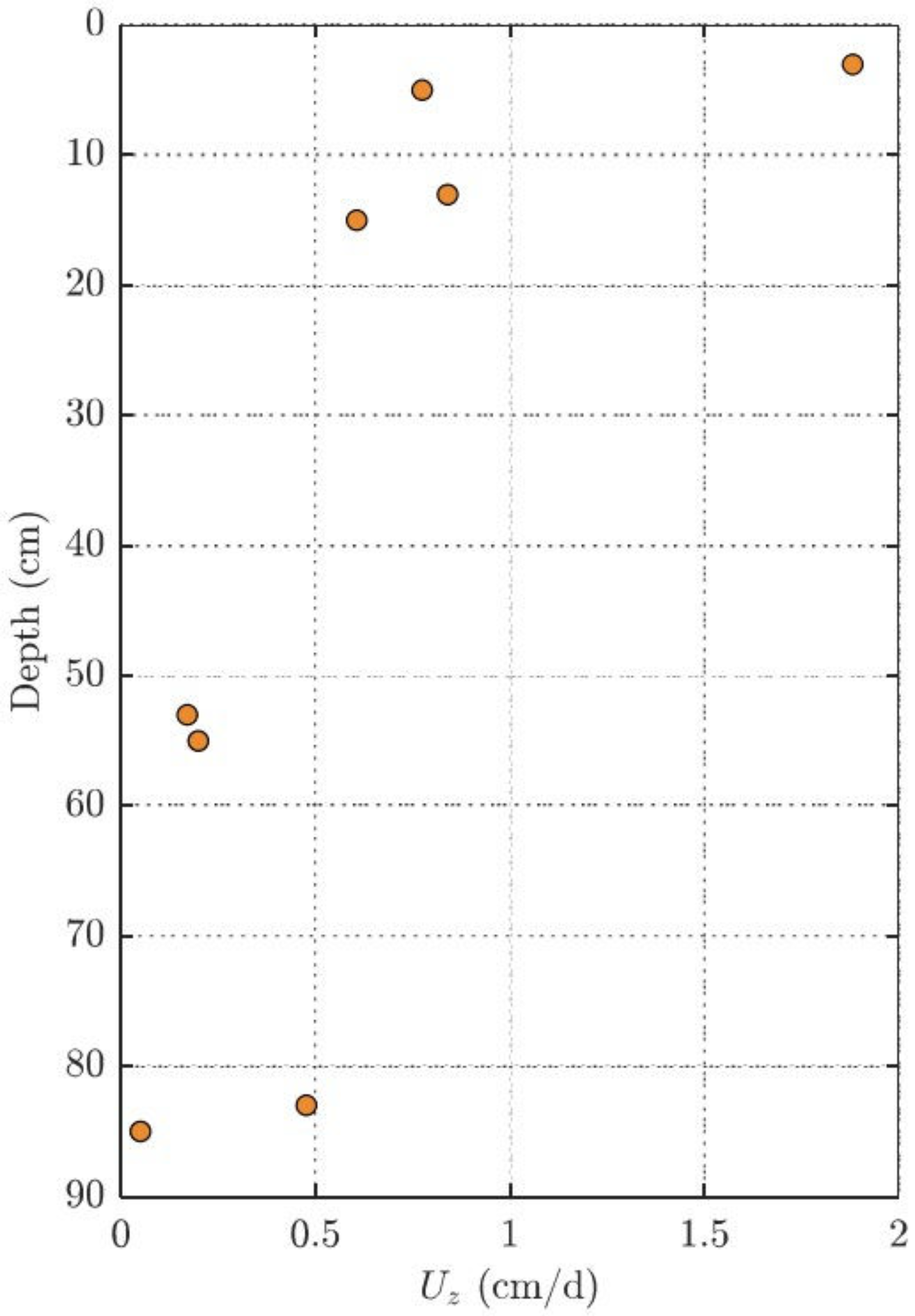
3.2. Estimating Groundwater Upwelling Velocity from Performance Reference Compounds
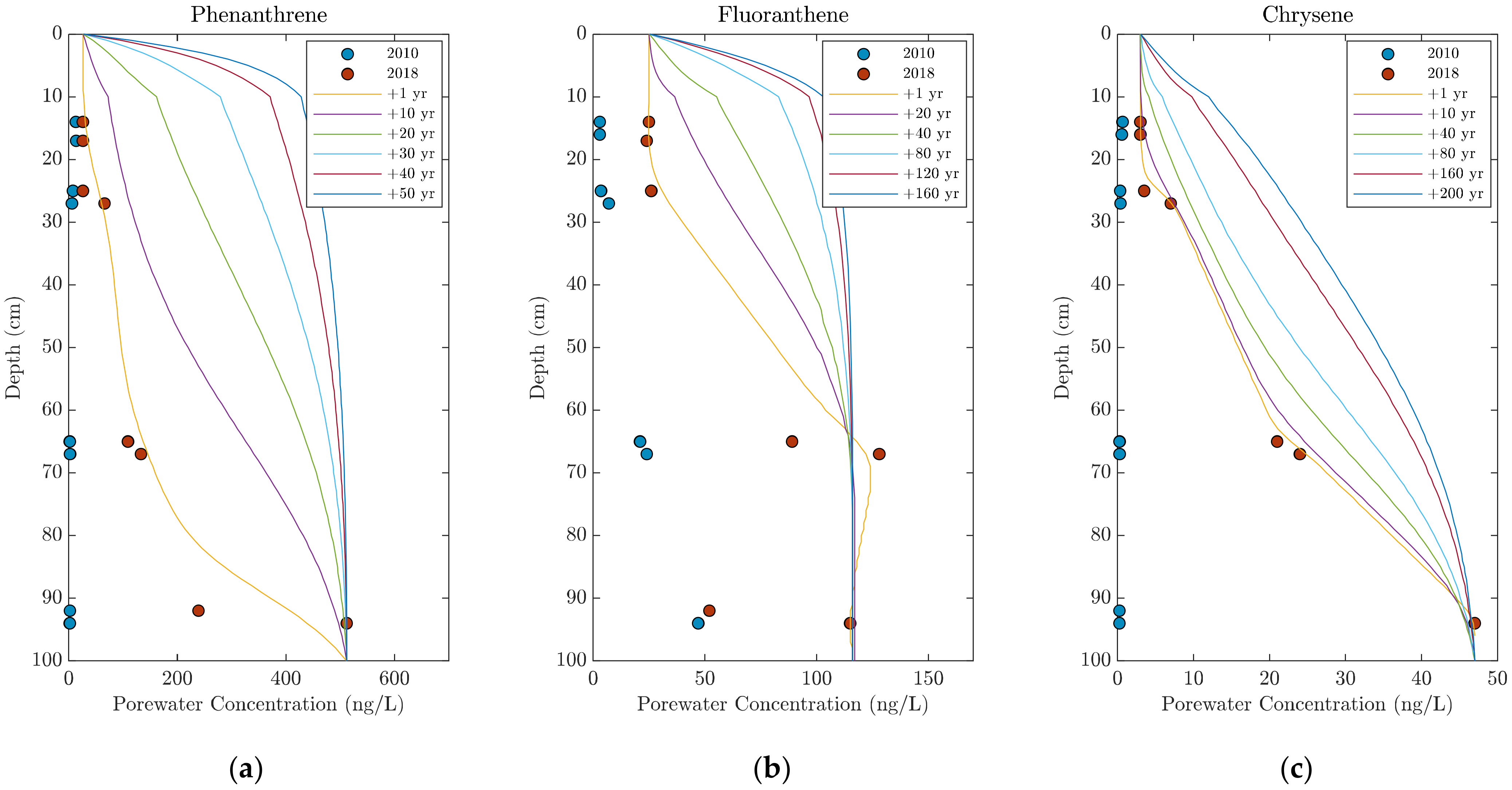
3.3. Modelled PAH Migration in the Cap
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Estimating Groundwater Upwelling Velocity from Performance Reference Compounds
References
- Eek, E.; Cornelissen, G.; Kibsgaard, A.; Breedveld, G.D. Diffusion of PAH and PCB from Contaminated Sediments with and without Mineral Capping; Measurement and Modelling. Chemosphere 2008, 71, 1629–1638. [Google Scholar] [CrossRef] [PubMed]
- Reible, D. Contaminant Processes in Sediments. In Processes, Assessment and Remediation of Contaminated Sediments; Springer Science + Business Media: New York, NY, USA, 2014. [Google Scholar]
- Mayer, P.; Parkerton, T.F.; Adams, R.G.; Cargill, J.G.; Gan, J.; Gouin, T.; Gschwend, P.M.; Hawthorne, S.B.; Helm, P.; Witt, G.A.; et al. Passive Sampling Methods for Contaminated Sediments: Scientific Rationale Supporting Use of Freely Dissolved Concentrations. Integr. Environ. Assess. Manag. 