Potassium and Silicon Synergistically Increase Cadmium and Lead Tolerance and Phytostabilization by Quinoa through Modulation of Physiological and Biochemical Attributes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Description
2.2. Plant Sampling and Growth Measurements
2.3. Determination of Metal Contents
2.4. Leaf Pigments and Stomatal Conductance
2.5. Oxidative Stress Attributes
2.6. Enzymatic Activities
2.7. Metal Accumulation and Translocation
2.8. Statistical Analyses
3. Results
3.1. Plant Growth
3.2. Pigment Contents and Stomatal Conductance
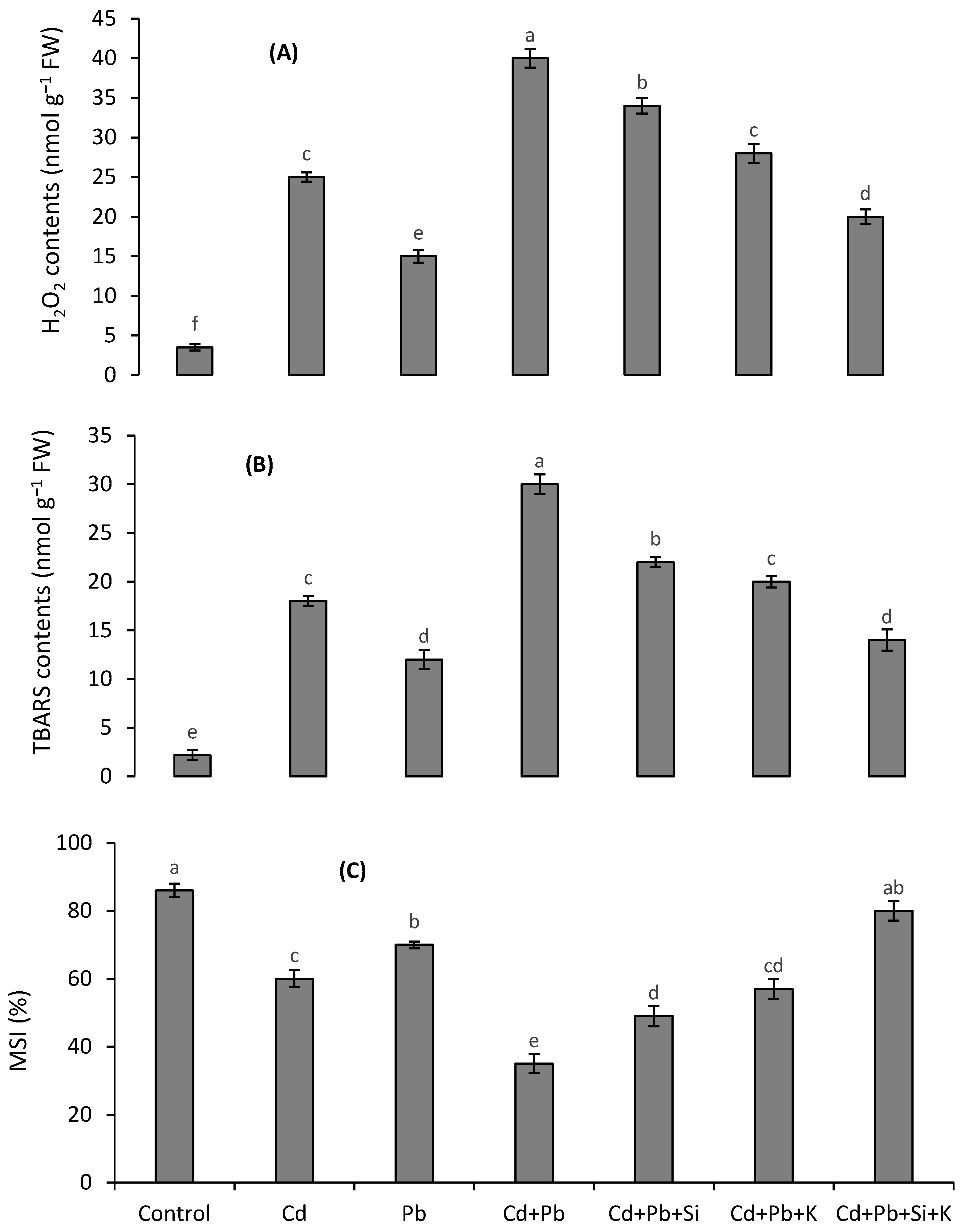
3.3. Oxidative Stress Attributes
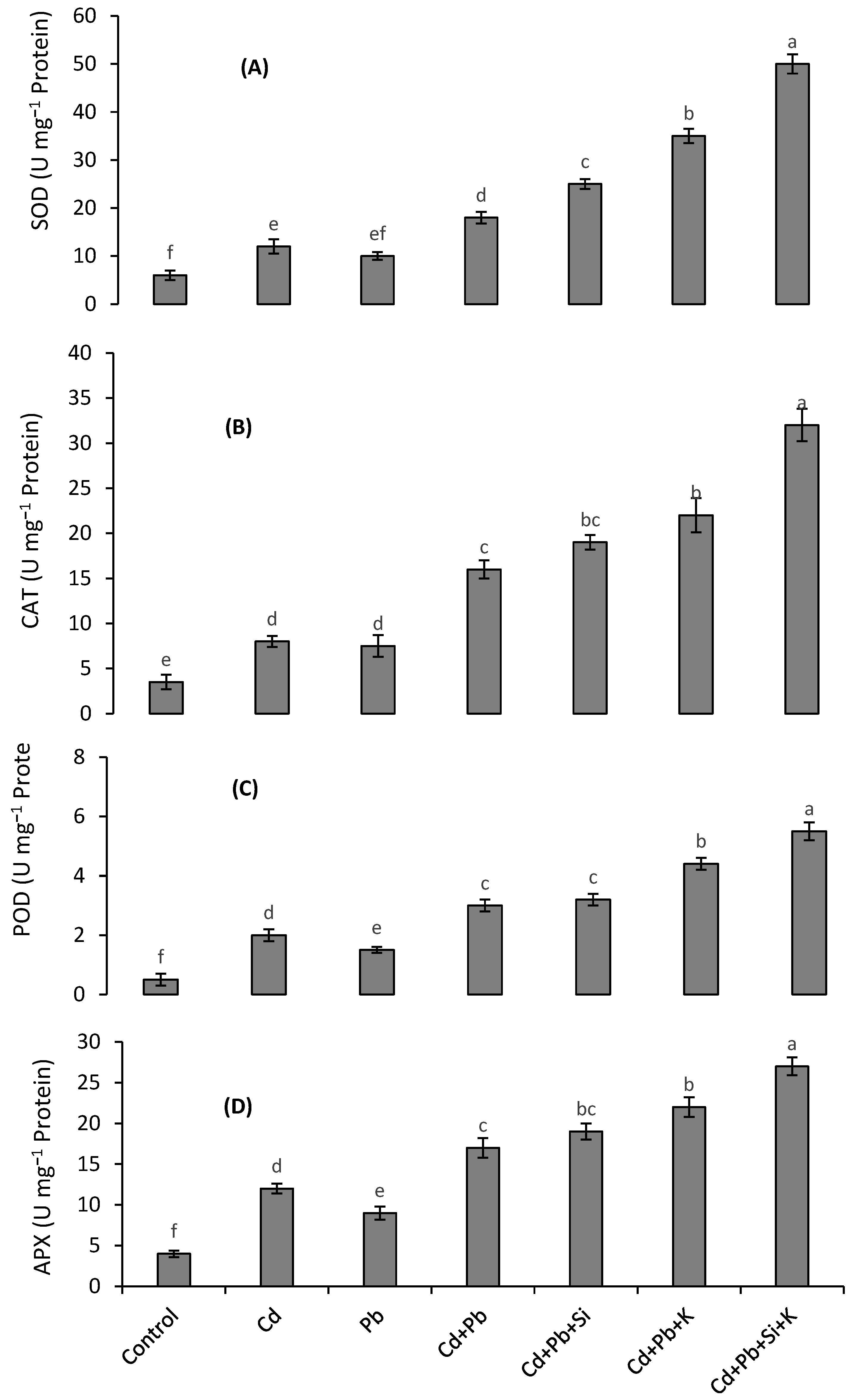
3.4. Antioxidant Enzymes
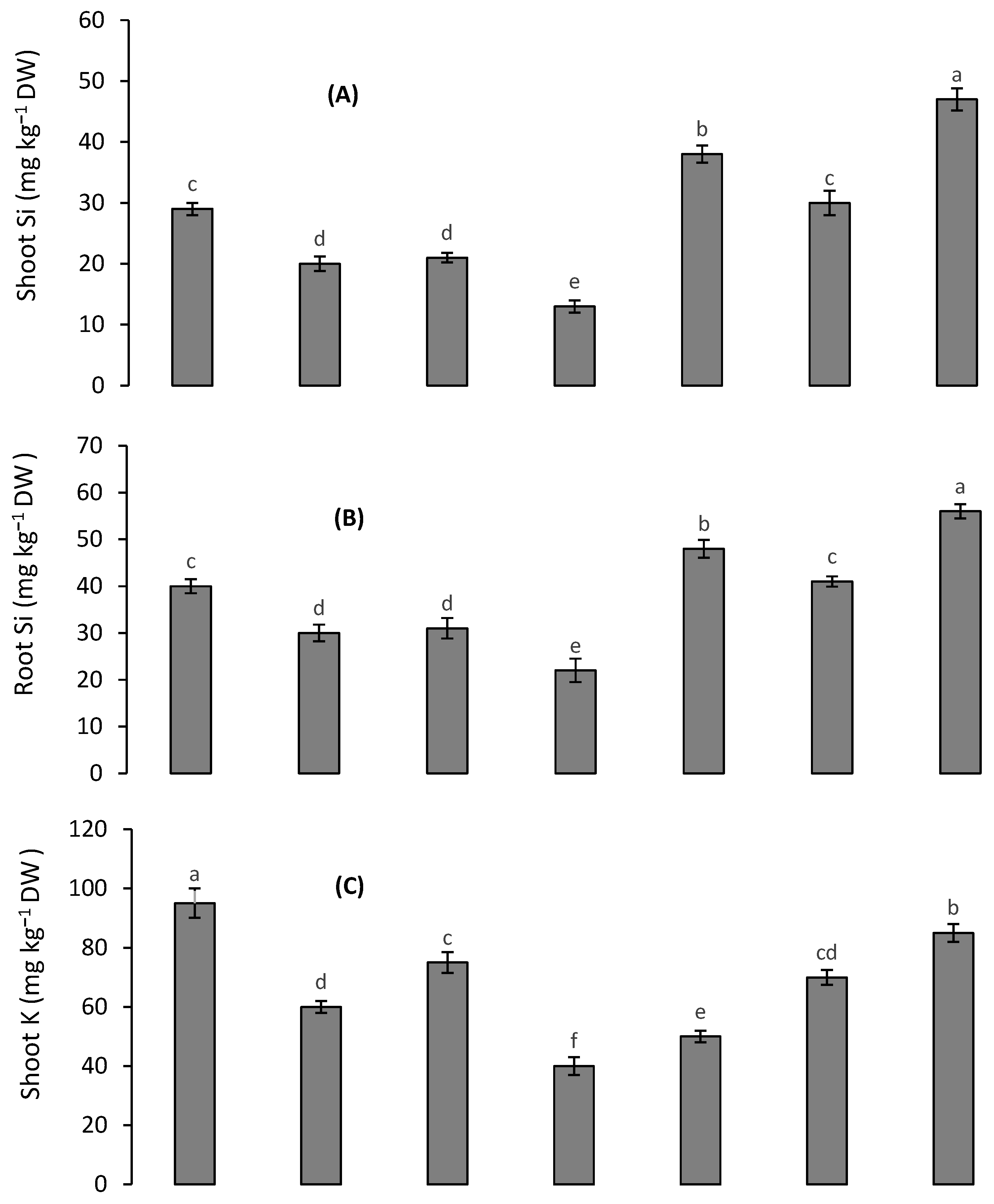
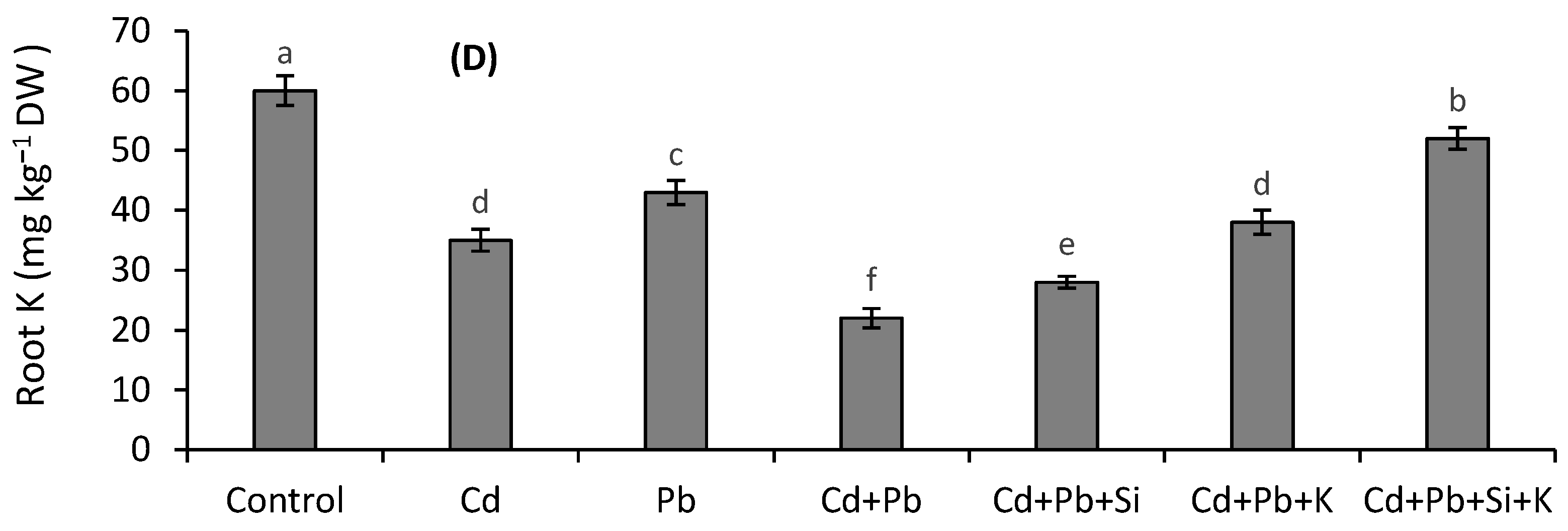
3.5. Silicon and K Concentrations
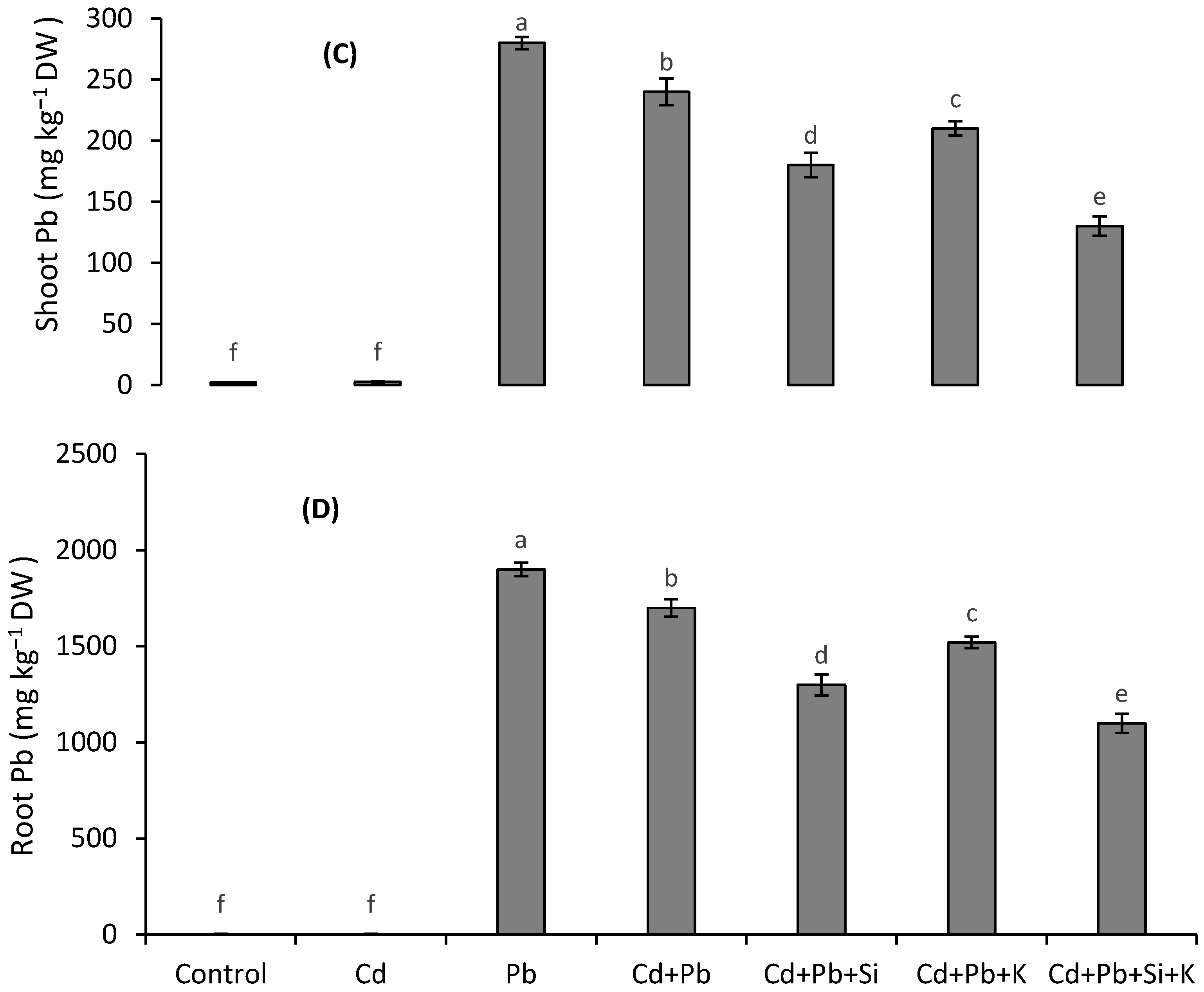
3.6. Metal Accumulation and Translocation
3.7. Multivariate Comparison of Variables
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Palansooriya, K.N.; Shaheen, S.M.; Chen, S.S.; Tsang, D.C.W.; Hashimoto, Y.; Hou, D.; Bolan, N.S.; Rinklebe, J.; Ok, Y.S. Soil amendments for immobilization of potentially toxic elements in contaminated soils: A critical review. Environ. Int. 2020, 134, 105046. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Guan, X.; Li, C.; Zhao, K.; Yang, X.; Fu, R.; Li, Y.; Yu, F. Global perspectives and future research directions for the phytoremediation of heavy metal-contaminated soil: A knowledge mapping analysis from 2001 to 2020. Front. Environ. Sci. Eng. 2022, 16, 1–20. [Google Scholar] [CrossRef]
- Ministry of Environmental Protection and Ministry of Land and Resources of China. Bulletin of National Soil Pollution Survey. 2014. Available online: http://www.zhb.gov.cn/gkml/hbb/qt/201404/W020140417558995804588.pdf (accessed on 7 March 2021).
- Mahar, A.; Wang, P.; Ali, A.; Awasthi, M.K.; Lahori, A.H.; Wang, Q.; Li, R.; Zhang, Z. Challenges and opportunities in the phytoremediation of heavy metals contaminated soils: A review. Ecotoxicol. Environ. Saf. 2016, 126, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Yadav, K.K.; Gupta, N.; Kumar, A.; Reece, L.M.; Singh, N.; Rezania, S.; Khan, S.A. Mechanistic understanding and holistic approach of phytoremediation: A review on application and future prospects. Ecol. Engin. 2018, 120, 274–298. [Google Scholar] [CrossRef]
- Rasafi, T.E.; Oukarroum, A.; Haddioui, A.; Song, H.; Kwon, E.E.; Bolan, N.; Tack, F.M.; Sebastian, A.; Prasad, M.N.V.; Rinklebe, J. Cadmium stress in plants: A critical review of the effects, mechanisms, and tolerance strategies. Crit. Rev. Environ. Sci. Technol. 2021, 52, 1–52. [Google Scholar] [CrossRef]
