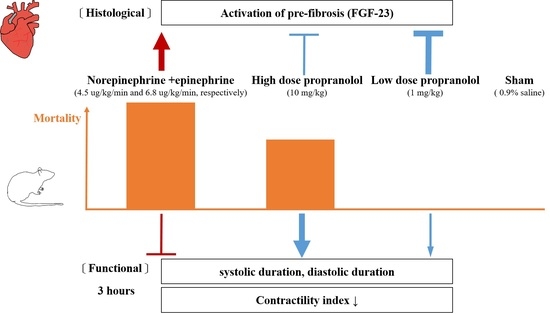
Low-Dose Propranolol Prevents Functional Decline in Catecholamine-Induced Acute Heart Failure in Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Hemodynamic Data Acquisition
2.3. Serum Analysis of Cardiac Markers
2.4. Examination of Cardiac Congestion and Lung Edema
2.5. Histological Studies
2.6. Statistical Analysis
3. Results
3.1. Overall Survival, Organ-to-Body Weight Ratios and Serum Cardiac Biomarkers
3.2. Hemodynamic Changes of the LV
3.3. Hemodynamic Changes of the RV
3.4. Acute Cardiac Injury in Histopathology
3.5. Lung Injury Mediated through Apoptosis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferreira, J.A.; Bissell, B.D. Misdirected Sympathy: The Role of Sympatholysis in Sepsis and Septic Shock. J. Intensive Care Med. 2018, 33, 74–86. [Google Scholar] [CrossRef] [PubMed]
- Kaye, D.M.; Lefkovits, J.; Cox, H.; Lambert, G.; Jennings, G.; Turner, A.; Esler, M.D. Regional epinephrine kinetics in human heart failure: Evidence for extra-adrenal, nonneural release. Am. J. Physiol. 1995, 269 Pt 2, H182–H188. [Google Scholar] [CrossRef] [PubMed]
- Muders, F.; Friedrich, E.; Luchner, A.; Pfeifer, M.; Ickenstein, G.; Hamelbeck, B.; Riegger, G.A.; Elsner, D. Hemodynamic changes and neurohumoral regulation during development of congestive heart failure in a model of epinephrine-induced cardiomyopathy in conscious rabbits. J. Card. Fail. 1999, 5, 109–116. [Google Scholar] [CrossRef]
- Barth, W.; Deten, A.; Bauer, M.; Reinohs, M.; Leicht, M.; Zimmer, H.G. Differential remodeling of the left and right heart after norepinephrine treatment in rats: Studies on cytokines and collagen. J. Mol. Cell. Cardiol. 2000, 32, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Leifheit-Nestler, M.; Kirchhoff, F.; Nespor, J.; Richter, B.; Soetje, B.; Klintschar, M.; Heineke, J.; Haffner, D. Fibroblast growth factor 23 is induced by an activated renin–angiotensin–aldosterone system in cardiac myocytes and promotes the pro-fibrotic crosstalk between cardiac myocytes and fibroblasts. Nephrol. Dial. Transplant. 2018, 33, 1722–1734. [Google Scholar] [CrossRef] [PubMed]
- Grabner, A.; Schramm, K.; Silswal, N.; Hendrix, M.; Yanucil, C.; Czaya, B.; Singh, S.; Wolf, M.; Hermann, S.; Stypmann, J.; et al. FGF23/FGFR4-mediated left ventricular hypertrophy is reversible. Sci. Rep. 2017, 7, 1993. [Google Scholar] [CrossRef]
- Kanwar, M.; Irvin, C.B.; Frank, J.J.; Weber, K.; Rosman, H. Confusion About Epinephrine Dosing Leading to Iatrogenic Overdose: A Life-Threatening Problem With a Potential Solution. Ann. Emerg. Med. 2010, 55, 341–344. [Google Scholar] [CrossRef]
- Tarvasmäki, T.; Lassus, J.; Varpula, M.; Sionis, A.; Sund, R.; Køber, L.; Spinar, J.; Parissis, J.; Banaszewski, M.; Silva Cardoso, J.; et al. Current real-life use of vasopressors and inotropes in cardiogenic shock—Adrenaline use is associated with excess organ injury and mortality. Crit. Care 2016, 20, 208. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.R.; Seth, M.S.; Soney, D.; Dai, H. Benefits of Beta-Blockade in Sepsis and Septic Shock: A Systematic Review. Clin. Drug Investig. 2019, 39, 429–440. [Google Scholar] [CrossRef]
- Lu, W.-H.; Chen, H.-H.; Chen, B.-H.; Lee, J.-C.; Lai, C.-C.; Li, C.-H.; Tseng, C.-J. Norepinephrine Leads to More Cardiopulmonary Toxicities than Epinephrine by Catecholamine Overdose in Rats. Toxics 2020, 8, 69. [Google Scholar] [CrossRef]
