The Toxicological Assessment of Content and Exposure of Heavy Metals (Pb and Cd) in Traditional Herbal Medicinal Products with Marshmallow Root (Althaea officinalis L., radix) from Polish Pharmacies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
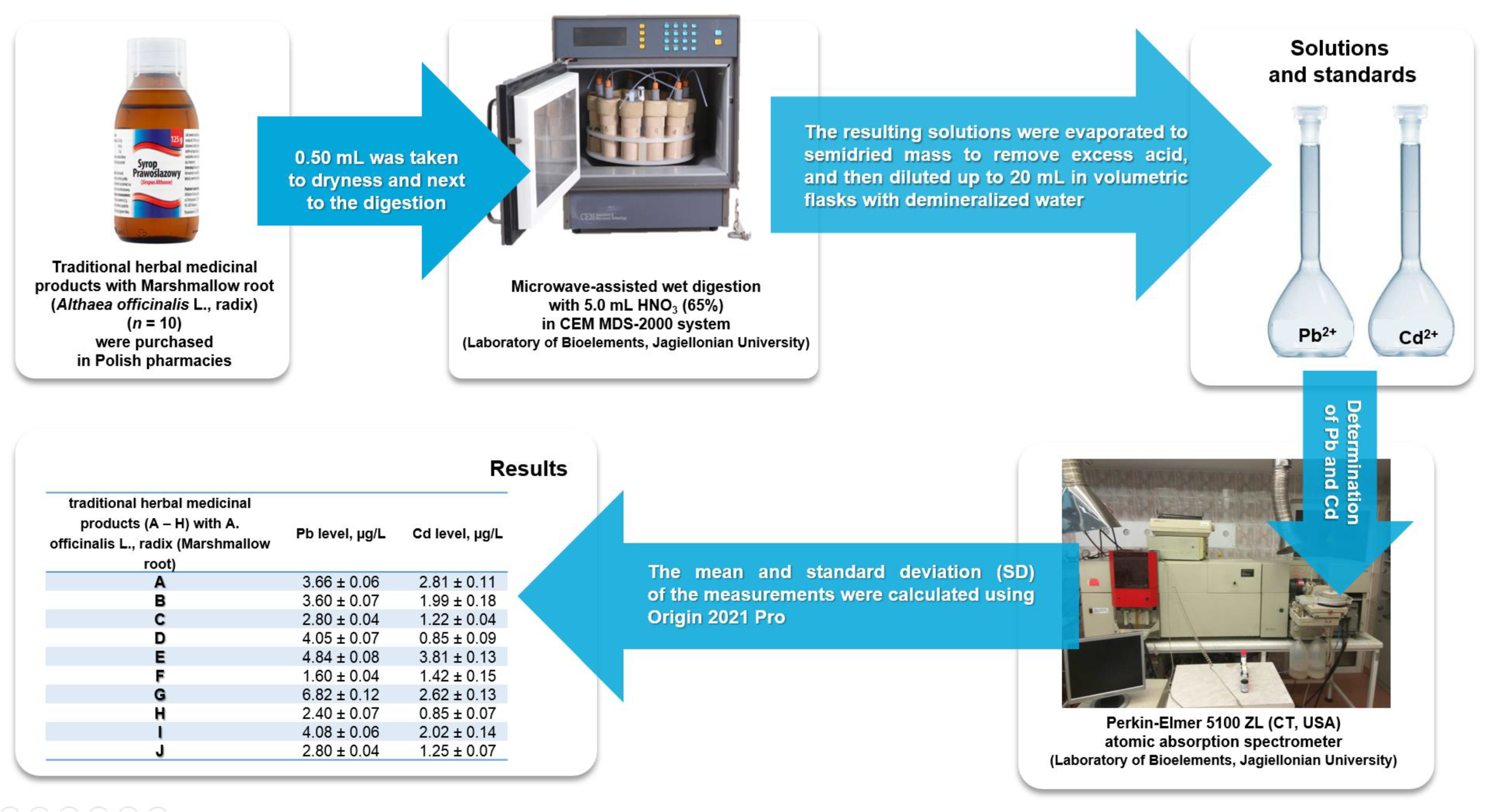
2.2. Samples
2.3. Determination of Pb and Cd
2.4. Data Analysis
2.5. Toxicological Assessment of Heavy Metals Impurities in Investigated Pharmaceutical Products
3. Results
3.1. The Lead and Cadmium Impurities (Raw Results) in Traditional Herbal Medicinal Products with A. officinalis L., radix (Marshmallow Root) in Polish Pharmacies
3.2. The Assessment of Exposure of Pb and Cd Impurities after Application of THMPs with A. officinalis L., radix (Marshmallow Root) Available in Polish Pharmacies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- ICH Guideline Q3D (R1) on Elemental Impurities. 28 March 2019EMA/CHMP/ICH/353369/2013 Committee for Human Medicinal Products. Available online: https://www.ema.europa.eu/en/ich-q3d-elemental-impurities (accessed on 7 April 2022).
