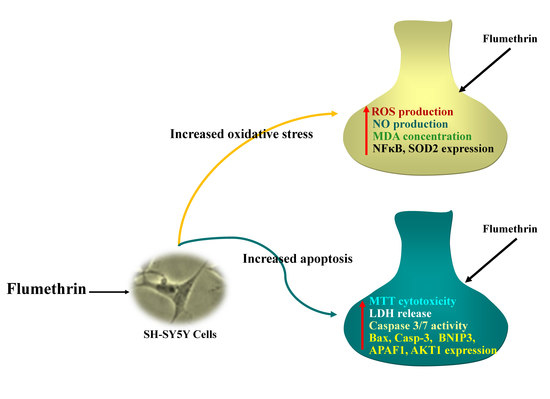
In Vitro Neurotoxicity of Flumethrin Pyrethroid on SH-SY5Y Neuroblastoma Cells: Apoptosis Associated with Oxidative Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Cell Line and Culture Condition
2.3. Cell Viability (Cytotoxicity-MTT Assay)
2.4. Lactate Dehydrogenase (LDH) Assay
2.5. Reactive Oxygen Species (ROS) Generation
2.6. Nitric Oxide (NO) Production
2.7. Lipid Peroxidation Determination
2.8. Caspase 3/7 Activity-Fluorescence Assay
2.9. RNA Extraction/Purification, cDNA Synthesis and Real-Time PCR
2.10. Statistical Analysis
3. Results
3.1. Flumethrin Reduced Cell Viability (Cytotoxicity)