- Zhao, H.; Guan, J.; Liang, Q.; Zhang, X.; Hu, H.; Zhang, J. Effects of cadmium stress on growth and physiological characteristics of sassafras seedlings. Sci. Rep. 2021, 11, 9913. [Google Scholar] [CrossRef]
- Rehman, S.; Abbas, G.; Shahid, M.; Saqib, M.; Farooq, A.B.U.; Hussain, M.; Farooq, A. Effect of salinity on cadmium tolerance, ionic homeostasis and oxidative stress responses in conocarpus exposed to cadmium stresss. Ecotoxico. Environ. 2019, 171, 146–153. [Google Scholar] [CrossRef]
- Mitsopoulou, N.; Lakiotis, K.; Golia, E.E.; Khah, E.M.; Pavli, O.I. Response of hrpZPsph-transgenic N. benthamiana plants under cadmium stress. Environ. Sci. Pollut. Res. 2021, 28, 3787–3796. [Google Scholar] [CrossRef]
- Kaya, C. Salicylic acid-induced hydrogen sulphide improves lead stress tolerance in pepper plants by upraising the ascorbate-glutathione cycle. Physiol. Plantr. 2021, 173, 8–19. [Google Scholar] [CrossRef]
- Guedes, F.R.C.M.; Maia, C.F.; da Silva, B.R.S.; Batista, B.L.; Alyemeni, M.N.; Ahmad, P.; da Silva Lobato, A.K. Exogenous 24-Epibrassinolide stimulates root protection, and leaf antioxidant enzymes in lead stressed rice plants: Central roles to minimize Pb content and oxidative stress. Environ. Pollut. 2021, 280, 116992. [Google Scholar] [CrossRef]
- Abdal, N.; Abbas, G.; Asad, S.A.; Ghfar, A.A.; Shah, G.M.; Rizwan, M.; Ali, S.; Shahbaz, M. Salinity mitigates cadmium-induced phytotoxicity in quinoa (Chenopodium quinoa Willd.) by limiting the Cd uptake and improved responses to oxidative stress: Implications for phytoremediation. Environ. Geochem. Health 2021, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Iftikhar, A.; Abbas, G.; Saqib, M.; Shabbir, A.; Amjad, M.; Shahid, M.; Ahmad, I.; Iqbal, S.; Qaisrani, S.A. Salinity modulates lead (Pb) tolerance and phytoremediation potential of quinoa: A multivariate comparison of physiological and biochemical attributes. Environ. Geochem. Health 2022, 44, 257–272. [Google Scholar] [CrossRef] [PubMed]
- 14 Abbas, G.; Amjad, M.; Saqib, M.; Murtaza, B.; Asif Naeem, M.; Shabbir, A.; Murtaza, G. Soil sodicity is more detrimental than salinity for quinoa (Chenopodium quinoa Willd.): A multivariate comparison of physiological, biochemical and nutritional quality attributes. J. Agron. Crop. Sci. 2021, 207, 59–73. [Google Scholar] [CrossRef]
- Fatemi, H.; Pour, B.E.; Rizwan, M. Foliar application of silicon nanoparticles affected the growth, vitamin C, flavonoid, and antioxidant enzyme activities of coriander (Coriandrum sativum L.) plants grown in lead (Pb)-spiked soil. Environ. Sci. Pollut. Res. 2021, 28, 1417–1425. [Google Scholar] [CrossRef] [PubMed]