- EMA/HMPC/436679/2015 Committee on Herbal Medicinal Products (HMPC) European Union Herbal Monograph on Althaea officinalis L., radix. Available online: https://www.ema.europa.eu/en/documents/herbal-monograph/draft-european-union-herbal-monograph-althaea-officinalis-l-radix_en.pdf (accessed on 7 April 2022).
- EMA/HMPC/436680/2015 Committee on Herbal Medicinal Products (HMPC) Assessment report on Althaea officinalis L., radix. Available online: https://www.ema.europa.eu/en/documents/herbal-report/final-assessment-report-althaea-officinalis-l-radix_en.pdf (accessed on 7 April 2022).
- Farmakopea Polska, 6th ed.; Office for Registration of Medicinal Products, Medical Devices and Biocidal Products: Warszawa, Poland, 2002; p. 913.
- Blumenthal, M.; Busse, W.R.; Goldberg, A.; Gruenwald, J. The Complete German Commission E Monographs, 1st ed.; The American Botanical Council: Austin, TX, USA, 2000; p. 167. [Google Scholar]
- Blumenthal, M.; Goldberg, A.; Brinckmann, J. Expanded Commission E Monographs; The American Botanical Council: Austin, TX, USA, 2000; pp. 244–248. [Google Scholar]
- Dorsch, W.; Loew, D.; Meyer-Buchtela, E.; Schilcher, H. Kinderdosierungen von Phytopharmaka; Kooperation Phytopharmaka: Bonn, Germany, 2002; pp. 30–31. [Google Scholar]
- Deutscher Arznimittel-Codex 2004. E-020 Eibischsirup. Available online: https://www.ema.europa.eu/en/documents/herbal-references/final-list-references-supporting-assessment-althaea-officinalis-l-radix_en.pdf (accessed on 23 March 2022).
- Abadin, H.; Ashizawa, A.; Llados, F.; Stevens, Y.W. Toxicological Profile of Lead; Agency for Toxic Substances and Disease Registry, Public Health Service, U.S. Department of Health and Human Services: Atlanta, GA, USA, 2000. Available online: https://www.atsdr.cdc.gov/toxprofiles/tp13.pdf (accessed on 7 April 2022).
- Jurowski, K.; Krośniak, M.; Fołta, M.; Cole, M.; Piekoszewski, W. The toxicological analysis of Pb and Cd by ETAAS in local anaesthetics for teething (teething gels) based on herbs available in Polish pharmacies. J. Trace Elem. Biol. Med. 2019, 52, 18–21. [Google Scholar] [CrossRef] [Green Version]
- Fenga, C.; Gangemi, S.; Di Salvatore, V.; Falzone, L.; Libra, M. Immunological effects of occupational exposure to lead. Mol. Med. Rep. 2017, 15, 3355–3360. [Google Scholar] [CrossRef] [Green Version]
- Assi, M.A.; Hezmee, M.N.M.; Abd Wahid Haron, M.Y.M.; Sabri, M.A.R. The detrimental effects of lead on human and animal health. Vet. World 2016, 9, 660. [Google Scholar] [CrossRef]
- LeBrón, A.M.; Torres, I.R.; Valencia, E.; Dominguez, M.L.; Garcia-Sanchez, D.G.; Logue, M.D.; Wu, J. The state of public health lead policies: Implications for urban health inequities and recommendations for health equity. Int. J. Environ. Res. 2019, 16, 1064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tjell, J.; Hovmand, E.; Mosbaek, H. Atmospheric Lead Pollution of Grass in a Background Area in Denmark. Nature 1979, 280, 425–426. [Google Scholar] [CrossRef]
- Zheljazkov, V.; Jeliazkova, E.; Kovacheva, N.; Dzhurmanski, A. Metal uptake by medicinal plant species grown in soils contaminated by a smelter. Environ. Exp. Bot. 2008, 64, 207–216. [Google Scholar] [CrossRef]