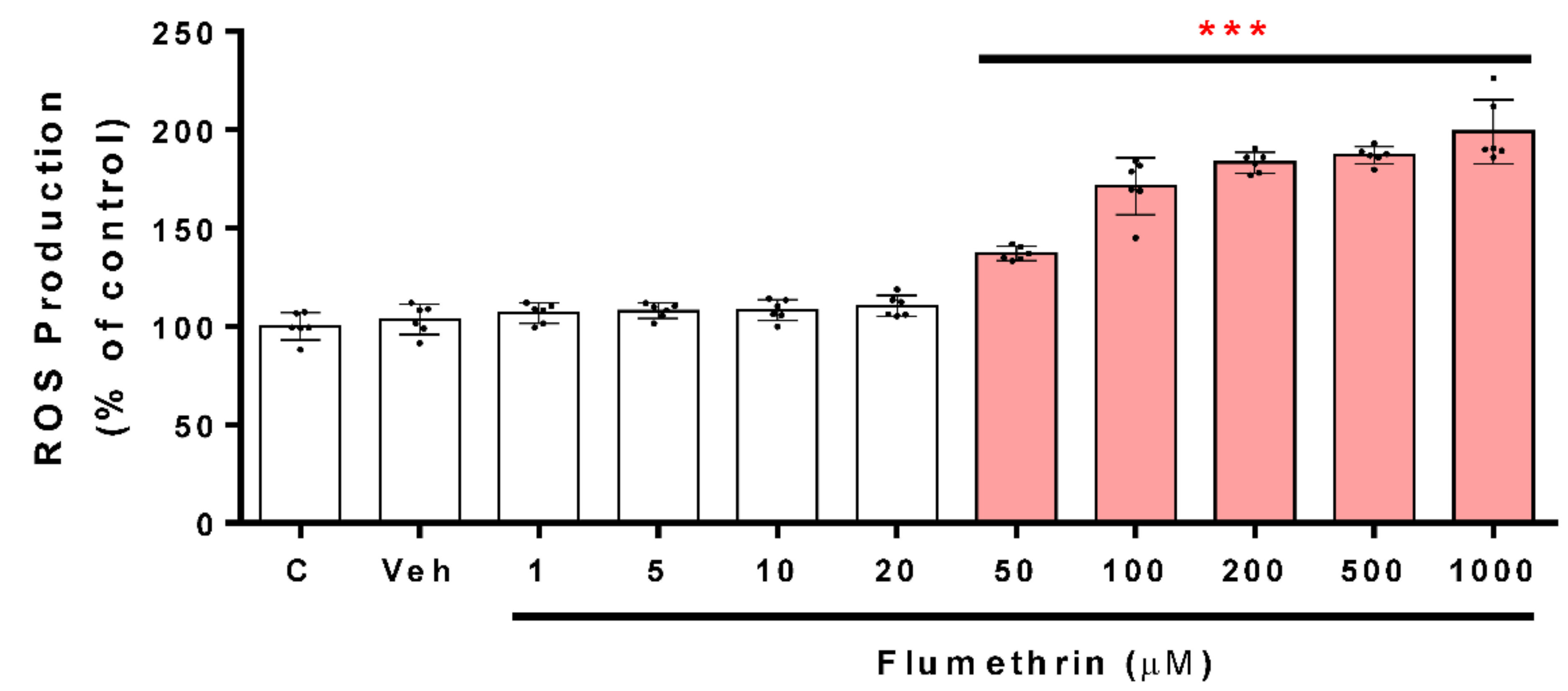
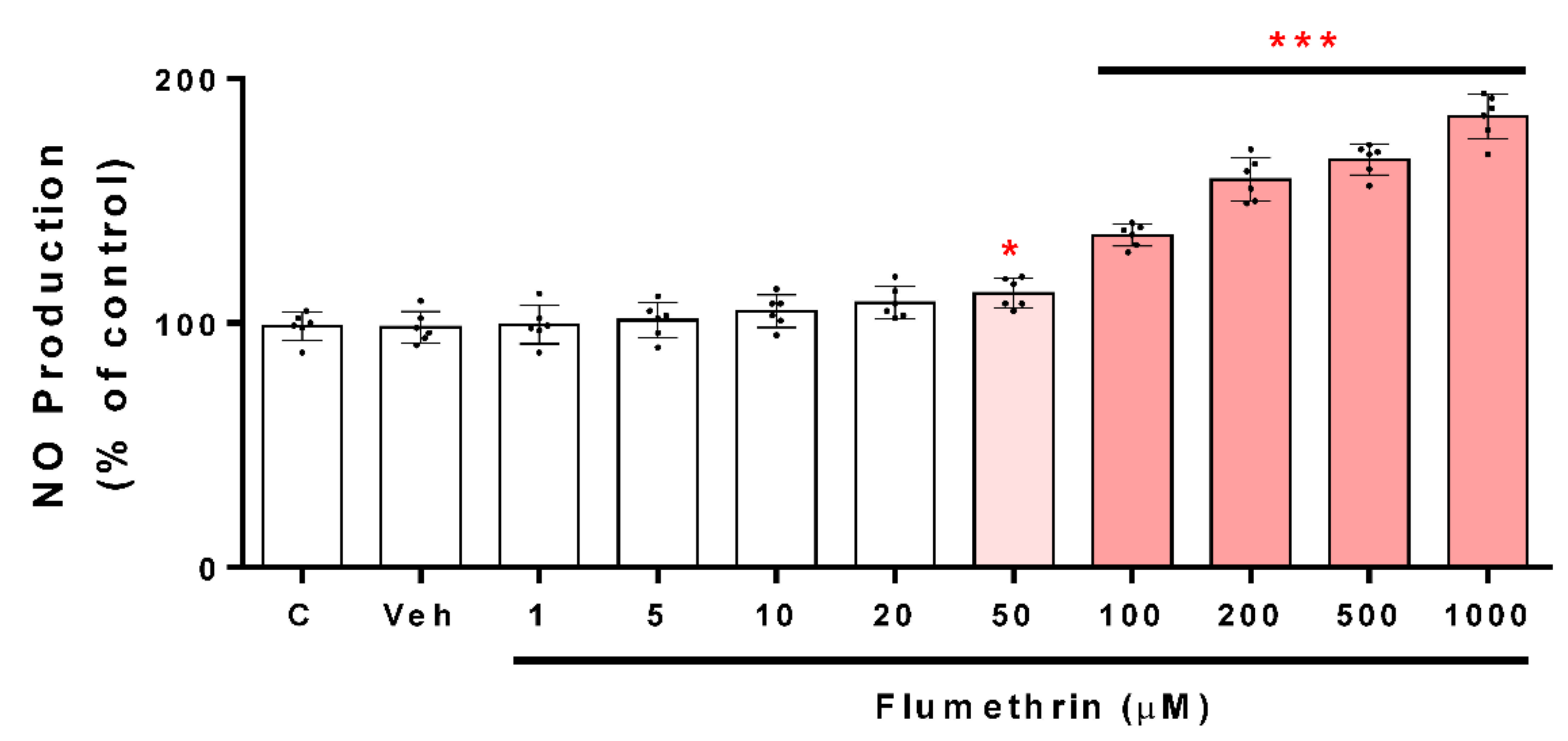
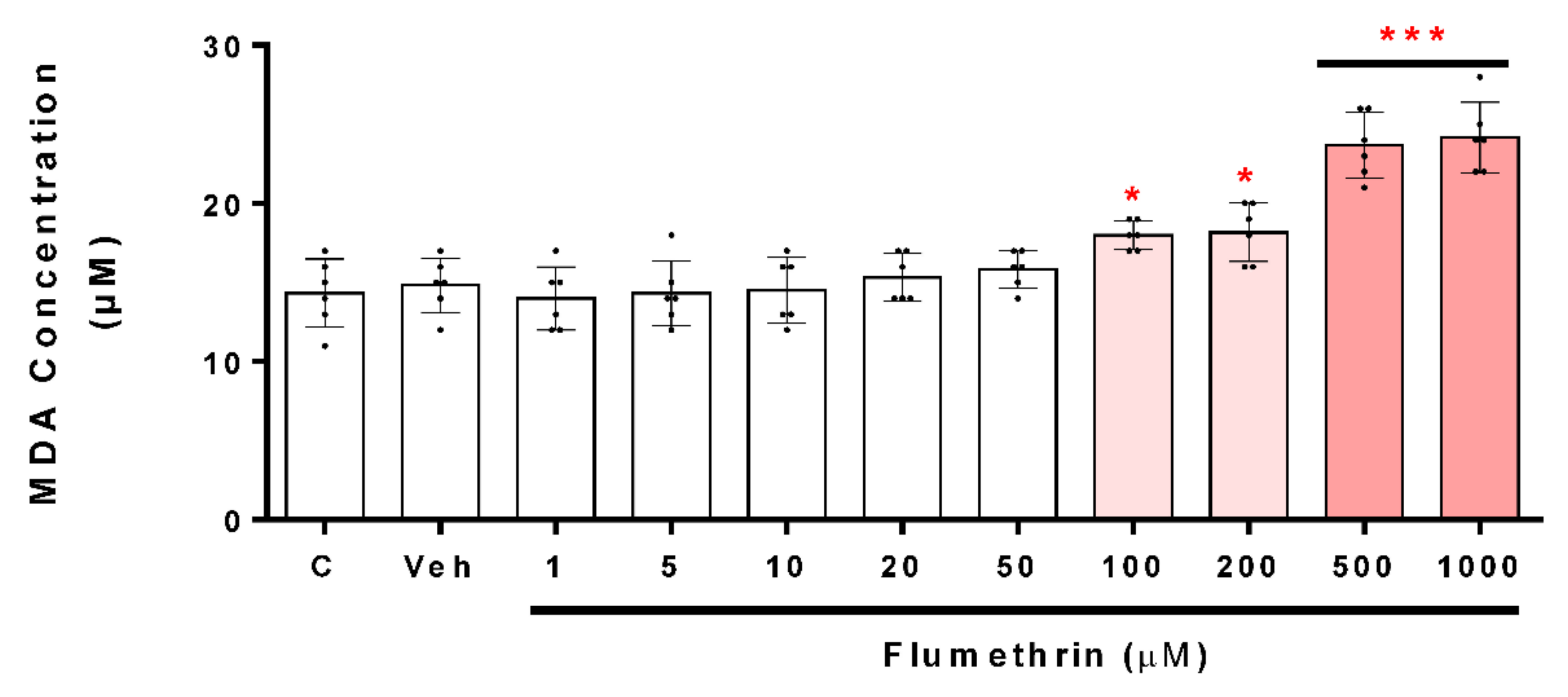
3.2. Flumethrin Induces Oxidative Stress through the Production of ROS, NO, and MDA Levels
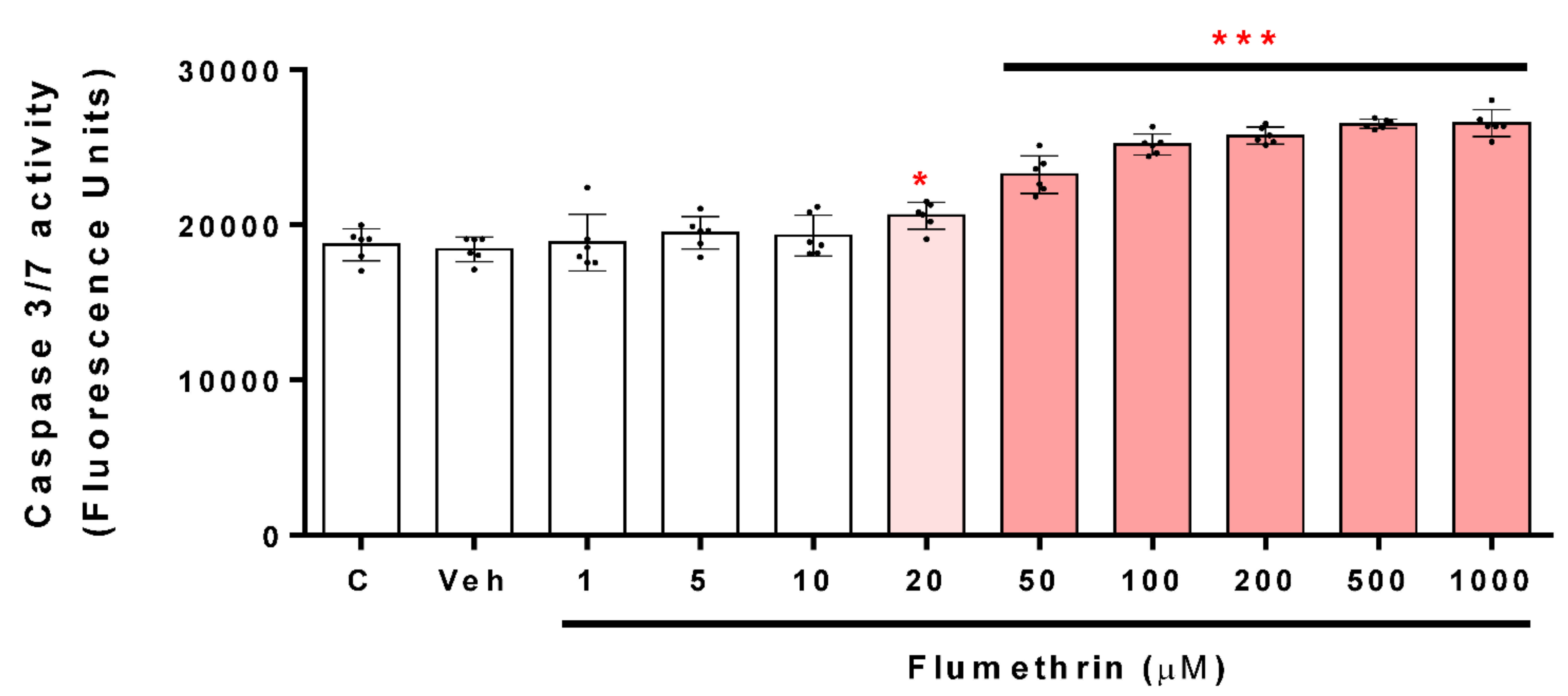
3.3. Flumethrin Induces the Caspase 3/7 Activity, an Apoptosis Mediator
3.4. Flumethrin Effect on Apoptosis and Oxidative Stress and Antioxidative Gene Transcriptions in the SH-SY5Y Cell Line
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, L.N. Residue and Risk Assessment of 7 Kinds of Pyrethroids in Water Environment in the Pearl