2014, 10, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Lampert, D.J.; Sarchet, W.V.; Reible, D.D. Assessing the Effectiveness of Thin-Layer Sand Caps for Contaminated Sediment Management through Passive Sampling. Environ. Sci. Technol. 2011, 45, 8437–8443. [Google Scholar] [CrossRef] [PubMed]
- Rakowska, M.I.; Kupryianchyk, D.; Harmsen, J.; Grotenhuis, T.; Koelmans, A.A. In Situ Remediation of Contaminated Sediments Using Carbonaceous Materials. Environ. Toxicol. Chem. 2012, 31, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, L.A.; Lao, W.; Maruya, K.A.; Burgess, R.M. Calculating the Diffusive Flux of Persistent Organic Pollutants between Sediments and the Water Column on the Palos Verdes Shelf Superfund Site Using Polymeric Passive Samplers. Environ. Sci. Technol. 2014, 48, 3925–3934. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.M.; Hsieh, C.H.; Luthy, R.G. Modeling Uptake of Hydrophobic Organic Contaminants into Polyethylene Passive Samplers. Environ. Sci. Technol. 2015, 49, 2270–2277. Available online: https://pubs.acs.org/sharingguidelines (accessed on 28 June 2021). [CrossRef] [PubMed]
- Reible, D.; Lu, X. Solid-Phase Microextraction Field Deployment and Analysis Pacific Sound Resources; Report to USACE; University of Texas: Austin, TX, USA, 2012. [Google Scholar]
- Kimura, S. Forced Convection Heat Transfer about a Cylinder Placed in Porous Media with Longitudinal Flows. Int. J. Heat Fluid Flow 1988, 9, 83–86. [Google Scholar] [CrossRef]
- Shen, X.; Lampert, D.; Ogle, S.; Reible, D. A Software Tool for Simulating Contaminant Transport and Remedial Effectiveness in Sediment Environments. Environ. Model. Softw. 2018, 109, 104–113. [Google Scholar] [CrossRef]
- EPA. Method 8310: Polynuclear Aromatic Hydrocarbons; U.S. Environmental Protection Agency: Washington, DC, USA, 1986.
- ATSDR. Toxicological Profile for Polycyclic Aromatic Hydrocarbons; ATSDR: Atlanta, GA, USA, 1995.
