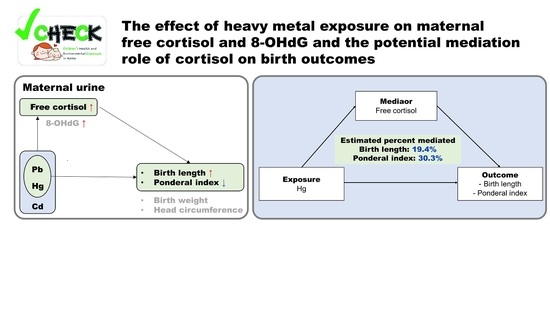
Free Cortisol Mediates Associations of Maternal Urinary Heavy Metals with Neonatal Anthropometric Measures: A Cross-Sectional Study
Abstract
:1. Introduction
2. Subjects and Methods
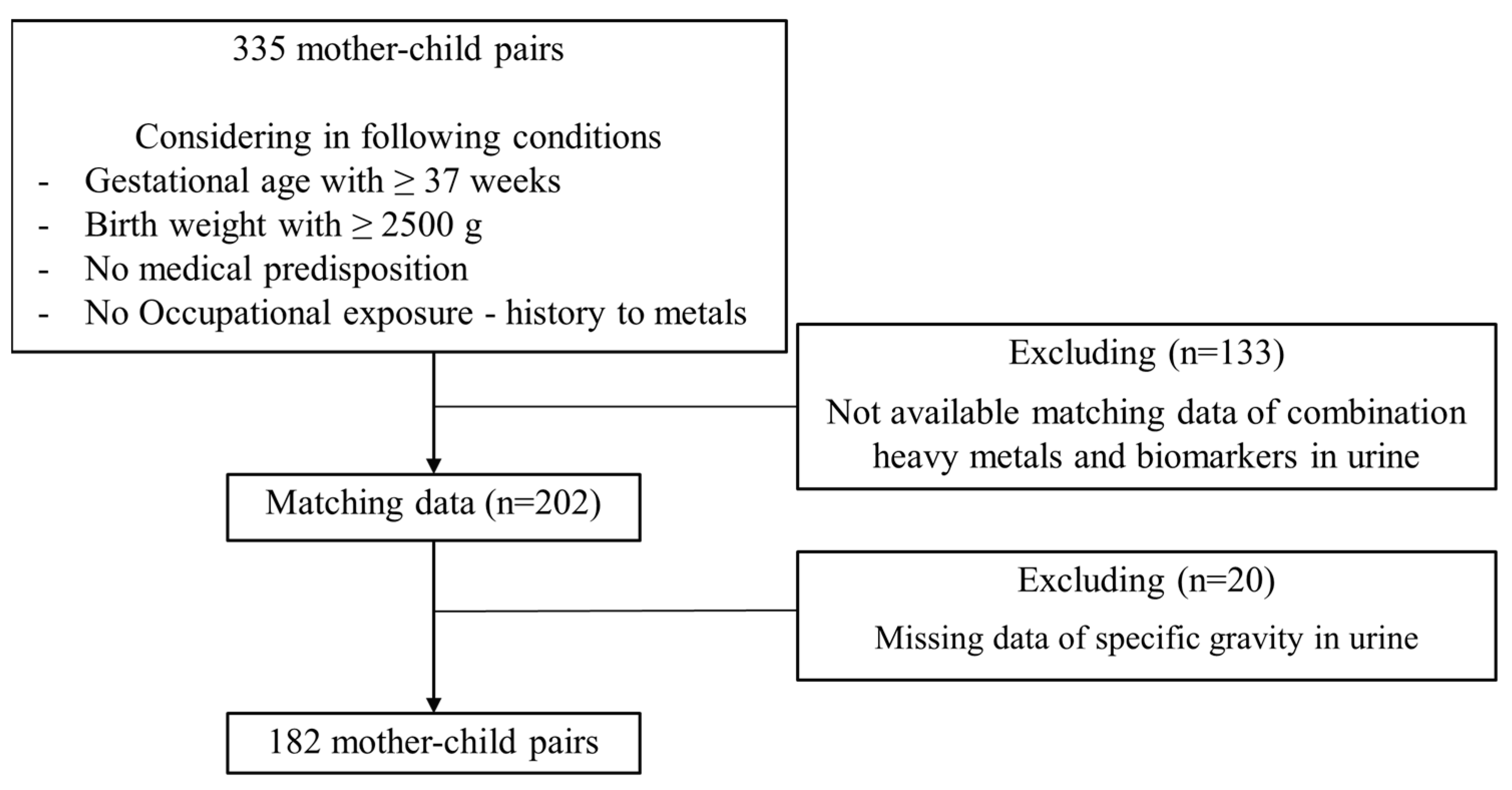
2.1. Study Population
2.2. Questionnaries and Birth Data Collection
2.3. Sampling and Urinary Measurement of Heavy Metals, Free Cortisol, and 8-OHdG
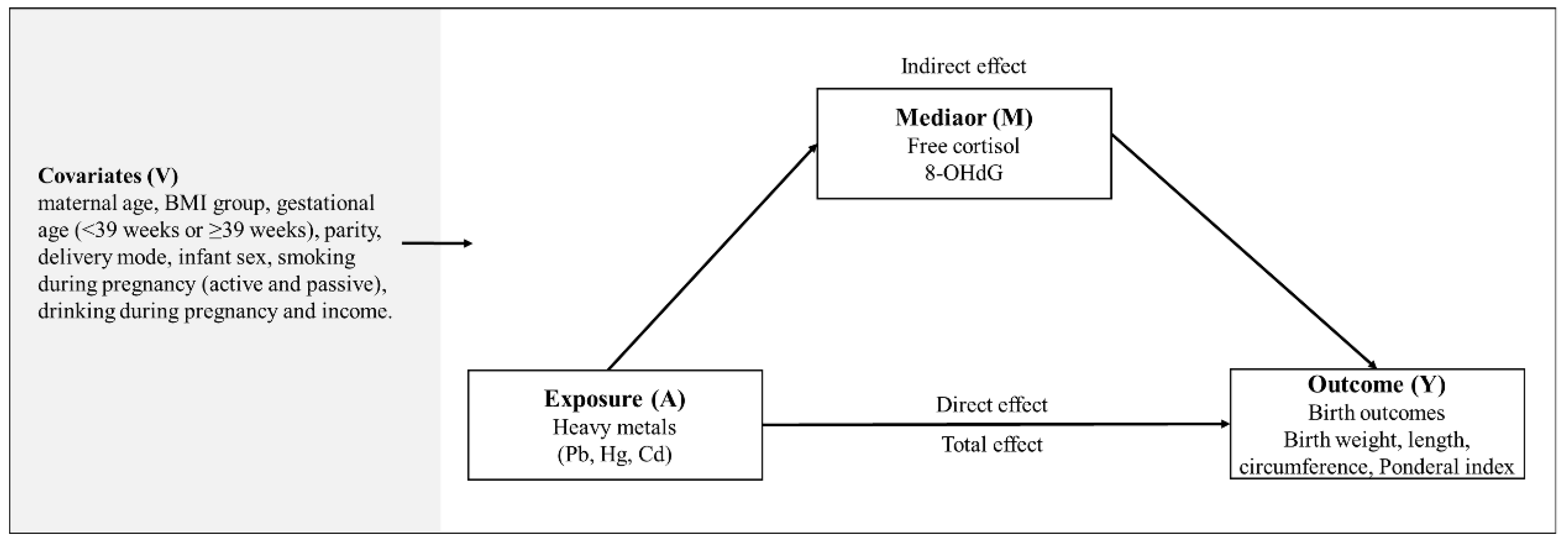
2.4. Statistical Analyses
3. Results
3.1. Urinary Concentrations of Heavy Metals, Free-Cortisol, and 8-OHdG
3.2. Associations between Urinary Heavy Metals and Free Cortisol or 8-OHdG
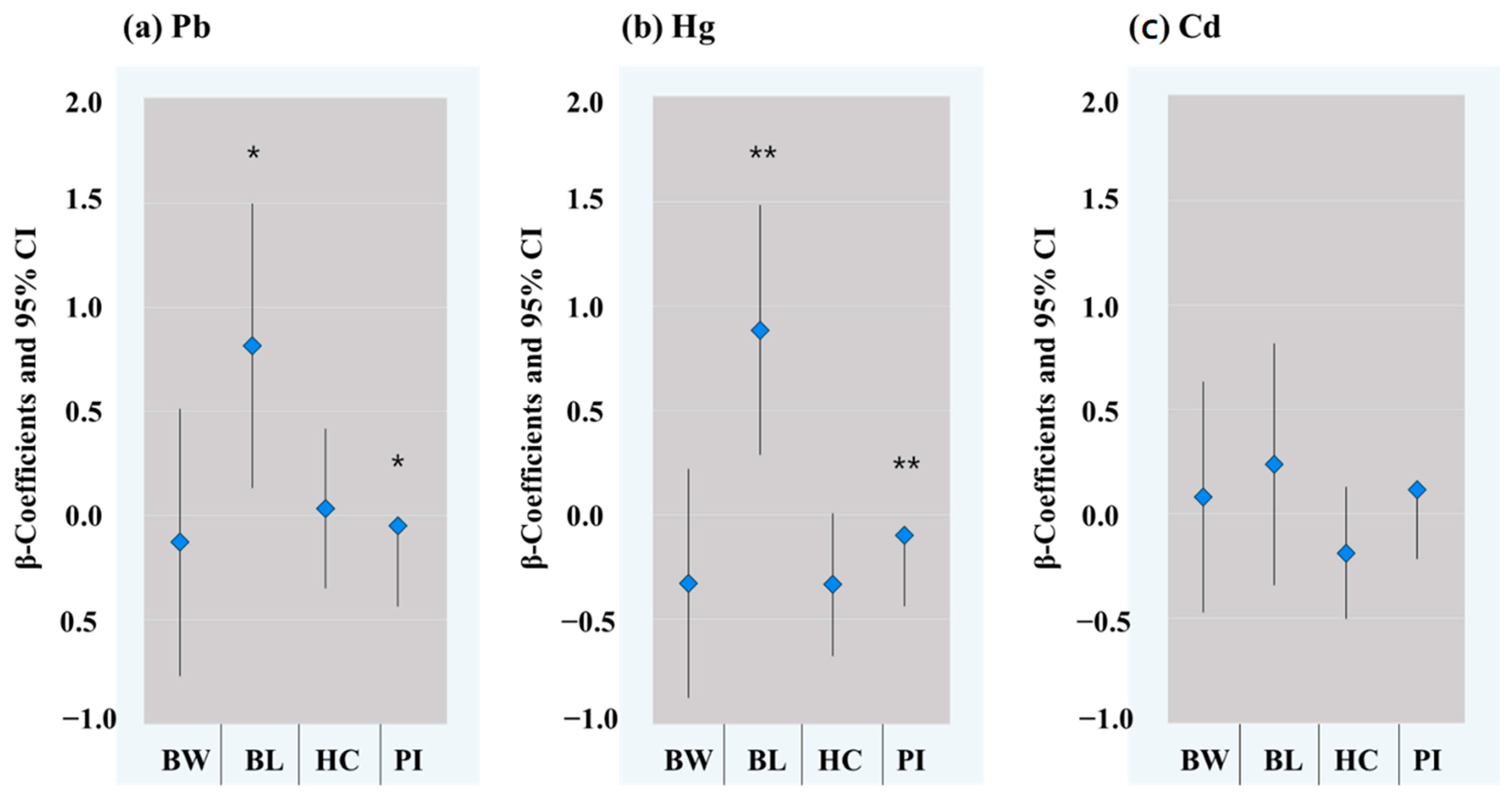
3.3. Associations between Maternal Urinary Metals and Birth Outcomes
3.4. Associations between Levels of Maternal Urinary Biomarkers and Birth Outcomes
3.5. Mediation Effect of Biomarkers on the Association between Heavy Metals and Birth Outcomes
4. Discussion
4.1. Comparison of Heavy Metals Concentrations in Maternal Urine
4.2. Association between Heavy Metals and Biomarkers in Maternal Urine Samples
4.3. Maternal Pb Exposure and Birth Outcomes
4.4. Maternal Hg Exposure and Birth Outcomes
4.5. Maternal Cd Exposure and Birth Outcomes
4.6. Free Cortisol and 8-OHdG in Maternal Urine with Anthropometric Measures of Newborns
4.7. Strength and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Clarkson, T.W.; Nordberg, G.F.; Sager, P.R. Reproductive and Developmental Toxicity of Metals. Scand. J. Work. Environ. Health 1985, 11, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Myers, G.J.; Davidson, P.W.; Cox, C.; Shamlaye, C.F.; Palumbo, D.; Cernichiari, E.; Sloane-Reeves, J.; Wilding, G.E.; Kost, J.; Huang, L.S.; et al. Prenatal Methylmercury Exposure from Ocean Fish Consumption in the Seychelles Child Development Study. Lancet 2003, 361, 1686–1692. [Google Scholar] [CrossRef]
- Bauer, J.A.; Fruh, V.; Howe, C.G.; White, R.F.; Claus Henn, B. Associations of Metals and Neurodevelopment: A Review of Recent Evidence on Susceptibility Factors. Curr. Epidemiol. Rep. 2020, 7, 237–262. [Google Scholar] [CrossRef] [PubMed]
- Al-Saleh, I.; Al-Rouqi, R.; Obsum, C.A.; Shinwari, N.; Mashhour, A.; Billedo, G.; Al-Sarraj, Y.; Rabbah, A. Mercury (Hg) and Oxidative Stress Status in Healthy Mothers and Its Effect on Birth Anthropometric Measures. Int. J. Hyg. Environ. Health 2014, 217, 567–585. [Google Scholar] [CrossRef]
- Kippler, M.; Tofail, F.; Gardner, R.; Rahman, A.; Hamadani, J.; Bottai, M.; Vahter, M. Maternal Cadmium Exposure during Pregnancy and Size at Birth: A Prospective Cohort Study. Environ. Health Perspect. 2012, 120, 284–289. [Google Scholar] [CrossRef] [Green Version]
- Sun, H.; Chen, W.; Wang, D.; Jin, Y.; Chen, X.; Xu, Y. The Effects of Prenatal Exposure to Low-Level Cadmium, Lead and Selenium on Birth Outcomes. Chemosphere 2014, 108, 33–39. [Google Scholar] [CrossRef]
- Halliwell, B. Reactive Oxygen Species in Living Systems: Source, Biochemistry, and Role in Human Disease. Am. J. Med. 1991, 91, S14–S22. [Google Scholar] [CrossRef]
- Barregard, L.; Møller, P.; Henriksen, T.; Mistry, V.; Koppen, G.; Rossner, P.; Sram, R.J.; Weimann, A.; Poulsen, H.E.; Nataf, R.; et al. Human and Methodological Sources of Variability in the Measurement of Urinary 8-Oxo-7,8-Dihydro-2′-Deoxyguanosine. Antioxid. Redox Signal. 2013, 18, 2377–2391. [Google Scholar] [CrossRef]