- Murtaza, B.; Naeem, F.; Shahid, M.; Abbas, G.; Shah, N.S.; Amjad, M.; Bakhat, H.F.; Imran, M.; Niazi, N.K.; Murtaza, G. A multivariate analysis of physiological and antioxidant responses and health hazards of wheat under cadmium and lead stress. Environ. Sci. Pollut. Res. 2019, 26, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Amjad, M.; Iqbal, M.M.; Abbas, G.; Farooq, A.B.U.; Naeem, M.A.; Imran, M.; Murtaza, B.; Nadeem, M.; Jacobsen, S.-E. Assessment of cadmium and lead tolerance potential of quinoa (Chenopodium quinoa Willd) and its implications for phytoremediation and human health. Environ. Geochem. Health 2021, 1–14. [Google Scholar] [CrossRef]
- Parvez, S.; Abbas, G.; Shahid, M.; Amjad, M.; Hussain, M.; Asad, S.A.; Imran, M.; Naeem, M.A. Effect of salinity on physiological, biochemical and photostabilizing attributes of two genotypes of quinoa (Chenopodium quinoa Willd.) exposed to arsenic stress. Ecotoxicol. Environ. 2020, 187, 109814. [Google Scholar] [CrossRef]
- Adrees, M.; Ali, S.; Rizwan, M.; Zia-ur-Rehman, M.; Ibrahim, M.; Abbas, F.; Farid, M.; Qayyum, M.F.; Irshad, M.K. Mechanisms of silicon-mediated alleviation of heavy metal toxicity in plants: A review. Ecotoxicol. Environ. Saf. 2015, 119, 186–197. [Google Scholar] [CrossRef]
- Ali, S.; Bharwana, S.A.; Rizwan, M.; Farid, M.; Kanwal, S.; Ali, Q.; Ibrahim, M.; Gill, R.A.; Khan, M.D. Fulvic acid mediates chromium (Cr) tolerance in wheat (Triticum aestivum L.) through lowering of Cr uptake and improved antioxidant defense system. Environ. Sci. Pollut. Res. 2015, 22, 10601–10609. [Google Scholar] [CrossRef]
- Cakmak, I. The role of potassium in alleviating detrimental effects of abiotic stresses in plants. J. Plant. Nutr. Soil Sci. 2005, 168, 521–530. [Google Scholar] [CrossRef]
- Abbas, G.; Rehman, S.; Siddiqui, M.H.; Ali, H.M.; Farooq, M.A.; Chen, Y. Potassium and humic acid synergistically increase salt tolerance and nutrient uptake in contrasting wheat genotypes through ionic homeostasis and activation of antioxidant enzymes. Plants 2022, 11, 263. [Google Scholar] [CrossRef] [PubMed]
- Siddique, M.H.; Al-Whaibi, A.H.; Sakran, M.O.; Basalah, H.M.A. Effect of calcium and potassium on antioxidant system of Vicia faba L. under cadmium stress. Int. J. Mol. Sci. 2012, 13, 6604–6619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bybordi, A. Influence of exogenous application of silicon and potassium on physiological responses, yield and yield components of salt-stressed wheat. Commun. Soil Sci. Plant Anal. 2015, 46, 109–122. [Google Scholar] [CrossRef]
- Zorb, C.; Senbayramb, M.; Peiter, E. Potassium in agriculture—Status and perspectives. J. Plant Physiol. 2014, 171, 656–669. [Google Scholar] [CrossRef] [PubMed]
- Zaheer, M.M.; Yasin, N.A.; Ahmad, S.R.; Khan, W.U.; Ahmad, A.; Ali, A.; Rehman, S.U. Amelioration of cadmium stress in gladiolus (Gladiolus grandiflora L.) by application of potassium and silicon. J. Plant Nutr. 2018, 41, 461–476. [Google Scholar] [CrossRef]