- Elenkov, I.J.; Wilder, R.L.; Chrousos, G.P.; Vizi, E.S. The sympathetic nerve--an integrative interface between two supersystems: The brain and the immune system. Pharmacol. Rev. 2000, 52, 595–638. [Google Scholar] [PubMed]
- Liu, W.S.; Chan, S.H.; Chang, H.T.; Li, G.C.; Tu, Y.T.; Tseng, H.H.; Fu, T.Y.; Chang, H.Y.; Liou, H.H.; Ger, L.P. Isocitrate dehydrogenase 1–snail axis dysfunction significantly correlates with breast cancer prognosis and regulates cell invasion ability. Breast Cancer Res. 2018, 20, 25. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, U.; Walzel, H.; Mylonas, I.; Papadopoulos, P.; Shabani, N.; Kuhn, C.; Schulze, S.; Friese, K.; Karsten, U.; Anz, D.; et al. The Human Endometrium Expresses the Glycoprotein Mucin-1 and Shows Positive Correlation for Thomsen-Friedenreich Epitope Expression and Galectin-1 Binding. J. Histochem. Cytochem. 2009, 57, 871–881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macala, K.F.; Khadaroo, R.G.; Panahi, S.; Gragasin, F.S.; Bourque, S.L. Low dose Intralipid resuscitation improves survival compared to ClinOleic in propranolol overdose in rats. PLoS ONE 2018, 13, e0202871. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Sinovas, A.; Sánchez, J.A.; Valls-Lacalle, L.; Consegal, M.; Ferreira-González, I. Connexins in the Heart: Regulation, Function and Involvement in Cardiac Disease. Int. J. Mol. Sci. 2021, 22, 4413. [Google Scholar] [CrossRef]
- Paur, H.; Wright, P.T.; Sikkel, M.B.; Tranter, M.H.; Mansfield, C.; O’gara, P.; Stuckey, D.J.; Nikolaev, V.O.; Diakonov, I.; Pannell, L.; et al. High Levels of Circulating Epinephrine Trigger Apical Cardiodepression in a β2-Adrenergic Receptor/Gi–Dependent Manner. Circulation 2012, 126, 697–706. [Google Scholar] [CrossRef] [Green Version]
- Downing, S.E.; Chen, V. Myocardial injury following endogenous catecholamine release in rabbits. J. Mol. Cell. Cardiol. 1985, 17, 377–387. [Google Scholar] [CrossRef]
- Izumi, Y.; Okatani, H.; Shiota, M.; Nakao, T.; Ise, R.; Kito, G.; Miura, K.; Iwao, H. Effects of metoprolol on epinephrine-induced takotsubo-like left ventricular dysfunction in non-human primates. Hypertens. Res. Off. J. Jpn. Soc. Hypertens. 2009, 32, 339–346. [Google Scholar] [CrossRef]
- Briest, W.; Hölzl, A.; Raßler, B.; Deten, A.; Leicht, M.; Baba, H.A.; Zimmer, H.-G. Cardiac remodeling after long term norepinephrine treatment in rats. Cardiovasc. Res. 2001, 52, 265–273. [Google Scholar] [CrossRef] [Green Version]
- Floras, J.S. Sympathetic nervous system activation in human heart failure: Clinical implications of an updated model. J. Am. Coll. Cardiol. 2009, 54, 375–385. [Google Scholar] [CrossRef] [Green Version]
- Dünser, M.W.; Hasibeder, W.R. Sympathetic overstimulation during critical illness: Adverse effects of adrenergic stress. J. Intensive Care Med. 2009, 24, 293–316. [Google Scholar] [CrossRef] [PubMed]
- Morelli, A.; Ertmer, C.; Westphal, M.; Rehberg, S.; Kampmeier, T.; Ligges, S.; Orecchioni, A.; D’Egidio, A.; D’Ippoliti, F.; Raffone, C.; et al. Effect of Heart Rate Control With Esmolol on Hemodynamic and Clinical Outcomes in Patients With Septic Shock: A Randomized Clinical Trial. JAMA 2013, 310, 1683. [Google Scholar] [CrossRef] [PubMed]
- Nies, A.S.; Shand, D.G. Clinical pharmacology of propranolol. Circulation 1975, 52, 6–15. [Google Scholar] [CrossRef] [Green Version]
- Macala, K.; Tabrizchi, R. The Effect of Fat Emulsion on Hemodynamics Following Treatment With Propranolol and Clonidine in Anesthetized Rats. Acad. Emerg. Med. 2014, 21, 1220–1225. [Google Scholar] [CrossRef] [PubMed]