- Jurowski, K.; Krośniak, M.; Fołta, M.; Cole, M.; Piekoszewski, W. The toxicological analysis of lead and cadmium in prescription food for special medical purposes and modified milk products for new-borns and infants available in Polish pharmacies. J. Trace Elem. Med. Biol. 2019, 51, 73–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jurowski, K.; Fołta, M.; Tatar, B.; Berkoz, M.; Krośniak, M. The toxicological risk assessment of lead and cadmium in Valeriana officinalis L., radix (Valerian root) as Herbal Medicinal Product for the Relief of Mild Nervous Tension and Sleep Disorders Available in Polish Pharmacies. Biol. Trace Elem. Res. 2022, 200, 904–909. [Google Scholar] [CrossRef] [PubMed]
- Jurowski, K.; Krośniak, M.; Fołta, M.; Tatar, B.; Cole, M.; Piekoszewski, W. Safety assessment of the trace element impurities Ni and Cr in pharmaceutical herbal products for teething from Polish pharmacies. Biol. Trace Elem. Res. 2019, 191, 517–521. [Google Scholar] [CrossRef] [PubMed]
- Jurowski, K.; Krośniak, M.; Fołta, M.; Cole, M.; Piekoszewski, W. The toxicological analysis of Cu, Mn and Zn as elemental impurities in pharmaceutical herbal products for teething available in pharmacies in Poland. J. Trace Elem. Med. Biol. 2019, 53, 109–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jurowski, K.; Fołta, M.; Tatar, B.; Berkoz, M.; Krośniak, M. Ni and Cr impurities profile in Valeriana officinalis L., radix-based herbal medicinal product available in polish pharmacies due to ICH Q3D guideline. Regul. Toxicol. Pharmacol. 2021, 123, 104945. [Google Scholar] [CrossRef] [PubMed]
- Jurowski, K.; Fołta, M.; Tatar, B.; Berkoz, M.; Krośniak, M. The toxicological risk assessment of Cu, Mn, and Zn as essential elemental impurities in herbal medicinal products with valerian root (Valeriana officinalis L., radix) available in Polish Pharmacies. Biol. Trace Elem. Res. 2021, 200, 1949–1955. [Google Scholar] [CrossRef] [PubMed]
- Azizov, U.M.; Mirakilova, D.B.; Umarova, N.T.; Salikhov, S.A.; Rakhimov, D.A.; Mezhlumyan, L.G. Chemical composition of dry extracts from Alcea rosea. Chem. Nat. Compd. 2007, 43, 508–511. [Google Scholar] [CrossRef]
- Al-Snafi, A.E. The pharmaceutical importance of Althaea officinalis and Althaea rosea: A review. Int. J. Pharm. Tech. Res. 2013, 5, 1378–1385. [Google Scholar]
- WHO. WHO Guidelines for Assessing Quality of Herbal Medicines with Reference to Contaminants and Residues; World Health Organization: Geneva, Switzerland, 2007; Available online: https://apps.who.int/iris/handle/10665/43510 (accessed on 7 April 2022).
- Hogan, K.; Marcus, A.; Smith, R.; White, P. Integrated exposure uptake biokineticmodel for lead in children: Empirical comparisons with epidemiologic data. Environ. Health Perspect. 1998, 106, 1557–1567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buchet, J.; Lauwerys, R. Renal effects of cadmium body burden of the general population. Lancet 1990, 336, 699–702. [Google Scholar] [CrossRef]

| Descriptor | Sample | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | H | I | J | |
| Amount of API in 10 mL of syrup | 0.33 | 0.27 | 0.29 | 0.21 | 0.28 | 0.31 | 0.30 | 0.25 | 0.21 | 0.29 |
| Posology | Orally: 15 mL, 3 times daily | Orally: 10–15 mL, 3 times daily | Orally: 15 mL, 3 times daily | Orally: 15 mL, 3 times daily | Orally: 15 mL, 3–4 times daily | Orally: 15 mL, 3–4 times daily | Orally: 15 mL, 3 times daily | Orally: 5 mL, 5 times daily | Orally: 15 mL, 3 times daily | Orally: 10–15 mL, 3 times daily |