- Alzahrani, Y. The defensive role of silicon in wheat against stress conditions induced by drought, salinity or cadmium. Ecotoxicol. Environ. Saf. 2018, 154, 187–196. [Google Scholar] [CrossRef]
- Ali, M.; Afzal, S.; Parveen, A.; Kamran, M.; Javed, M.R.; Abbasi, G.H.; Malik, Z.; Riaz, M.; Ahmad, S.; Chattha, M.S.; et al. Silicon mediated improvement in the growth and ion homeostasis by decreasing Na+ uptake in maize (Zea mays L.) cultivars exposed to salinity stress. Plant Physiol. Biochem. 2021, 158, 208–218. [Google Scholar] [CrossRef]
- Huang, H.; Rizwan, M.; Li, M.; Song, F.; Zhou, S.; He, X.; Ding, R.; Dai, Z.; Yuan, Y.; Cao, M.; et al. Comparative efficacy of organic and inorganic silicon fertilizers on antioxidant response, Cd/Pb accumulation and health risk assessment in wheat (Triticum aestivum L.). Environ. Pollut. 2019, 255, 113146. [Google Scholar] [CrossRef]
- Khan, A.; Bilal, S.; Khan, A.L.; Imran, M.; Al-Harrasi, A.; Al-Rawahi, A.; Lee, I.J. Silicon-mediated alleviation of combined salinity and cadmium stress in date palm (Phoenix dactylifera L.) by regulating physio-hormonal alteration. Ecotoxicol. Environ. Saf. 2020, 188, 109885. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Circular. Calif. Agric. Exp. Stn. 1950, 347, 32. [Google Scholar]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Islam, E.; Liu, D.; Li, T.; Yang, X.; Jin, X.; Mahmood, Q.; Tian, S.; Li, J. Effect of Pb toxicity on leaf growth, physiology and ultrastructure in the two ecotypes of Elsholtzia argyi. J. Hazard. Mater. 2008, 154, 914–926. [Google Scholar] [CrossRef] [PubMed]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Sairam, R.K.; Rao, K.V.; Srivastava, G.C. Differential response of wheat genotypes to long term salinity stress in relation to oxidative stress, antioxidant activity and osmolyte concentration. Plant Sci. 2002, 163, 1037–1046. [Google Scholar] [CrossRef]
- Dhindsa, R.S.; Plumb-Dhindsa, P.; Thorpe, T.A. Leaf senescence: Correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J. Exp. Bot. 1981, 32, 93–101. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar]
- Hemeda, H.M.; Klein, B.P. Effects of naturally occurring antioxidants on peroxidase activity of vegetable extracts. J. Food Sci. 1990, 55, 184–185. [Google Scholar] [CrossRef]
- Steel, R.; Torrie, J.; Dickey, D. Principles and Procedures of Statistics: A Biometrical Approach; McGraw-Hill: New York, NY, USA, 1997. [Google Scholar]
- Shabir, R.; Abbas, G.; Saqib, M.; Shahid, M.; Shah, G.M.; Akram, M.; Niazi, N.K.; Naeem, M.A.; Hussain, M.; Ashraf, F. Cadmium tolerance and phytoremediation potential of acacia (Acacia nilotica L.) under salinity stress. Int. J. Phytorem. 2018, 20, 739–746. [Google Scholar] [CrossRef]