River Delta. Master’s Thesis, Shanghai Ocean University, Shanghai, China, 2014. [Google Scholar]
- Thatheyus, A.; Selvam, A.D.G. Synthetic Pyrethroids: Toxicity and Biodegradation. Appl. Ecol. Environ. Sci. 2013, 1, 33–36. [Google Scholar] [CrossRef] [Green Version]
- Narahashi, T.; Ginsburg, K.S.; Nagata, K. Ion channels as targets for insecticides. Neurotoxicology 1998, 19, 581–590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soderlund, D.M.; Clark, J.M.; Sheets, L.P.; Mullin, L.S.; Piccirillo, V.J.; Sargent, D.; Stevens, J.T.; Weiner, M.L. Mechanisms of pyrethroid neurotoxicity: Implications for cumulative risk assessment. Toxicology 2002, 171, 3–59. [Google Scholar] [CrossRef]
- Verschoyle, R.D.; Aldridge, W.N. Structure-activity relationships of some pyrethroids in rats. Arch. Toxicol. 1980, 45, 325–329. [Google Scholar] [CrossRef]
- Anadón, A.; Martínez-Larrañaga, M.R.; Martínez, M.A. Use and abuse of pyrethrins and synthetic pyrethroids in veterinary medicine. Vet. J. 2009, 182, 7–20. [Google Scholar] [CrossRef]
- USEPA. Chlorpyrifos Revised Risk Assessment and Agreement with Registrants. 2013. Available online: http://www.ibiblio.org/london/NAFEX/message-archives/old/pdf00000.pdf (accessed on 1 May 2021).