- Thomas, C.; Lampert, D.; Reible, D. Remedy performance monitoring at contaminated sediment sites using profiling solid phase microextraction (SPME) polydimethylsiloxane (PDMS) fibers. Environ. Sci. Processes Impacts 2014, 16, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, U.; Kane Driscoll, S.; Burgess, R.M.; Jonker, M.T.; Reible, D.; Gobas, F.; Choi, Y.; Apitz, S.E.; Maruya, K.A.; Beegan, C.; et al. Passive Sampling Methods for Contaminated Sediments: Practical Guidance for Selection, Calibration, and Implementation. Integr. Environ. Assess. Manag. 2014, 10, 210–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, X.; Danny, R. An Analytical Model for the Fate and Transport of Performance Reference Compounds and Target Compounds around Cylindrical Passive Samplers. Chemosphere 2019, 232, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Lampert, D.J.; Thomas, C.; Reible, D.D. Internal and External Transport Significance for Predicting Contaminant Uptake Rates in Passive Samplers. Chemosphere 2015, 119, 910–916. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.; Lu, X.; Reible, D.D. Solid-Phase Microextraction Field Deployment and Analysis Wyckoff/Eagle Harbor; Report to USACE; University of Texas: Austin, TX, USA, 2012. [Google Scholar]
- Bejan, A. Convection Heat Transfer; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Boudreau, B.P. The diffusive tortuosity of fine-grained unlithified sediments. Geochim. Cosmochim. Acta 1996, 60, 3139–3142. [Google Scholar] [CrossRef]
- Millington, R.J.; Quirk, J.P. Permeability of porous solids. Trans. Faraday Soc. 1961, 57, 1200–1207. [Google Scholar] [CrossRef]
- Baker, J.R. Evaluation of Estimation Methods for Organic Carbon Normalized Sorption Coefficients. Water Environ. Res. 1997, 69, 136–145. [Google Scholar] [CrossRef]
- Bejan, A. Convection Heat Transfer; John wiley & sons, 2003. [Google Scholar]
- Crank, J. The Mathematics of Diffusion; Oxford, Clarendon Press, 1975. [Google Scholar]