- Crystal, G.J.; Pagel, P.S. Right Ventricular Perfusion: Physiology and Clinical Implications. Anesthesiology 2018, 128, 202–218. [Google Scholar] [CrossRef] [PubMed]
- Horinouchi, T.; Morishima, S.; Tanaka, T.; Suzuki, F.; Tanaka, Y.; Koike, K.; Muramatsu, I. Pharmacological evaluation of plasma membrane beta-adrenoceptors in rat hearts using the tissue segment binding method. Life Sci. 2006, 79, 941–948. [Google Scholar] [CrossRef] [PubMed]
- Salameh, A.; Frenzel, C.; Boldt, A.; Rassler, B.; Glawe, I.; Schulte, J.; Mühlberg, K.; Zimmer, H.G.; Pfeiffer, D.; Dhein, S. Subchronic alpha- and beta-adrenergic regulation of cardiac gap junction protein expression. FASEB J. 2006, 20, 365–367. [Google Scholar] [CrossRef]
- Salameh, A.; Blanke, K.; Dhein, S.; Janousek, J. Cardiac Gap Junction Channels Are Upregulated by Metoprolol: An Unexpected Effect of Beta-Blockers—Abstract—Pharmacology 2010, Vol. 85, No. 4—Karger Publishers. Pharmacology 2010, 85, 203–210. [Google Scholar] [CrossRef]
- Faul, C.; Amaral, A.P.; Oskouei, B.; Hu, M.C.; Sloan, A.; Isakova, T.; Gutiérrez, O.M.; Aguillon-Prada, R.; Lincoln, J.; Hare, J.M.; et al. FGF23 induces left ventricular hypertrophy. J. Clin. Investig. 2011, 121, 4393–4408. [Google Scholar] [CrossRef] [Green Version]
- Grabner, A.; Amaral, A.P.; Schramm, K.; Singh, S.; Sloan, A.; Yanucil, C.; Li, J.; Shehadeh, L.A.; Hare, J.M.; David, V.; et al. Activation of Cardiac Fibroblast Growth Factor Receptor 4 Causes Left Ventricular Hypertrophy. Cell Metab. 2015, 22, 1020–1032. [Google Scholar] [CrossRef] [Green Version]
- Moe, S.M.; Chertow, G.M.; Parfrey, P.S.; Kubo, Y.; Block, G.A.; Correa-Rotter, R.; Drüeke, T.B.; Herzog, C.A.; London, G.M.; Mahaffey, K.W.; et al. Cinacalcet, Fibroblast Growth Factor-23, and Cardiovascular Disease in Hemodialysis. Circulation 2015, 132, 27–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrukhova, O.; Slavic, S.; Odörfer, K.I.; Erben, R.G. Experimental Myocardial Infarction Upregulates Circulating Fibroblast Growth Factor-23. J. Bone Miner. Res. 2015, 30, 1831–1839. [Google Scholar] [CrossRef] [PubMed]
- Hao, H.; Li, X.; Li, Q.; Lin, H.; Chen, Z.; Xie, J.; Xuan, W.; Liao, W.; Bin, J.; Huang, X.; et al. FGF23 promotes myocardial fibrosis in mice through activation of β-catenin. Oncotarget 2016, 7, 64649–64664. [Google Scholar] [CrossRef] [PubMed] [Green Version]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, C.-K.; Chen, B.-H.; Chen, H.-H.; Hsieh, R.J.-L.; Lee, J.-C.; Chu, Y.-T.; Lu, W.-H. Low-Dose Propranolol Prevents Functional Decline in Catecholamine-Induced Acute Heart Failure in Rats. Toxics 2022, 10, 238. https://doi.org/10.3390/toxics10050238
Tsai C-K, Chen B-H, Chen H-H, Hsieh RJ-L, Lee J-C, Chu Y-T, Lu W-H. Low-Dose Propranolol Prevents Functional Decline in Catecholamine-Induced Acute Heart Failure in Rats. Toxics. 2022; 10(5):238. https://doi.org/10.3390/toxics10050238
Chicago/Turabian StyleTsai, Cheng-Ken, Bo-Hau Chen, Hsin-Hung Chen, Rebecca Jen-Ling Hsieh, Jui-Chen Lee, Yi-Ting Chu, and Wen-Hsien Lu. 2022. "Low-Dose Propranolol Prevents Functional Decline in Catecholamine-Induced Acute Heart Failure in Rats" Toxics 10, no. 5: 238. https://doi.org/10.3390/toxics10050238
APA StyleTsai, C.-K., Chen, B.-H., Chen, H.-H., Hsieh, R. J.-L., Lee, J.-C., Chu, Y.-T., & Lu, W.-H. (2022). Low-Dose Propranolol Prevents Functional Decline in Catecholamine-Induced Acute Heart Failure in Rats. Toxics, 10(5), 238. https://doi.org/10.3390/toxics10050238