- Cantalamessa, F. Acute toxicity of two pyrethroids, permethrin, and cypermethrin in neonatal and adult rats. Arch. Toxicol. 1993, 67, 510–513. [Google Scholar] [CrossRef]
- Liu, R.; Liu, I.Y.; Bi, X.; Thompson, R.F.; Doctrow, S.R.; Malfroy, B.; Baudry, M. Reversal of age-related learning deficits and brain oxidative stress in mice with superoxide dismutase/catalase mimetics. Proc. Natl. Acad. Sci. USA 2003, 100, 8526–8531. [Google Scholar] [CrossRef] [Green Version]
- Ansari, R.W.; Shukla, R.K.; Yadav, R.S.; Seth, K.; Pant, A.B.; Singh, D.; Agrawal, A.K.; Islam, F.; Khanna, V.K. Involvement of dopaminergic and serotonergic systems in the neurobehavioral toxicity of lambda-cyhalothrin in developing rats. Toxicol. Lett. 2012, 211, 1–9. [Google Scholar] [CrossRef]
- Gargouri, B.; Yousif, N.M.; Attaai, A.; Bouchard, M.; Chtourou, Y.; Fiebich, B.L.; Fetoui, H. Pyrethroid bifenthrin induces oxidative stress, neuroinflammation, and neuronal damage, associated with cognitive and memory impairment in murine hippocampus. Neurochem. Int. 2018, 120, 121–133. [Google Scholar] [CrossRef]
- Rodriguez-Gutierrez, J.-L.; Ares, I.; Martínez, M.; Martínez-Larrañaga, M.-R.; Anadón, A.; Martínez, M.-A. Bioavailability and nervous tissue distribution of pyrethroid insecticide cyfluthrin in rats. Food Chem. Toxicol. 2018, 118, 220–226. [Google Scholar] [CrossRef]
- The European Agency for the Evaluation of Medicinal Products Veterinary Medicines and Information Technology Unit. EMEA/MRL, 469/98, Committee for Veterinary Medicinal Products: Flumethrin; EMEA: London, UK, 1998. [Google Scholar]
- Kaneko, H.; Miyamoto, J. Pyrethroid Chemistry and Metabolism. In Handbook of Pesticide Toxicology: Agents; Krieger, R., Ed.; Academic Press: New York, NY, USA, 2001; Volume 1, pp. 1263–1288. [Google Scholar]
- Başçi, Z.; Eraslan, G. Toxicokinetic of flumethrin in rabbits. Drug Chem. Toxicol. 2014, 38, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Oruc, H.H.; Hranitz, J.M.; Sorucu, A.; Duell, M.; Cakmak, I.; Aydin, L.; Orman, A. Determination of acute oral toxicity of flumethrin in honey bees. J. Econ. Entomol. 2012, 105, 1890–1894. [Google Scholar] [CrossRef] [PubMed]
- Påhlman, S.; Mamaeva, S.; Meyerson, G.; Mattsson, M.; Bjelfman, C.; Ortoft, E.; Hammerling, U. Human neuroblastoma cells in culture: A model for neuronal cell differentiation and function. Acta Physiol. Scand. Suppl. 1990, 592, 25–37. [Google Scholar] [PubMed]
- Denizot, F.; Lang, R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J. Immunol. Methods 1986, 89, 271–277. [Google Scholar] [CrossRef]
- Wang, H.; Joseph, J.A. Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radic. Biol. Med. 1999, 27, 612–616. [Google Scholar] [CrossRef]
- Moncada, S.; Palmer, R.M.; Higgs, E.A. Nitric oxide: Physiology, pathophysiology, and pharmacology. Pharmacol. Rev. 1991, 43, 109–142. [Google Scholar]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Ramakers, C.; Ruijter, J.M.; Deprez, R.H.L.; Moorman, A.F.M. Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neurosci. Lett. 2003, 339, 62–66. [Google Scholar] [CrossRef]
- Gupta, S.; Gajbhiye, V.T.; Kalpana, A.; Agnihotri, N.P. Leaching Behavior of Imidacloprid Formulations in Soil. Bull. Environ. Contam. Toxicol. 2002, 68, 502–508. [Google Scholar] [CrossRef]