- Gu, H.; Qiu, H.; Tian, T.; Zhan, S.; Deng, T.; Chaney, R.L.; Wang, S.; Tang, Y.; Morel, J.; Qiu, R. Mitigation effects of silicon rich amendments on heavy metal accumulation in rice (Oryza sativa L.) planted on multi-metal contaminated acidic soil. Chemosphere 2011, 83, 1234–1240. [Google Scholar] [CrossRef]
- Chen, R.; Zhang, C.; Zhao, Y.; Huang, Y.; Liu, Z.; Chen, R. Foliar application with nano-silicon reduced cadmium accumulation in grains by inhibiting cadmium translocation in rice plants. Environ. Sci. Pollut. Res. 2018, 25, 2361–2368. [Google Scholar] [CrossRef]
- Huang, F.; Wen, X.; Cai, Y.; Cai, K. Silicon-Mediated enhancement of heavy metal tolerance in rice at different growth stages. Int. J. Environ. Res. Public Health 2018, 15, 2193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ke, Y.C.; Cao, M.; Yang, X.F.; Huang, Z.; Song, L.Q.; Liu, Y. Effects of spraying different concentrations of organic silicon fertilizer on yield and quality of tropical melon. J. South. Agric. 2015, 46, 53–57. [Google Scholar] [CrossRef]
- Ma, J.; Cai, H.; He, C.; Zhang, W.; Wang, L. A hemicellulose-bound form of silicon inhibits cadmium ion uptake in rice (Oryza sativa) cells. New Phytol. 2015, 206, 1063–1074. [Google Scholar] [CrossRef]
- Li, X.; Long, J.; Peng, P.; Chen, Q.; Dong, X.; Jiang, K. Evaluation of calcium oxide of quicklime and SieCaeMg fertilizer for remediation of Cd uptake in rice plants and Cd mobilization in two typical Cd-polluted paddy soils. Int. J. Environ. Res. 2018, 12, 877–885. [Google Scholar] [CrossRef]
- Zhang, S.; Ni, X.; Arif, M.; Zheng, J.; Stubbs, A.; Li, C. NaCl improved Cd tolerance of the euhalophyte Suaeda glauca but not the recretohalophyte Limonium aureum. Plant Soil. 2020, 449, 303–318. [Google Scholar] [CrossRef]
- Gallego, S.M.; Pena, L.B.; Barcia, R.A.; Azpilicueta, C.E.; Iannone, M.F.; Rosales, E.P.; Zawoznik, M.S.; Groppa, M.D.; Benavides, M.P. Unravelling cadmium toxicity and tolerance in plants: Insight into regulatory mechanisms. Environ. Exp. Bot. 2012, 83, 33–46. [Google Scholar] [CrossRef]
- Lu, Y.G.; Ma, J.; Teng, Y.; He, J.Y.; Christie, P.; Zhu, L.J.; Ren, W.J.; Zhang, M.Y.; Deng, S.P. Effect of silicon on growth, physiology, and cadmium translocation of tobacco (Nicotiana tabacum L.) in cadmium-contaminated soil. Pedosph. Int. J. 2018, 28, 680–689. [Google Scholar] [CrossRef]
- Song, Z.Z.; Duan, C.L.; Guo, S.L.; Yang, Y.; Feng, Y.F.; Ma, R.J.; Yu, M.L. Potassium contributes to zinc stress tolerance in peach (Prunus persica) seedlings by enhancing photosynthesis and the antioxidant defense system. Genet. Mol. Res. 2015, 14, 8338–8835. [Google Scholar] [CrossRef]