- Ercal, N.; Gurer-Orhan, H.; Aykin-Burns, N. Toxic metals and oxidative stress part I: Mechanisms involved in metal-induced oxidative damage. Curr. Top. Med. Chem. 2001, 1, 529–539. [Google Scholar] [CrossRef]
- Meister, A. Glutathione Metabolism and Its Selective Modification. J. Biol. Chem. 1988, 263, 17205–17208. [Google Scholar] [CrossRef]
- Pizzino, G.; Bitto, A.; Interdonato, M.; Galfo, F.; Irrera, N.; Mecchio, A.; Pallio, G.; Ramistella, V.; De Luca, F.; Minutoli, L.; et al. Oxidative Stress and DNA Repair and Detoxification Gene Expression in Adolescents Exposed to Heavy Metals Living in the Milazzo-Valle Del Mela Area (Sicily, Italy). Redox Biol. 2014, 2, 686–693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ni, W.; Huang, Y.; Wang, X.; Zhang, J.; Wu, K. Associations of Neonatal Lead, Cadmium, Chromium and Nickel Co-Exposure with DNA Oxidative Damage in an Electronic Waste Recycling Town. Sci. Total Environ. 2014, 472, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Valavanidis, A.; Vlachogianni, T.; Fiotakis, C. 8-Hydroxy-2′-Deoxyguanosine (8-OHdG): A Critical Biomarker of Oxidative Stress and Carcinogenesis. J. Environ. Sci. Health Part C 2009, 27, 120–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scholl, T.O.; Stein, T.P. Oxidant Damage to DNA and Pregnancy Outcome. J. Matern.-Fetal Neonatal Med. 2001, 10, 182–185. [Google Scholar] [CrossRef]
- Kim, Y.J.; Hong, Y.C.; Lee, K.H.; Park, H.J.; Park, E.A.; Moon, H.S.; Ha, E.H. Oxidative Stress in Pregnant Women and Birth Weight Reduction. Reprod. Toxicol. 2005, 19, 487–492. [Google Scholar] [CrossRef]
- Min, J.; Park, B.; Kim, Y.J.; Lee, H.; Ha, E.; Park, H. Effect of Oxidative Stress on Birth Sizes: Consideration of Window from Mid Pregnancy to Delivery. Placenta 2009, 30, 418–423. [Google Scholar] [CrossRef]
- Ferguson, K.K.; McElrath, T.F.; Chen, Y.H.; Loch-Caruso, R.; Mukherjee, B.; Meeker, J.D. Repeated Measures of Urinary Oxidative Stress Biomarkers during Pregnancy and Preterm Birth. Am. J. Obstet. Gynecol. 2015, 212, 208.e1–208.e8. [Google Scholar] [CrossRef] [Green Version]
- Becker, K.L. Principles and Practice of Endocrinology and Metabolism; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 1995. [Google Scholar]
- McEwen, B.S. Protective and Damaging Effects of Stress Mediators. Library 1998, 338, 171–179. [Google Scholar] [CrossRef] [Green Version]
- Giurgescu, C. Are Maternal Cortisol Levels Related to Preterm Birth? JOGNN 2009, 38, 377–390. [Google Scholar] [CrossRef]
- Webster Marketon, J.I.; Sternberg, E.M. The Glucocorticoid Receptor: A Revisited Target for Toxins. Toxins 2010, 2, 1357–1380. [Google Scholar] [CrossRef] [Green Version]
- Gharaei, A.; Ghaffari, M.; Keyvanshokooh, S.; Akrami, R. Changes in Metabolic Enzymes, Cortisol and Glucose Concentrations of Beluga (Huso Huso) Exposed to Dietary Methylmercury. Fish Physiol. Biochem. 2011, 37, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Lee, S.J.; Kim, S.Y.; Choi, G.; Lee, J.J.; Kim, H.J.; Kim, S.; Park, J.; Moon, H.B.; Choi, K.; et al. Association of Food Consumption during Pregnancy with Mercury and Lead Levels in Cord Blood. Sci. Total Environ. 2016, 563–564, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Park, Y.; Kim, S.K.; Moon, H.B.; Park, J.; Choi, K.; Kim, S. Timing of an Accelerated Body Mass Increase in Children Exposed to Lead in Early Life: A Longitudinal Study. Sci. Total Environ. 