- Dantas-Torres, F.; Latrofa, M.S.; Annoscia, G.; Giannelli, A.; Parisi, A.; Otranto, D. Morphological and genetic diversity of Rhipicephalus sanguineus sensu lato from the New and Old Worlds. Parasites Vectors 2013, 6, 213–217. [Google Scholar] [CrossRef] [Green Version]
- Romero, A.; Ramos, E.; Ares, I.; Castellano, V.; Martínez, M.; Martínez-Larrañaga, M.-R.; Anadón, A.; Martínez, M.-A. Oxidative stress and gene expression profiling of cell death pathways in alpha-cypermethrin-treated SH-SY5Y cells. Arch. Toxicol. 2017, 91, 2151–2164. [Google Scholar] [CrossRef] [PubMed]
- Martínez, M.-A.; Rodríguez, J.-L.; Lopez-Torres, B.; Martínez, M.; Martínez-Larrañaga, M.-R.; Anadón, A.; Ares, I. Oxidative stress and related gene expression effects of cyfluthrin in human neuroblastoma SH-SY5Y cells: Protective effect of melatonin. Environ. Res. 2019, 177, 108579. [Google Scholar] [CrossRef] [PubMed]
- Raszewski, G.; Lemieszek, M.K.; Łukawski, K.; Juszczak, M.; Rzeski, W. Chlorpyrifos and Cypermethrin Induce Apoptosis in Human Neuroblastoma Cell Line SH-SY5Y. Basic Clin. Pharmacol. Toxicol. 2014, 116, 158–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hatcher, J.M.; Pennell, K.D.; Miller, G.W. Parkinson’s disease and pesticides: A toxicological perspective. Trends Pharmacol. Sci. 2008, 29, 322–329. [Google Scholar] [CrossRef] [Green Version]
- Zhao, M.; Zhang, Y.; Wang, C.; Fu, Z.; Liu, W.; Gan, J. Induction of Macrophage Apoptosis by an Organochlorine Insecticide Acetofenate. Chem. Res. Toxicol. 2009, 22, 504–510. [Google Scholar] [CrossRef]
- Mansour, S.A.; Mossa, A.-T.H. Oxidative damage, biochemical and histopathological alterations in rats exposed to chlorpyrifos and the antioxidant role of zinc. Pestic. Biochem. Physiol. 2009, 96, 14–23. [Google Scholar] [CrossRef]
- Giray, B.; Gürbay, A.; Hincal, F. Cypermethrin-induced oxidative stress in rat brain and liver is prevented by Vitamin E or allopurinol. Toxicol. Lett. 2001, 118, 139–146. [Google Scholar] [CrossRef]
- Patel, M.; Patil, P. Synthetic Pyrethroids: Toxicity and Metabolism. IOSR J. Agric. Vet. Sci. 2016, 9, 55–60. [Google Scholar] [CrossRef]
- Martínez, M.-A.; Lopez-Torres, B.; Rodriguez-Gutierrez, J.-L.; Martínez, M.; Maximiliano, J.-E.; Martínez-Larrañaga, M.-R.; Anadón, A.; Ares, I. Toxicologic evidence of developmental neurotoxicity of Type II pyrethroids cyfluthrin and alpha-cypermethrin in SH-SY5Y cells. Food Chem. Toxicol. 2020, 137, 111173. [Google Scholar] [CrossRef]
- Dreher, D.; Junod, A. Role of oxygen free radicals in cancer development. Eur. J. Cancer 1996, 32, 30–38. [Google Scholar] [CrossRef]
- Westhuyzen, J. The oxidation hypothesis of atherosclerosis: An update. Ann. Clin. Lab. Sci. 1997, 27, 1–10. [Google Scholar] [PubMed]
- Knight, J. Reactive oxygen species and the neurodegenerative disorders. Ann. Clin. Lab. Sci. 1997, 27, 11–25. [Google Scholar] [PubMed]
- Siegel, D.; Gustafson, D.L.; Dehn, D.L.; Han, J.Y.; Boonchoong, P.; Berliner, L.J.; Ross, D. NAD(P)H:Quinone Oxidoreductase 1: Role as a Superoxide Scavenger. Mol. Pharmacol. 2004, 65, 1238–1247. [Google Scholar] [CrossRef] [Green Version]