- Shahid, M.; Farooq, A.B.U.; Rabbani, F.; Khalid, S.; Dumat, C. Risk assessment and biophysiochemical responses of spinach to foliar application of lead oxide nanoparticles: A multivariate analysis. Chemosphere 2019, 245, 125605. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant. Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pourrut, B.; Shahid, M.; Dumat, C.; Winterton, P.; Pinelli, E. Lead uptake, toxicity, and detoxification in plants. Rev. Environ. Contam. Toxicol. 2011, 213, 113–136. [Google Scholar] [PubMed] [Green Version]
- Li, X.; Zhang, X.; Wang, X.; Yang, X.; Cui, Z. Bioaugmentation-assisted phytoremediation of lead and salinity co-contaminated soil by Suaeda salsa and Trichoderma asperellum. Chemosphere 2019, 224, 716–725. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.E.; Harada, E.; Wada, M.; Tsuboi, H.; Mortia, Y.; Kusano, T.; Sano, H. Detoxification of cadmium in tobacco plant: Formation and active excretion of crystals containing cadmium and calcium through trichomes. Planta 2001, 213, 45–50. [Google Scholar] [CrossRef]
- Song, W.Y.; Martinoia, E.; Lee, J.; Kim, D.; Kim, D.-Y.; Vogt, E.; Shim, D.; Choi, K.S.; Hwang, I.; Lee, Y. A novel family of cys-rich membrane proteins mediates cadmium resistance in Arabidopsis. Plant Physiol. 2004, 135, 1027–1039. [Google Scholar] [CrossRef] [Green Version]
- Howladar, S.M.; Al-robai, S.A.; Al-zahrani, F.S.; Howladar, M.M.; Aldhebiani, A.Y. Silicon and its application method effects on modulation of cadmium stress responses in Triticum aestivum (L.) through improving the antioxidative defense system and polyamine gene expression. Ecotoxicol. Environ. Saf. 2018, 159, 143–152. [Google Scholar] [CrossRef]
- Kim, Y.H.; Khan, A.L.; Waqas, M.; Lee, I.J. Silicon regulates antioxidant activities of crop plants under abiotic-induced oxidative stress: A review. Front. Plant Sci. 2017, 8, 510. [Google Scholar] [CrossRef] [Green Version]
- Emamverdian, A.; Ding, Y.; Xie, Y.; Sangari, S. Silicon mechanisms to ameliorate heavy metal stress in plants. BioMed Res. Int. 2018, 2018, 8492898. [Google Scholar] [CrossRef] [Green Version]
- Imtiaz, M. Silicon occurrence, uptake, transport and mechanisms of heavy metals, minerals and salinity en hanced tolerance in plants with future prospects: A review. J. Environ. Manag. 2016, 183, 521–529. [Google Scholar] [CrossRef] [Green Version]
- Souri, Z.; Karimi, N.; de Oliveira, L.M. Antioxidant enzymes responses in shoots of arsenic hyperaccumulator, Isatis cappadocica Desv., under interaction of arsenate and phosphate. Environ. Technol. 2018, 39, 1316–1327. [Google Scholar] [CrossRef]
- Abbaspour, A.; Kalbasi, M.; Hajrasuliha, S.; Fotovat, A. Effect of organic matter and salinity on ethylenediaminetetraacetic acid–extractable and solution species of cadmium and lead in three agricultural soils. J. Commun. Soil. Sci. Plant Anal. 2008, 39, 983–1005. [Google Scholar] [CrossRef]