2017, 584–585, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Eom, S.; Kim, H.J.; Lee, J.J.; Choi, G.; Choi, S.; Kim, S.; Kim, S.Y.; Cho, G.; Kim, Y.D.; et al. Association between Maternal Exposure to Major Phthalates, Heavy Metals, and Persistent Organic Pollutants, and the Neurodevelopmental Performances of Their Children at 1 to 2 Years of Age- CHECK Cohort Study. Sci. Total Environ. 2018, 624, 377–384. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, A.; Kim, S.K.; Moon, H.B.; Park, J.; Choi, K.; Kim, S. Lead and Mercury Levels in Repeatedly Collected Urine Samples of Young Children: A Longitudinal Biomonitoring Study. Environ. Res. 2020, 189, 109901. [Google Scholar] [CrossRef]
- Florey, C.V. The Use and Interpretation of Ponderal Index and Other Weight-Height Ratios in Epidemiological Studies. J. Chronic Dis. 1970, 23, 93–103. [Google Scholar] [CrossRef]
- Roje, D.; Ivo, B.; Ivica, T.; Mirjana, V.; Vesna, C.; Aljosa, B.; Marko, V.; Zoran, M.; Marko, M.; Tomislav, M. Gestational Age—The Most Important Factor of Neonatal Ponderal Index. Yonsei Med. J. 2004, 45, 273–280. [Google Scholar] [CrossRef]
- Park, Y.; Lee, A.; Choi, K.; Kim, H.J.; Lee, J.J.; Choi, G.; Kim, S.; Kim, S.Y.; Cho, G.J.; Suh, E.; et al. Exposure to Lead and Mercury through Breastfeeding during the First Month of Life: A CHECK Cohort Study. Sci. Total Environ. 2018, 612, 876–883. [Google Scholar] [CrossRef]
- Hung, T.H.; Lo, L.M.; Chiu, T.H.; Li, M.J.; Yeh, Y.L.; Chen, S.F.; Hsieh, T.T.A. A Longitudinal Study of Oxidative Stress and Antioxidant Status in Women with Uncomplicated Pregnancies throughout Gestation. Reprod. Sci. 2010, 17, 401–409. [Google Scholar] [CrossRef]
- Kang, S.; Kim, S.; Park, J.; Kim, H.J.; Lee, J.; Choi, G.; Choi, S.; Kim, S.; Kim, S.Y.; Moon, H.B.; et al. Urinary Paraben Concentrations among Pregnant Women and Their Matching Newborn Infants of Korea, and the Association with Oxidative Stress Biomarkers. Sci. Total Environ. 2013, 461–462, 214–221. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, J.; Moon, H.-B.; Park, J.; Choi, K.; Kim, S.K.; Kim, S. Association of Phthalate Exposures with Urinary Free Cortisol and 8-Hydroxy-2′-Deoxyguanosine in Early Childhood. Sci. Total Environ. 2018, 627, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Nermell, B.; Lindberg, A.L.; Rahman, M.; Berglund, M.; Åke Persson, L.; El Arifeen, S.; Vahter, M. Urinary Arsenic Concentration Adjustment Factors and Malnutrition. Environ. Res. 2008, 106, 212–218. [Google Scholar] [CrossRef] [PubMed]
- White, A.J.; O’Brien, K.M.; Jackson, B.P.; Karagas, M.R. Urine and Toenail Cadmium Levels in Pregnant Women: A Reliability Study. Environ. Int. 2018, 118, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Braun, J.M.; Kalkbrenner, A.E.; Calafat, A.M.; Bernert, J.T.; Ye, X.; Silva, M.J.; Barr, D.B.; Sathyanarayana, S.; Lanphear, B.P. Variability and Predictors of Urinary Bisphenol a Concentrations during Pregnancy. Environ. Health Perspect. 2011, 119, 131–137. [Google Scholar] [CrossRef] [Green Version]
- Karin, S.; Groothuis-Oudshoorn, K. Mice: Multivariate Imputation by Chained Equations in R. J. Stat. Softw. 2011, 45, 1–67. [Google Scholar] [CrossRef] [Green Version]
- Lewis, R.C.; Meeker, J.D.; Basu, N.; Gauthier, A.M.; Cantoral, A.; Mercado-García, A.; Peterson, K.E.; Téllez-Rojo, M.M.; Watkins, D.J. Urinary Metal Concentrations among Mothers and Children in a Mexico City Birth Cohort Study. Int. J. Hyg. Environ. Health 2018, 221, 609–615. [Google Scholar] [CrossRef]