| Density, g/mL | 1.29 | 1.31 | 1.26 | 1.28 | 1.32 | 1.30 | 1.295 | 1.33 | 1.28 | 1.26 |
| Lot number | 20920 | 02AF0620 | 01AF0820 | 01AF0920 | 21220 | 61020 | 10420 | 454201 | 01AF0920 | 01AF0820 |
| License | IL-0692/LN | 12180 | Not applicable | Not applicable | IL-5954/LN | IL-4746/LN | 8147 | Not applicable | Not applicable | Not applicable |
| Classification | OTC | OTC | Diet supplement | Diet supplement | OTC | OTC | OTC | Medical product | Diet supplement | Diet supplement |
| Pb | Step | Temperature [°C] | Ramp [s] | Hold [s] | Gas Flow [mL/min] |
| 1 | 120 | 1 | 30 | 250 | |
| 2 | 950 | 10 | 20 | 250 | |
| 3 | 1450 | 0 | 5 | 0 | |
| 5 | 2400 | 1 | 2 | 250 | |
| Cd | Step | Temperature [°C] | Ramp [s] | Hold [s] | Gas Flow [mL/min] |
| 1 | 120 | 10 | 25 | 250 | |
| 2 | 300 | 5 | 15 | 250 | |
| 3 | 1600 | 0 | 3 | 0 | |
| 5 | 2400 | 1 | 2 | 250 |
| Operating Parameters | Pb | Cd |
|---|---|---|
| Wavelength, nm | 283.3 | 228.8 |
| Lamp current, mA | 8 | 5 |
| Slit width, nm | 0.7 | 0.7 |
| Optimum working range, µg/kg | 1.0–10.0 | 0.02–0.20 |
| Traditional Herbal Medicinal Products (A–H) with A. officinalis L., radix (Marshmallow Root) | Pb Level, µg/L | Cd Level, µg/L |
|---|---|---|
| A | 3.66 ± 0.06 | 2.81 ± 0.11 |
| B | 3.60 ± 0.07 | 1.99 ± 0.18 |
| C | 2.80 ± 0.04 | 1.22 ± 0.04 |
| D | 4.05 ± 0.07 | 0.85 ± 0.09 |
| E | 4.84 ± 0.08 | 3.81 ± 0.13 |
| F | 1.60 ± 0.04 | 1.42 ± 0.15 |
| G | 6.82 ± 0.12 | 2.62 ± 0.13 |
| H | 2.40 ± 0.07 | 0.85 ± 0.07 |
| I | 4.08 ± 0.06 | 2.02 ± 0.14 |
| J | 2.80 ± 0.04 | 1.25 ± 0.07 |
| Sample | Estimated Oral Exposure of Pb Based on Posology | |
|---|---|---|
| Single Administration, mg 10−6/single Dose | Maximum Daily Dose, mg 10−6/day | |
| A | 55.874 | 167.71 |
| B | 53.514 | 160.53 |
| C | 41.223 | 123.66 |
| D | 61.844 | 185.52 |
| E | 70.144 | 280.56 |
| F | 25.113 | 100.44 |
| G | 101.43 | 304.29 |
| H | 12.186 | 60.948 |
| I | 61.844 | 185.52 |
| J | 41.223 | 123.66 |
| Sample | Estimated Oral Exposure of Cd Based on Posology | |
| Single Administration, mg 10−6/single Dose | Maximum Daily Dose, mg 10−6/day | |
| A | 42.150 | 126.45 |
| B | 29.850 | 89.55 |
| C | 18.299 | 54.90 |
| D | 12.750 | 38.25 |
| E | 57.151 | 228.60 |
| F | 21.300 | 85.20 |
| G | 39.310 | 117.90 |
| H | 4.251 | 21.25 |
| I | 10.101 | 50.50 |
| J | 6.250 | 31.25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jurowski, K.; Krośniak, M. The Toxicological Assessment of Content and Exposure of Heavy Metals (Pb and Cd) in Traditional Herbal Medicinal Products with Marshmallow Root (Althaea officinalis L., radix) from Polish Pharmacies. Toxics 2022, 10, 188. https://doi.org/10.3390/toxics10040188
Jurowski K, Krośniak M. The Toxicological Assessment of Content and Exposure of Heavy Metals (Pb and Cd) in Traditional Herbal Medicinal Products with Marshmallow Root (Althaea officinalis L., radix) from Polish Pharmacies. Toxics. 2022; 10(4):188. https://doi.org/10.3390/toxics10040188
Chicago/Turabian StyleJurowski, Kamil, and Mirosław Krośniak. 2022. "The Toxicological Assessment of Content and Exposure of Heavy Metals (Pb and Cd) in Traditional Herbal Medicinal Products with Marshmallow Root (Althaea officinalis L., radix) from Polish Pharmacies" Toxics 10, no. 4: 188. https://doi.org/10.3390/toxics10040188