- Kimura, S. Forced convection heat transfer about a cylinder placed in porous media with longitudinal flows. Int. J. Heat Fluid Flow 1988, 9, 83–86. [Google Scholar]



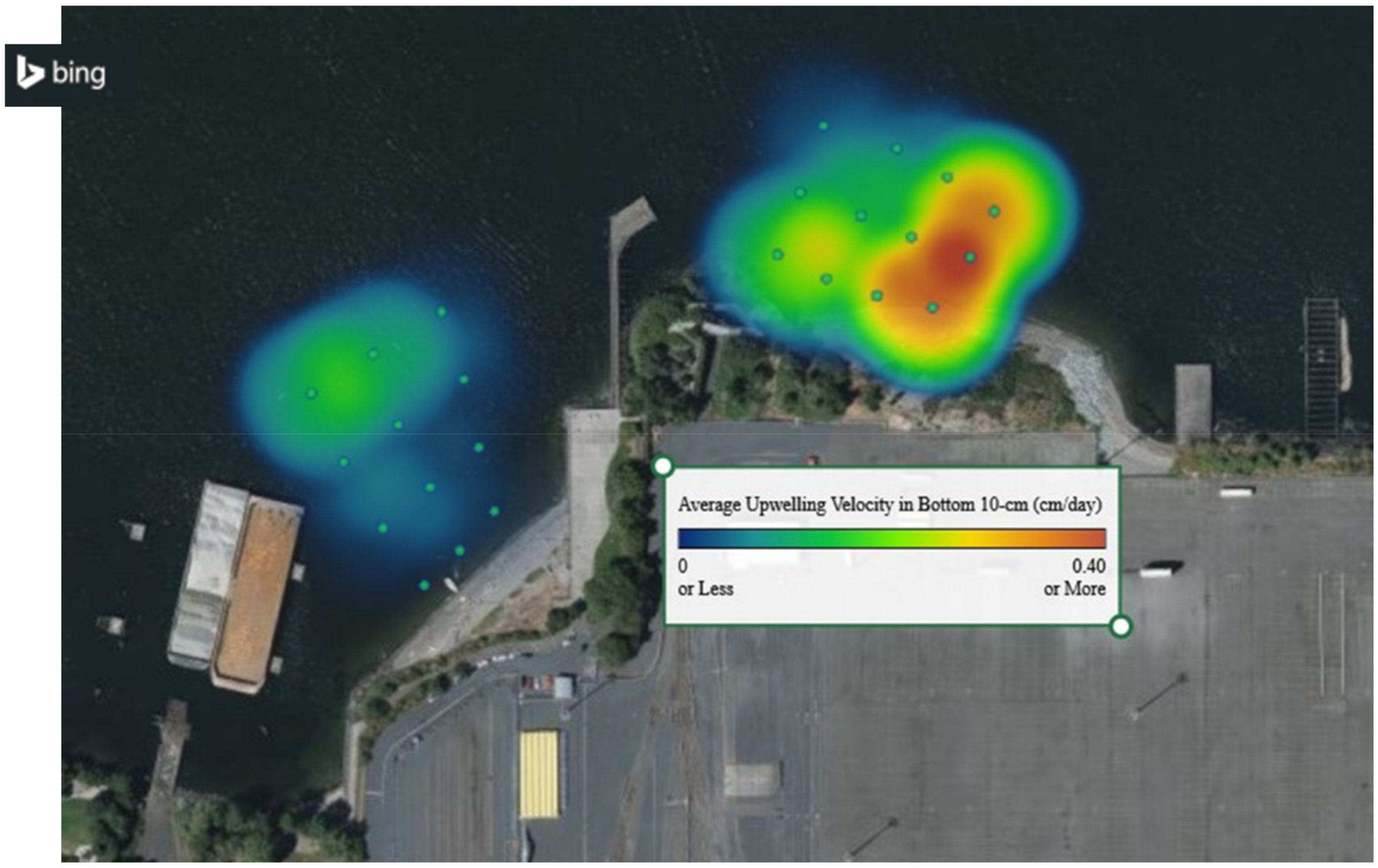
| Sample Location | ∑PAH (2010) ng/L | ∑PAH (2018) ng/L | |
|---|---|---|---|
| Northwest | 1 | 70 | 121 |
| 2 | 170 | 92 | |
| 3 | 34 | 125 | |
| 4 | 97 | 85 | |
| 5 | 490 | 124 | |
| 6 | 71 | 27 | |
| 7 | 104 | 21 | |
| 8 | 170 | - | |
| 9 | 89 | - | |
| 10 | 66 | 66 | |
| 11 | 315 | 111 | |
| 12 | 67 | 12 | |
| Northeast | 13 | 89 | 384 |
| 14 | 66 | 186 | |
| 15 | 75 | 324 | |
| 16 | 58 | 809 | |
| 17 | 74 | 690 | |
| 18 | 68 | 567 | |
| 19 | 170 | 33 | |
| 20 | 83 | 876 | |
| 21 | 99 | 413 | |
| 22 | 47 | 439 | |
| 23 | 56 | 378 | |
| 24 | 86 | 576 |
| Top 10-cm Depth (0–10 cm) | Bottom 10-cm Depth (80–90 cm) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Station ID | ∑PAH16 ng/L | Uz (cm/d) | Porewater Concentration (ng/L) | Uz (cm/d) | Porewater Concentration (ng/L) | ||||
| Phenanthrene | Fluoranthene | Chrysene | Phenanthrene | Fluoranthene | Chrysene | ||||
| Northwest | |||||||||
| 1 | 121 | 1.4 | 2.5 | <1.0 | <0.1 | 0.02 | 11.6 | 9.8 | 0.8 |
| 2 | 92 | 2.4 | 5.7 | 21.6 | 2.3 | 0.08 | 1.3 | 7.8 | 2.5 |
| 3 | 125 | 8.3 | <1.0 | 10.1 | <0.1 | 0.10 | 0.1 | <1.0 | <0.1 |
| 4 | 85 | 1.7 | 15.7 | 20.3 | <0.1 | 0.01 | 12.3 | 8.1 | <0.1 |
| 5 | 124 | 5.7 | 0.8 | 9.9 | 1.1 | 0.02 | 48.6 | 12.4 | 1.2 |
| 6 | 27 | 10.1 | 2.1 | 7.9 | <0.1 | 0.02 | 12.1 | 9.1 | <0.1 |
| 7 | 21 | 6.5 | 1.3 | 14.1 | 2.5 | 0.00 | <1.0 | <1.0 | <0.1 |
| 10 | 66 | 3.4 | 15.7 | 20.5 | <0.1 | 0.02 | <1.0 | 8.1 | <0.1 |
| 11 | 111 | 2.7 | 1.2 | 0.1 | <0.1 | 0.02 | 61.2 | 12.4 | 1.2 |
| 12 | 12 | 10.4 | 1.3 | 14.1 | 1.8 | 0.01 | <1.0 | <1.0 | 0.8 |
| Northeast | |||||||||
| 13 | 384 | 6.7 | 15.5 | 9.3 | <0.1 | 0.02 | 8.3 | 16.5 | 1.1 |
| 14 | 186 | <0.1 | <1.0 | <1.0 | <0.1 | 0.10 | 10.9 | <1.0 | <0.1 |