- Hinwood, A.L.; Callan, A.C.; Ramalingam, M.; Boyce, M.; Heyworth, J.; McCafferty, P.; Odland, J. Cadmium, Lead and Mercury Exposure in Non Smoking Pregnant Women. Environ. Res. 2013, 126, 118–124. [Google Scholar] [CrossRef]
- Fort, M.; Cosín-Tomás, M.; Grimalt, J.O.; Querol, X.; Casas, M.; Sunyer, J. Assessment of Exposure to Trace Metals in a Cohort of Pregnant Women from an Urban Center by Urine Analysis in the First and Third Trimesters of Pregnancy. Environ. Sci. Pollut. Res. 2014, 21, 9234–9241. [Google Scholar] [CrossRef] [Green Version]
- Wai, K.M.; Mar, O.; Kosaka, S.; Umemura, M.; Watanabe, C. Prenatal Heavy Metal Exposure and Adverse Birth Outcomes in Myanmar: A Birth-Cohort Study. Int. J. Environ. Res. Public Health 2017, 14, 1339. [Google Scholar] [CrossRef] [Green Version]
- Cheng, L.; Zhang, B.; Huo, W.; Cao, Z.; Liu, W.; Liao, J.; Xia, W.; Xu, S.; Li, Y. Fetal Exposure to Lead during Pregnancy and the Risk of Preterm and Early-Term Deliveries. Int. J. Hyg. Environ. Health 2017, 220, 984–989. [Google Scholar] [CrossRef]
- Wu, J.; Ying, T.; Shen, Z.; Wang, H. Effect of Low-Level Prenatal Mercury Exposure on Neonate Neurobehavioral Development in China. Pediatric Neurol. 2014, 51, 93–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Badry, A.; Rezk, M.; El-Sayed, H. Mercury-Induced Oxidative Stress May Adversely Affect Pregnancy Outcome among Dental Staff: A Cohort Study. Int. J. Occup. Environ. Med. 2018, 9, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Engström, K.S.; Vahter, M.; Johansson, G.; Lindh, C.H.; Teichert, F.; Singh, R.; Kippler, M.; Nermell, B.; Raqib, R.; Strömberg, U.; et al. Chronic Exposure to Cadmium and Arsenic Strongly Influences Concentrations of 8-Oxo-7,8-Dihydro-2′-Deoxyguanosine in Urine. Free Radic. Biol. Med. 2010, 48, 1211–1217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Qi, L.; Peng, Y.; Xia, W.; Xu, S.; Li, Y.; Zhang, H. Urinary Concentrations of Environmental Metals and Associating Factors in Pregnant Women. Environ. Sci. Pollut. Res. 2019, 26, 13464–13475. [Google Scholar] [CrossRef]
- Shirai, S.; Suzuki, Y.; Yoshinaga, J.; Mizumoto, Y. Maternal Exposure to Low-Level Heavy Metals during Pregnancy and Birth Size. J. Environ. Sci. Health Part A 2010, 45, 1468–1474. [Google Scholar] [CrossRef]
- Dereumeaux, C.; Saoudi, A.; Pecheux, M.; Berat, B.; de Crouy-Chanel, P.; Zaros, C.; Brunel, S.; Delamaire, C.; le Tertre, A.; Lefranc, A.; et al. Biomarkers of Exposure to Environmental Contaminants in French Pregnant Women from the Elfe Cohort in 2011. Environ. Int. 2016, 97, 56–67. [Google Scholar] [CrossRef]
- Moynihan, M.; Peterson, K.E.; Cantoral, A.; Song, P.X.K.; Jones, A.; Solano-González, M.; Meeker, J.D.; Basu, N.; Téllez-Rojo, M.M. Dietary Predictors of Urinary Cadmium among Pregnant Women and Children. Sci. Total Environ. 2017, 575, 1255–1262. [Google Scholar] [CrossRef] [Green Version]
- Osorio-Yáñez, C.; Gelaye, B.; Enquobahrie, D.A.; Qiu, C.; Williams, M.A. Dietary Intake and Urinary Metals among Pregnant Women in the Pacific Northwest. Environ. Pollut. 2018, 236, 680–688. [Google Scholar] [CrossRef]
- Kippler, M.; Nermell, B.; Hamadani, J.; Tofail, F.; Moore, S.; Vahter Marie, M. Burden of Cadmium in Early Childhood: Longitudinal Assessment of Urinary Cadmium in Rural Bangladesh. Toxicol. Lett. 2010, 198, 20–25. [Google Scholar] [CrossRef]