- Limón-Pacheco, J.; Gonsebatt, M.E. The role of antioxidants and antioxidant-related enzymes in protective responses to environmentally induced oxidative stress. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2009, 674, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Dröge, W. Free Radicals in the Physiological Control of Cell Function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef]
- Kanbur, M.; Siliğ, Y.; Eraslan, G.; Karabacak, M.; Soyer-Sarıca, Z.; Şahin, S. The toxic effect of cypermethrin, amitraz and combinations of cypermethrin-amitraz in rats. Environ. Sci. Pollut. Res. 2016, 23, 5232–5242. [Google Scholar] [CrossRef]
- Gaweł, S.; Wardas, M.; Niedworok, E.; Wardas, P. Dialdehyd malonowy (MDA) jako wskaźnik procesów peroksydacji lipidów w organizmie [Malondialdehyde (MDA) as a lipid peroxidation marker]. Wiad Lek. 2004, 57, 453–455. [Google Scholar]
- Orrenius, S.; Gogvadze, V.; Zhivotovsky, B. Mitochondrial Oxidative Stress: Implications for Cell Death. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 143–183. [Google Scholar] [CrossRef]
- Lazebnik, Y.A.; Kaufmann, S.H.; Desnoyers, S.; Poirier, G.G.; Earnshaw, W. Cleavage of poly(ADP-ribose) polymerase by a proteinase with properties like ICE. Nature 1994, 371, 346–347. [Google Scholar] [CrossRef]
- Martínez, M.-A.; Rodriguez-Gutierrez, J.-L.; Lopez-Torres, B.; Martínez, M.; Martínez-Larrañaga, M.-R.; Maximiliano, J.-E.; Anadón, A.; Ares, I. Use of human neuroblastoma SH-SY5Y cells to evaluate glyphosate-induced effects on oxidative stress, neuronal development and cell death signaling pathways. Environ. Int. 2020, 135, 105414. [Google Scholar] [CrossRef]
- Murphy, K.M.; Ranganathan, V.; Farnsworth, M.L.; Kavallaris, M.; Lock, R. Bcl-2 inhibits Bax translocation from cytosol to mitochondria during drug-induced apoptosis of human tumor cells. Cell Death Differ. 2000, 7, 102–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Youle, R.J.; Strasser, A. The BCL-2 protein family: Opposing activities that mediate cell death. Nat. Rev. Mol. Cell Biol. 2008, 9, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Nijhawan, D.; Budihardjo, I.; Srinivasula, S.M.; Ahmad, M.; Alnemri, E.S.; Wang, X. Cytochrome c and dATP-Dependent Formation of Apaf-1/Caspase-9 Complex Initiates an Apoptotic Protease Cascade. Cell 1997, 91, 479–489. [Google Scholar] [CrossRef] [Green Version]
- Zimmermann, K.C.; Bonzon, C.; Green, D. The machinery of programmed cell death. Pharmacol. Ther. 2001, 92, 57–70. [Google Scholar] [CrossRef]
- Jänicke, R.U.; Sprengart, M.L.; Wati, M.R.; Porter, A.G. Caspase-3 Is Required for DNA Fragmentation and Morphological Changes Associated with Apoptosis. J. Biol. Chem. 1998, 273, 9357–9360. [Google Scholar] [CrossRef] [Green Version]
- Prabhakaran, K.; Li, L.; Zhang, L.; Borowitz, J.L.; Isom, G.E. Upregulation of BNIP3 and translocation to mitochondria mediates cyanide-induced apoptosis in cortical cells. Neuroscience 2007, 150, 159–167. [Google Scholar] [CrossRef] [Green Version]
- Yook, Y.-H.; Kang, K.-H.; Maeng, O.; Kim, T.-R.; Lee, J.-O.; Kang, K.-I.; Kim, Y.S.; Paik, S.-G.; Lee, H. Nitric oxide induces BNIP3 expression that causes cell death in macrophages. Biochem. Biophys. Res. Commun. 2004, 321, 298–305. [Google Scholar] [CrossRef]