| 15 | 324 | 4.6 | 8.6 | 6.4 | <0.1 | 0.12 | 1.0 | <1.0 | <0.1 |
| 16 | 809 | 2.8 | 28.3 | 49.6 | 1.7 | 0.01 | 44.9 | 38.2 | 1.0 |
| 17 | 690 | 6.6 | 147.7 | 162.5 | 2.1 | 0.08 | 28.8 | 13.9 | 1.6 |
| 18 | 567 | 1.1 | 18.3 | 32.9 | 0.7 | 0.02 | 461.4 | 132.2 | <0.1 |
| 19 | 33 | 2.2 | 2.2 | 18.7 | <0.1 | 0.06 | 6.6 | 27.3 | <0.1 |
| 20 | 876 | 1.3 | 26.3 | 24.6 | 3.1 | 0.26 | 350.5 | 78.3 | 46.8 |
| 21 | 412 | 2.7 | 39.6 | 39.3 | <0.1 | 0.05 | 24.6 | 12.3 | <0.1 |
| 22 | 439 | 0.7 | 21.9 | 25.5 | 0.9 | 0.02 | 38.3 | 17.2 | 0.8 |
| 23 | 378 | 1.0 | 50.8 | 21.2 | <0.1 | 0.03 | 17.2 | 11.6 | <0.1 |
| 24 | 576 | 14.9 | 26.2 | 54.6 | 1.7 | 0.32 | 84.1 | 159.3 | 14.4 |
| Layer | Depth (cm) | foc | Uz (cm/day) | Boundary Condition | Comments |
|---|---|---|---|---|---|
| Sediment | 10 | 0.01 | 0.21 | Mass transfer | Bioturbation in top 5 cm at 2 cm2/year |
| Sand/Gravel | 90 | 0.003 | 0.21 | Constant Concentration | No depletion in bottom concentration |
| Contaminant | Koc | Initial Concentrations | |||
| Phenanthrene | 3.93 | 2018 porewater concentrations as shown in Figure 7—Local equilibrium with adjacent solids assumed | |||
| Fluoranthene | 4.51 | ||||
| Chrysene | 5.09 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smith, A.V.; Shen, X.; Garza-Rubalcava, U.; Gardiner, W.; Reible, D. In Situ Passive Sampling to Monitor Long Term Cap Effectiveness at a Tidally Influenced Shoreline. Toxics 2022, 10, 106. https://doi.org/10.3390/toxics10030106
Smith AV, Shen X, Garza-Rubalcava U, Gardiner W, Reible D. In Situ Passive Sampling to Monitor Long Term Cap Effectiveness at a Tidally Influenced Shoreline. Toxics. 2022; 10(3):106. https://doi.org/10.3390/toxics10030106
Chicago/Turabian StyleSmith, Alex V., Xiaolong Shen, Uriel Garza-Rubalcava, William Gardiner, and Danny Reible. 2022. "In Situ Passive Sampling to Monitor Long Term Cap Effectiveness at a Tidally Influenced Shoreline" Toxics 10, no. 3: 106. https://doi.org/10.3390/toxics10030106