- Al-Saleh, I.; Abduljabbar, M.; Al-Rouqi, R.; Elkhatib, R.; Alshabbaheen, A.; Shinwari, N. Mercury (Hg) Exposure in Breast-Fed Infants and Their Mothers and the Evidence of Oxidative Stress. Biol. Trace Elem. Res. 2013, 153, 145–154. [Google Scholar] [CrossRef]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, Mechanism and Health Effects of Some Heavy Metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Cadahía, B.; Laffon, B.; Porta, M.; Lafuente, A.; Cabaleiro, T.; López, T.; Caride, A.; Pumarega, J.; Romero, A.; Pásaro, E.; et al. Relationship between Blood Concentrations of Heavy Metals and Cytogenetic and Endocrine Parameters among Subjects Involved in Cleaning Coastal Areas Affected by the “Prestige” Tanker Oil Spill. Chemosphere 2008, 71, 447–455. [Google Scholar] [CrossRef]
- Alves, J.; Barrientos, G.; Toro, V.; Grijota, F.J.; Muñoz, D.; Maynar, M. Correlations between Basal Trace Minerals and Hormones in Middle and Long-Distance High-Level Male Runners. Int. J. Environ. Res. Public Health 2020, 17, 9473. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.W.; Meiller, J.C.; Mahaffey, K.R. The Endocrine Effects of Mercury in Humans and Wildlife. Crit. Rev. Toxicol. 2009, 39, 228–269. [Google Scholar] [CrossRef]
- Aluru, N.; Vijayan, M.M. Stress Transcriptomics in Fish: A Role for Genomic Cortisol Signaling. Gen. Comp. Endocrinol. 2009, 164, 142–150. [Google Scholar] [CrossRef]
- Schreier, H.M.; Hsu, H.H.; Amarasiriwardena, C.; Coull, B.A.; Schnaas, L.; Téllez-Rojo, M.M.; Tamayo, Y.; Ortiz, M.; Wright, R.J.; Wright, R.O. Mercury and Psychosocial Stress Exposure Interact to Predict Maternal Diurnal Cortisol during Pregnancy. Environ. Health 2015, 14, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castiello, F.; Olmedo, P.; Gil, F.; Molina, M.; Mundo, A.; Romero, R.R.; Ruíz, C.; Gómez-Vida, J.; Vela-Soria, F.; Freire, C. Association of Urinary Metal Concentrations with Blood Pressure and Serum Hormones in Spanish Male Adolescents. Environ. Res. 2020, 182, 108958. [Google Scholar] [CrossRef]
- Xie, X.; Ding, G.; Cui, C.; Chen, L.; Gao, Y.; Zhou, Y.; Shi, R.; Tian, Y. The Effects of Low-Level Prenatal Lead Exposure on Birth Outcomes. Environ. Pollut. 2013, 175, 30–34. [Google Scholar] [CrossRef]
- Taylor, C.M.; Tilling, K.; Golding, J.; Emond, A.M. Low Level Lead Exposure and Pregnancy Outcomes in an Observational Birth Cohort Study: Dose-Response Relationships. BMC Res. Notes 2016, 9, 291. [Google Scholar] [CrossRef] [Green Version]
- Osman, K.; Åkesson, A.; Berglund, M.; Bremme, K.; Schütz, A.; Ask, K.; Vahter, M. Toxic and Essential Elements in Placentas of Swedish Women. Clin. Biochem. 2000, 33, 131–138. [Google Scholar] [CrossRef]
- Wang, J.; Gao, Z.Y.; Yan, J.; Ying, X.L.; Tong, S.L.; Yan, C.H. Sex Differences in the Effects of Prenatal Lead Exposure on Birth Outcomes. Environ. Pollut. 2017, 225, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.M.; Golding, J.; Emond, A.M. Adverse Effects of Maternal Lead Levels on Birth Outcomes in the ALSPAC Study: A Prospective Birth Cohort Study. BJOG Int. J. Obstet. Gynaecol. 2015, 122, 322–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- West, W.L.; Knight, E.M.; Edwards, C.H.; Manning, M.; Spurlock, B.; James, H.; Johnson, A.A.; Oyemade, U.J.; Cole, O.J.; Westney, O.E.; et al. Maternal Low Level Lead and Pregnancy Outcomes. J. Nutr. 1994, 124, 981–986. [Google Scholar]