| Treatment | Shoot Length (cm) | Root Length (cm) | Shoot Dry Weight (g plant−1) | Root Dry Weight (g plant−1) |
|---|---|---|---|---|
| Control | 20.3 ± 0.5 a | 19 ± 0.6 a | 1.8 ± 0.05 a | 0.40 ± 0.01 a |
| Cd | 12.1 ± 0.2 d | 12 ± 0.2 d | 1.05 ± 0.02 d | 0.24 ± 0.007 d |
| Pb | 14.2 ± 0.5 c | 13 ± 0.5 cd | 1.3 ± 0.03 c | 0.27 ± 0.013 cd |
| Cd + Pb | 7.5 ± 0.2 e | 6.0 ± 0.2 f | 0.6 ± 0.04 e | 0.13 ± 0.01 f |
| Cd + Pb + Si | 12.2 ± 0.2 d | 10 ± 0.5 e | 1.0 ± 0.03 d | 0.21 ± 0.008 e |
| Cd + Pb + K | 15.0 ± 0.4 c | 14 ± 0.4 c | 1.25 ± 0.04 c | 0.29 ± 0.011 c |
| Cd + Pb + Si + K | 17.1 ± 0.6 b | 16 ± 0.6 b | 1.5 ± 0.05 b | 0.33 ± 0.01 b |
| Treatment | Chl a (µg g−1 FW) | Chl b (µg g−1 FW) | Total Chl (µg g−1 FW) | Stomatal Conductance (mmol m−2 s−1) |
|---|---|---|---|---|
| Control | 180 ± 5 a | 250 ± 5 a | 430 ± 10 a | 480 ± 10 a |
| Cd | 105 ± 4 d | 125 ± 8 d | 230 ± 12 d | 270 ± 12 d |
| Pb | 130 ± 5 c | 190 ± 8 c | 320 ± 8 c | 350 ± 8 c |
| Cd + Pb | 60 ± 4 e | 94 ± 8 e | 154 ± 15 e | 145 ± 15 f |
| Cd + Pb + Si | 92 ± 6 de | 110 ± 6 d | 202 ± 12 de | 230 ± 12 e |
| Cd + Pb + K | 120 ± 5 c | 188 ± 5 c | 305 ± 10 c | 300 ± 10 cd |
| Cd + Pb + Si + K | 155 ± 4 b | 215 ± 4 b | 370 ± 12 b | 410 ± 12 b |
| Treatment | Pb | Cd | |||
|---|---|---|---|---|---|
| BCF | TF | BCF | TF | TI | |
| Cd | - | - | 2.23 ± 0.25 b | 0.53 ± 0.05 b | 58 ± 2.1 c |
| Pb | 2.70 ± 0.3 a | 0.15 ± 0.005 a | - | - | 72 ± 3.0 b |
| Cd + Pb | 2.31 ± 0.2 b | 0.14 ± 0.006 b | 3.35 ± 0.2 a | 0.61 ± 0.02 a | 33 ± 2.5 d |
| Cd + Pb + Si | 1.73 ± 0.1 d | 0.13 ± 0.005 b | 1.56 ± 0.2 c | 0.46 ± 0.03 d | 55 ± 1.9 c |
| Cd + Pb + K | 2.02 ± 0.2 b | 0.13 ± 0.003 b | 2.14 ± 0.15 b | 0.52 ± 0.02 bc | 69 ± 2.0 b |
| Cd + Pb + Si + K | 1.25 ± 0.1 e | 0.11 ± 0.003 c | 1.16 ± 0.1 d | 0.43 ± 0.01 e | 83 ± 2.1 a |
| Variables | RDW | SDW | Chla | Chlb | TChl | Cond | SOD | CAT | POD | APX | H2O2 | TBARS | MSI | RCd | SCd | SK | RK | SPb | RPb | RSi |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SDW | 0.9925 | |||||||||||||||||||
| Chla | 0.9553 | 0.9585 | ||||||||||||||||||
| Chlb | 0.9873 | 0.9950 | 0.9652 | |||||||||||||||||
| TChl | 0.9761 | 0.9820 | 0.9935 | 0.9887 | ||||||||||||||||
| Cond | 0.9774 | 0.9904 | 0.9614 | 0.9983 | 0.9862 | |||||||||||||||
| SOD | 0.0670 | 0.0421 | 0.1005 | 0.0547 | 0.0786 | 0.0270 | ||||||||||||||
| CAT | −0.0963 | −0.1134 | −0.0495 | −0.1002 | −0.0741 | −0.1250 | 0.9824 | |||||||||||||
| POD | −0.1478 | −0.1791 | −0.1025 | −0.1655 | −0.1338 | −0.1930 | 0.9730 | 0.9841 | ||||||||||||
| APX | −0.2400 | −0.2675 | −0.2084 | −0.2582 | −0.2348 | −0.2851 | 0.9501 | 0.9793 | 0.9923 | |||||||||||
| H2O2 | −0.9019 | −0.9244 | −0.8888 | −0.9322 | −0.9168 | −0.9451 | 0.2903 | 0.4321 | 0.4873 | 0.5716 | ||||||||||