- Nicholson, K.M.; Anderson, N.G. The protein kinase B/Akt signalling pathway in human malignancy. Cell. Signal. 2002, 14, 381–395. [Google Scholar] [CrossRef]
- Lindenboim, L.; Yuan, J.; Stein, R. Bcl-x(S) and Bax induce different apoptotic pathways in PC12 cells. Oncogene 2000, 19, 1783–1793. [Google Scholar] [CrossRef] [Green Version]
- Lu, Q.; Sun, Y.; Ares, I.; Anadón, A.; Martínez, M.; Martínez-Larrañaga, M.-R.; Yuan, Z.; Wang, X.; Martínez, M.-A. Deltamethrin toxicity: A review of oxidative stress and metabolism. Environ. Res. 2019, 170, 260–281. [Google Scholar] [CrossRef]
- Wang, X.; Martínez, M.-A.; Dai, M.; Chen, D.; Ares, I.; Romero, A.; Castellano, V.; Martínez, M.; Rodriguez-Gutierrez, J.-L.; Martínez-Larrañaga, M.-R.; et al. Permethrin-induced oxidative stress and toxicity and metabolism. A review. Environ. Res. 2016, 149, 86–104. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Wang, C.; Zhang, C.; Wen, Y.; Liu, W. Enantioselective Cytotoxicity Profile of o,p’-DDT in PC 12 Cells. PLoS ONE 2012, 7, e43823. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Genes | Primer Forward Sequence | Primer Reverse Sequence |
|---|---|---|
| Housekeeping gene | ||
| GAPDH | GAGAAGGCTGGGGCTCATTT | AGTGATGGCATGGACTGTGG |
| Apoptosis related genes | ||
| Bax | CCCCCGAGAGGTCTTTTTCC | CCTTGAGCACCAGTTTGCTG |
| Casp-3 | GTGGAGGCCGACTTCTTGTA | TTTCAGCATGGCACAAAGCG |
| Bcl-2 | TCTCATGCCAAGGGGGAAAC | TCCCGGTTATCGTACCCTGT |
| BNIP3 | CCTCAGCATGAGGAACACGA | GCCACCCCAGGATCTAACAG |
| AKT1 | GAAGGACGGGAGCAGGC | TGTACTCCCCTCGTTTGTGC |
| APAF1 | TCTTCCAGTGGTAAAGATTCAGTT | CGGAGACGGTCTTTAGCCTC |
| Oxidative stress and antioxidative related genes | ||
| NFκB1 | TTTTCGACTACGCGGTGACA | GTTACCCAAGCGGTCCAGAA |
| Nrf2 | CTGGTCATCGGAAAACCCCA | TCTGCAATTCTGAGCAGCCA |
| SOD2 | CCACTGCTGGGGATTGATGT | CGTGGTTTACTTTTTGCAAGCC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barrios-Arpi, L.; Arias, Y.; Lopez-Torres, B.; Ramos-Gonzalez, M.; Ticli, G.; Prosperi, E.; Rodríguez, J.-L. In Vitro Neurotoxicity of Flumethrin Pyrethroid on SH-SY5Y Neuroblastoma Cells: Apoptosis Associated with Oxidative Stress. Toxics 2022, 10, 131. https://doi.org/10.3390/toxics10030131
Barrios-Arpi L, Arias Y, Lopez-Torres B, Ramos-Gonzalez M, Ticli G, Prosperi E, Rodríguez J-L. In Vitro Neurotoxicity of Flumethrin Pyrethroid on SH-SY5Y Neuroblastoma Cells: Apoptosis Associated with Oxidative Stress. Toxics. 2022; 10(3):131. https://doi.org/10.3390/toxics10030131
Chicago/Turabian StyleBarrios-Arpi, Luis, Yurie Arias, Bernardo Lopez-Torres, Mariella Ramos-Gonzalez, Giulio Ticli, Ennio Prosperi, and José-Luis Rodríguez. 2022. "In Vitro Neurotoxicity of Flumethrin Pyrethroid on SH-SY5Y Neuroblastoma Cells: Apoptosis Associated with Oxidative Stress" Toxics 10, no. 3: 131. https://doi.org/10.3390/toxics10030131
APA StyleBarrios-Arpi, L., Arias, Y., Lopez-Torres, B., Ramos-Gonzalez, M., Ticli, G., Prosperi, E., & Rodríguez, J.-L. (2022). In Vitro Neurotoxicity of Flumethrin Pyrethroid on SH-SY5Y Neuroblastoma Cells: Apoptosis Associated with Oxidative Stress. Toxics, 10(3), 131. https://doi.org/10.3390/toxics10030131