- Wells, E.M.; Herbstman, J.B.; Lin, Y.H.; Jarrett, J.; Verdon, C.P.; Ward, C.; Caldwell, K.L.; Hibbeln, J.R.; Witter, F.R.; Halden, R.U.; et al. Cord Blood Methylmercury and Fetal Growth Outcomes in Baltimore Newborns: Potential Confounding and Effect Modification by Omega-3 Fatty Acids, Selenium, and Sex. Environ. Health Perspect. 2016, 124, 373–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bloom, M.S.; Buck Louis, G.M.; Sundaram, R.; Maisog, J.M.; Steuerwald, A.J.; Parsons, P.J. Birth Outcomes and Background Exposures to Select Elements, the Longitudinal Investigation of Fertility and the Environment (LIFE). Environ. Res. 2015, 138, 118–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dack, K.; Fell, M.; Taylor, C.M.; Havdahl, A.; Lewis, S.J. Mercury and Prenatal Growth: A Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 7140. [Google Scholar] [CrossRef]
- Eriksson, J.G.; Tuomilehto, J.; Osmond, C.; Barker, D.J.P. Early Growth and Coronary Heart Disease in Later Life: Longitudinal Study. BMJ 2001, 322, 949–953. [Google Scholar] [CrossRef] [Green Version]
- Larnkjær, A.; Ingstrup, H.K.; Schack-Nielsen, L.; Mølgaard, C.; Michaelsen, K.F. Thin Newborns Are More Insulin Resistant at 10 Years of Age. Acta Paediatr. Int. J. Paediatr. 2011, 100, 511–514. [Google Scholar] [CrossRef]
- Al-Saleh, I.; Shinwari, N.; Mashhour, A.; Rabah, A. Birth Outcome Measures and Maternal Exposure to Heavy Metals (Lead, Cadmium and Mercury) in Saudi Arabian Population. Int. J. Hyg. Environ. Health 2014, 217, 205–218. [Google Scholar] [CrossRef]
- Salafia, C.M.; Charles, A.K.; Maas, E.M. Placenta and Fetal Growth Restriction. Clin. Obstet. Gynecol. 2006, 49, 236–256. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, X.; Chen, A.; Davuljigari, C.B.; Zheng, X.; Kim, S.S.; Dietrich, K.N.; Ho, S.M.; Reponen, T.; Huo, X. Maternal Urinary Cadmium Levels during Pregnancy Associated with Risk of Sex-Dependent Birth Outcomes from an e-Waste Pollution Site in China. Reprod. Toxicol. 2018, 75, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Huo, W.; Zhang, B.; Zheng, T.; Li, Y.; Pan, X.; Liu, W.; Chang, H.; Jiang, M.; Zhou, A.; et al. Maternal Urinary Cadmium Concentrations in Relation to Preterm Birth in the Healthy Baby Cohort Study in China. Environ. Int. 2016, 94, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Zhong, H.; Guo, Z.; Wu, Z.; Zhang, H.; Wang, C.; Zhou, Y.; Zuo, Z. Levels of Heavy Metals and Trace Elements in Umbilical Cord Blood and the Risk of Adverse Pregnancy Outcomes: A Population-Based Study. Biol. Trace Elem. Res. 2014, 160, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Kippler, M.; Wagatsuma, Y.; Rahman, A.; Nermell, B.; Persson, L.-Å.; Raqib, R.; Vahter, M. Environmental Exposure to Arsenic and Cadmium during Pregnancy and Fetal Size: A Longitudinal Study in Rural Bangladesh. Reprod. Toxicol. 2012, 34, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Andersson, L.; Sundström-Poromaa, I.; Wulff, M.; Åström, M.; Bixo, M. Neonatal Outcome Following Maternal Antenatal Depression and Anxiety: A Population-Based Study. Am. J. Epidemiol. 2004, 159, 872–881. [Google Scholar] [CrossRef] [Green Version]
- Rahman, A.; Bunn, J.; Lovel, H.; Creed, F. Association between Antenatal Depression and Low Birthweight in a Developing Country. Acta Psychiatr. Scand. 2007, 115, 481–486. [Google Scholar] [CrossRef]
- Bolten, M.I.; Wurmser, H.; Buske-Kirschbaum, A.; Papoušek, M.; Pirke, K.M.; Hellhammer, D. Cortisol Levels in Pregnancy as a Psychobiological Predictor for Birth Weight. Arch. Women’s Ment. Health 2011, 14, 33–41. [Google Scholar] [CrossRef]