| TBARS | −0.9260 | −0.9490 | −0.8821 | −0.9476 | −0.9191 | −0.9565 | 0.2495 | 0.3926 | 0.4578 | 0.5345 | 0.9900 | |||||||||
| MSI | 0.9471 | 0.9644 | 0.9205 | 0.9808 | 0.9555 | 0.9875 | 0.0282 | −0.1242 | −0.1874 | −0.2768 | −0.9402 | −0.9490 | ||||||||
| RCd | −0.7228 | −0.7926 | −0.7572 | −0.7854 | −0.7790 | −0.8099 | 0.3429 | 0.4350 | 0.5242 | 0.5800 | 0.8926 | 0.8899 | −0.8010 | |||||||
| SCd | −0.7673 | −0.8348 | −0.7714 | −0.8224 | −0.8023 | −0.8422 | 0.2256 | 0.3295 | 0.4203 | 0.4744 | 0.8843 | 0.9006 | −0.8409 | 0.9839 | ||||||
| SK | 0.9768 | 0.9820 | 0.9831 | 0.9925 | 0.9957 | 0.9919 | 0.0683 | −0.0899 | −0.1429 | −0.2444 | −0.9296 | −0.9302 | 0.9746 | −0.7770 | −0.8051 | |||||
| RK | 0.9704 | 0.9779 | 0.9640 | 0.9932 | 0.9858 | 0.9950 | 0.0326 | −0.1176 | −0.1835 | −0.2797 | −0.9385 | −0.9419 | 0.9820 | −0.7758 | −0.7991 | 0.9912 | ||||
| SPb | −0.5084 | −0.4574 | −0.2852 | −0.4647 | −0.3642 | −0.4540 | 0.2144 | 0.3111 | 0.3177 | 0.3377 | 0.4667 | 0.5092 | −0.4830 | 0.1254 | 0.1637 | −0.4038 | −0.4751 | |||
| RPb | −0.4906 | −0.4420 | −0.2677 | −0.4465 | −0.3463 | −0.4382 | 0.3096 | 0.4045 | 0.4063 | 0.4239 | 0.4832 | 0.5209 | −0.4664 | 0.1543 | 0.1815 | −0.3867 | −0.4568 | 0.9947 | ||
| RSi | 0.5461 | 0.5556 | 0.4590 | 0.5339 | 0.4928 | 0.5101 | 0.7044 | 0.6313 | 0.5450 | 0.5230 | −0.2378 | −0.3385 | 0.5010 | −0.2257 | −0.3733 | 0.4831 | 0.4759 | −0.1747 | −0.0983 | |
| SSi | 0.5085 | 0.5206 | 0.4278 | 0.5038 | 0.4622 | 0.4819 | 0.7301 | 0.6647 | 0.5760 | 0.5564 | −0.2044 | −0.3030 | 0.4789 | −0.1985 | −0.3460 | 0.4539 | 0.4504 | −0.1543 | −0.0748 | 0.9975 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alharby, H.F.; Al-Zahrani, H.S.; Abbas, G. Potassium and Silicon Synergistically Increase Cadmium and Lead Tolerance and Phytostabilization by Quinoa through Modulation of Physiological and Biochemical Attributes. Toxics 2022, 10, 169. https://doi.org/10.3390/toxics10040169
Alharby HF, Al-Zahrani HS, Abbas G. Potassium and Silicon Synergistically Increase Cadmium and Lead Tolerance and Phytostabilization by Quinoa through Modulation of Physiological and Biochemical Attributes. Toxics. 2022; 10(4):169. https://doi.org/10.3390/toxics10040169
Chicago/Turabian StyleAlharby, Hesham F., Hassan S. Al-Zahrani, and Ghulam Abbas. 2022. "Potassium and Silicon Synergistically Increase Cadmium and Lead Tolerance and Phytostabilization by Quinoa through Modulation of Physiological and Biochemical Attributes" Toxics 10, no. 4: 169. https://doi.org/10.3390/toxics10040169
APA StyleAlharby, H. F., Al-Zahrani, H. S., & Abbas, G. (2022). Potassium and Silicon Synergistically Increase Cadmium and Lead Tolerance and Phytostabilization by Quinoa through Modulation of Physiological and Biochemical Attributes. Toxics, 10(4), 169. https://doi.org/10.3390/toxics10040169