- Kajantie, E.; Eriksson, J.; Osmond, C.; Wood, P.J.; Forsén, T.; Barker, D.J.P.; Phillips, D.I.W. Size at Birth, the Metabolic Syndrome and 24-h Salivary Cortisol Profile. Clin. Endocrinol. 2004, 60, 201–207. [Google Scholar] [CrossRef]
- Antolic, A.; Feng, X.; Wood, C.E.; Richards, E.M.; Keller-Wood, M. Increased Maternal Nighttime Cortisol Concentrations in Late Gestation Alter Glucose and Insulin in the Neonatal Lamb. Physiol. Rep. 2015, 3, e12548. [Google Scholar] [CrossRef]
- Matsubasa, T.; Uchino, T.; Karashima, S.; Tanimura, M.; Endo, F. Oxidative Stress in Very Low Birth Weight Infants as Measured by Urinary 8-OHdG. Free Radic. Res. 2002, 36, 189–193. [Google Scholar] [CrossRef]
- Hsieh, T.T.A.; Chen, S.F.; Lo, L.M.; Li, M.J.; Yeh, Y.L.; Hung, T.H. The Association between Maternal Oxidative Stress at Mid-Gestation and Subsequent Pregnancy Complications. Reprod. Sci. 2012, 19, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.; Ho, J.T.; Torpy, D.J.; Rogers, A.; Doogue, M.; Lewis, J.G.; Czajko, R.J.; Inder, W.J. A Longitudinal Study of Plasma and Urinary Cortisol in Pregnancy and Postpartum. J. Clin. Endocrinol. Metab. 2011, 96, 1533–1540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conde, A.; Figueiredo, B. 24-h Urinary Free Cortisol from Mid-Pregnancy to 3-Months Postpartum: Gender and Parity Differences and Effects. Psychoneuroendocrinology 2014, 50, 264–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Epstein, J. Cortisol Urine Test. Available online: http://www.healthline.com/health/cortisol-level?pr (accessed on 30 January 2021).

| Variables | N (%) or Mean ± SD | Median (Range) | ||||
|---|---|---|---|---|---|---|
| Pb (μg/L) | Hg (μg/L) | Cd (μg/L) | Free cortisol (μg/dL) | 8–OHdG (ng/mL) | ||
| Total | 182 (100) | 4.37 (0.92–92.36) | 1.25 (0.03–18.3) | 0.72 (0.05–7.80) | 34.2 (5.19–178) | 60.5 (29.0–601) |
| Detection frequency (%) | 91.8 | 99.5 | 96.7 | 100 | 100 | |
| Maternal age (year) | 33.4 ± 3.94 | |||||
| 20–29 | 25 (13.7) | 3.82 (0.92–7.20) ** | 1.78 (0.59–18.3) | 0.84 (0.12–3.28) ** | 38.8 (22.3–89.0) | 76.6 (29.0–137) |
| 30–39 | 147 (80.8) | 4.37 (0.92–22.2) | 1.24 (0.05–15.9) | 0.68 (0.05–7.80) | 33.3 (5.19–178) | 60.0 (29.0–602) |
| 40–49 | 10 (5.5) | 5.73 (1.64–92.4) | 1.30 (0.03–4.75) | 1.94 (0.33–6.17) | 24.6 (7.50–59.0) | 63.0 (38.1–346) |
| Prepregnancy BMI (kg/m2) | 21.7 ±3.92 | |||||
| <18.5 | 29 (15.9) | 4.95 (0.92–22.2) | 1.74 (0.48–18.3) * | 0.97 (0.09–6.17) | 40.5 (6.33–178) | 67.5 (38.6–199) |
| 18.5–24.9 | 103 (56.6) | 4.37 (0.92–19.6) | 1.24 (0.03–9.14) | 0.65 (0.05–7.80) | 30.6 (5.19–178) | 58.6 (29.0–602) |
| >25 | 50 (27.5) | 4.19 (0.92–92.4) | 1.08 (0.05–14.5) | 0.74 (0.05–7.03) | 31.5 (7.60–69.2) | 63.9 (29.0–346) |
| Monthly household income (USD) | ||||||
| <3000 | 47 (25.8) | 4.30 (1.46–22.2) | 1.15 (0.03–6.35) * | 0.65 (0.05–3.04) | 37.7 (7.54–178) | 60.4 (29.0–602) |
| 3000–6000 | 65 (35.7) | 4.48 (0.92–19.6) | 1.46 (0.47–15.9) | 0.68 (0.05–7.80) | 30.0 (5.93–178) | 60.0 (29.0–200) |
| ≥6000 | 70 (38.5) | 4.20 (0.92–92.4) | 1.18 (0.05–18.3) | 0.74 (0.05–7.03) | 35.3 (5.19–121) | 60.9 (29.0–346) |
| Smoking status (active or passive) during pregnancy | ||||||
| No | 83 (45.6) | 4.43 (0.92–17.2) | 1.29 (0.13–14.8) | 0.62 (0.05–7.80) | 31.7 (5.19–178) | 63.9 (29.0–602) |
| Yes | 99 (54.4) | 4.42 (0.92–92.4) | 1.35 (0.03–18.4) | 0.73 (0.05–6.17) | 33.5 (7.54–178) | 66.6 (29.0–346) |
| Drinking during pregnancy | ||||||
| No | 159 (87.4) | 4.31 (0.92–92.4) * | 1.29 (0.05–18.3) | 0.71 (0.05–7.03) | 5.19 (5.19–178) | 60.4 (29.0–602) |
| Yes | 23 (12.6) | 5.83 (2.27–22.2) | 1.11 (0.03–9.14) | 0.73 (0.33–7.80) | 6.33 (6.33–178) | 60.7 (38.1–200) |
| Gestational period (days) | 276 ± 7.14 | |||||
| <39 weeks | 57 | 4.67 (0.92–17.2) | 1.13 (0.05–18.3) * | 0.68 (0.05–2.65) | 28.9 (5.19–83.1) * | 56.5 (29.0–158) * |
| ≥39 weeks | 125 | 4.30 (0.92–92.4) | 1.36 (0.03–15.9) | 0.76 (0.05–7.80) | 37.7 (5.93–178) | 62.7 (29.0–601) |
| Delivery mode | ||||||
| Vaginal delivery | 129 (70.9) | 4.29 (0.92–22.2) | 1.35 (0.03–15.9) | 0.68 (0.05–7.80) | 34.6 (5.93–178) | 61.0 (29.0–602) |
| Cesarean-section | 53 (29.1) | 4.74 (0.92–92.4) | 1.16 (0.05–18.3) | 0.74 (0.05–3.40) | 33.0 (5.19–89.0) | 59.5 (29.0–346) |
| Parity | ||||||
| 0 | 69 | 4.14 (0.92–92.4) | 1.34 (0.03–18.3) | 0.83 (0.09–3.41) | 35.6 (7.54–178) | 65.1 (33.3–346) |
| ≥1 | 113 | 4.48 (0.92–19.6) | 1.24 (0.05–15.9) | 0.64 (0.05–7.80) | 33.6 (5.19–178) | 58.6 (29.0–602) |
| Heavy Metals | Free Cortisol | 8-OHdG | ||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted Model | Adjusted Model * | Unadjusted Model | Adjusted Model * | |||||
| β (95% CI) | p | β (95% CI) | p | β (95% CI) | p | β (95% CI) | p | |
| Pb | 0.11 (−0.02–0.23) | 0.093 | 0.12 (−0.00–0.25) | 0.058 | 0.16 (0.08–0.25) | <0.001 | 0.18 (0.09–0.26) | <0.001 |
| Hg | 0.18 (0.08–0.28) | <0.001 | 0.17 (0.06–0.27) | 0.003 | 0.16 (0.09–0.22) | <0.001 | 0.17 (0.10–0.24) | <0.001 |
| Cd | 0.25 (0.15–0.36) | <0.001 | 0.24 (0.14–0.35) | <0.001 | 0.14 (0.06–0.21) | <0.001 | 0.12 (0.04–0.20) | 0.003 |
| Biomarkers | Birth Weight (g) | Birth Length (cm) | Birth Circumference (cm) | Ponderal Index (g/cm3) | |||||
|---|---|---|---|---|---|---|---|---|---|
| β (95% CI) | p | β (95% CI) | p | β (95% CI) | p | β (95% CI) | p | ||
| Free cortisol | Unadjusted | 1.80 (−76.8–80.4) | 0.964 | 1.11 (0.36–1.86) | 0.004 | −0.48 (−0.92–−0.03) | 0.037 | −0.31 (−0.52–−0.11) | 0.003 |
| Adjusted | −0.27 (−75.1–74.6) | 0.994 | 1.10 (0.32–1.88) | 0.006 | −0.36 (−0.79–0.07) | 0.103 | −0.32 (−0.54–−0.10) | 0.005 | |
| 8-OHdG | Unadjusted | 27.4 (−84.5–139) | 0.632 | 0.75 (−0.33–1.83) | 0.175 | −0.06 (−0.69–0.58) | 0.862 | −0.15 (−0.45–0.15) | 0.341 |
| Adjusted | 7.79 (−97.5–113) | 0.885 | 0.64 (−0.48–1.75) | 0.263 | −0.10 (−0.71–0.52) | 0.754 | −0.15 (−0.46–0.17) | 0.370 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, S.; Lee, A.; Choi, G.; Moon, H.-B.; Kim, S.; Choi, K.; Park, J. Free Cortisol Mediates Associations of Maternal Urinary Heavy Metals with Neonatal Anthropometric Measures: A Cross-Sectional Study. Toxics 2022, 10, 167. https://doi.org/10.3390/toxics10040167
Choi S, Lee A, Choi G, Moon H-B, Kim S, Choi K, Park J. Free Cortisol Mediates Associations of Maternal Urinary Heavy Metals with Neonatal Anthropometric Measures: A Cross-Sectional Study. Toxics. 2022; 10(4):167. https://doi.org/10.3390/toxics10040167
Chicago/Turabian StyleChoi, Sohyeon, Aram Lee, Gyuyeon Choi, Hyo-Bang Moon, Sungkyoon Kim, Kyungho Choi, and Jeongim Park. 2022. "Free Cortisol Mediates Associations of Maternal Urinary Heavy Metals with Neonatal Anthropometric Measures: A Cross-Sectional Study" Toxics 10, no. 4: 167. https://doi.org/10.3390/toxics10040167
APA StyleChoi, S., Lee, A., Choi, G., Moon, H.-B., Kim, S., Choi, K., & Park, J. (2022). Free Cortisol Mediates Associations of Maternal Urinary Heavy Metals with Neonatal Anthropometric Measures: A Cross-Sectional Study. Toxics, 10(4), 167. https://doi.org/10.3390/toxics10040167