Establishment of Repeated In Vitro Exposure System for Evaluating Pulmonary Toxicity of Representative Criteria Air Pollutants Using Advanced Bronchial Mucosa Models
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bronchial Mucosa Model (Bro-ALI)
2.2. Bro-ALI Model Exposure Systems
- Particulate matter (PM): diesel exhaust particles (DEP).
- Gaseous air pollutants: nitrogen dioxides (NO2); sulfur dioxide (SO2).
2.2.1. DEP Generation and Exposure System
2.2.2. Gaseous Air Pollutants (Nitrogen Dioxides and Sulphur Dioxides) and Exposure System
2.2.3. Repeated Exposures to Diesel Exposure Particles and Gaseous Air Pollutants
2.2.4. Single Combined Exposure to DEP and Gaseous (NO2 and SO2) Air Pollutants
2.3. Sample Analysis
2.3.1. Cell Viability: Lactate Dehydrogenase (LDH) Assay
2.3.2. Secreted Protein Concentration Measurements by ELISA
2.3.3. Transcript Expression Analysis by Real-Time Quantitative Reverse Transcription-PCR (RT-qPCR)
2.3.4. Statistics
3. Results
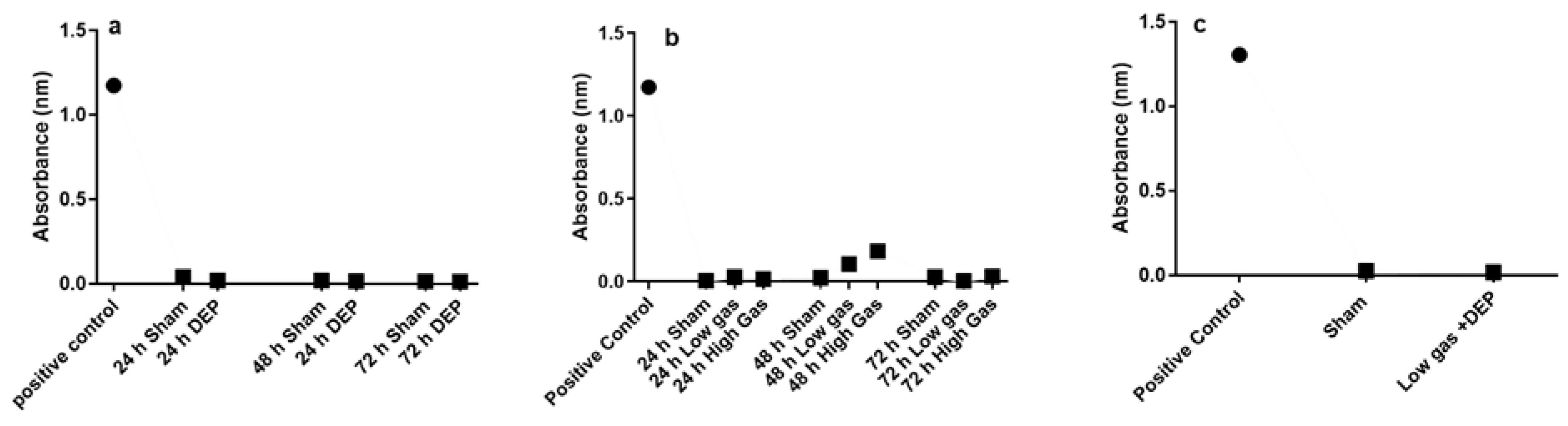
3.1. Effects of DEP and Gaseous Air Pollutants Exposure on Cell Viability (Lactate Dehydrogenase Assay)
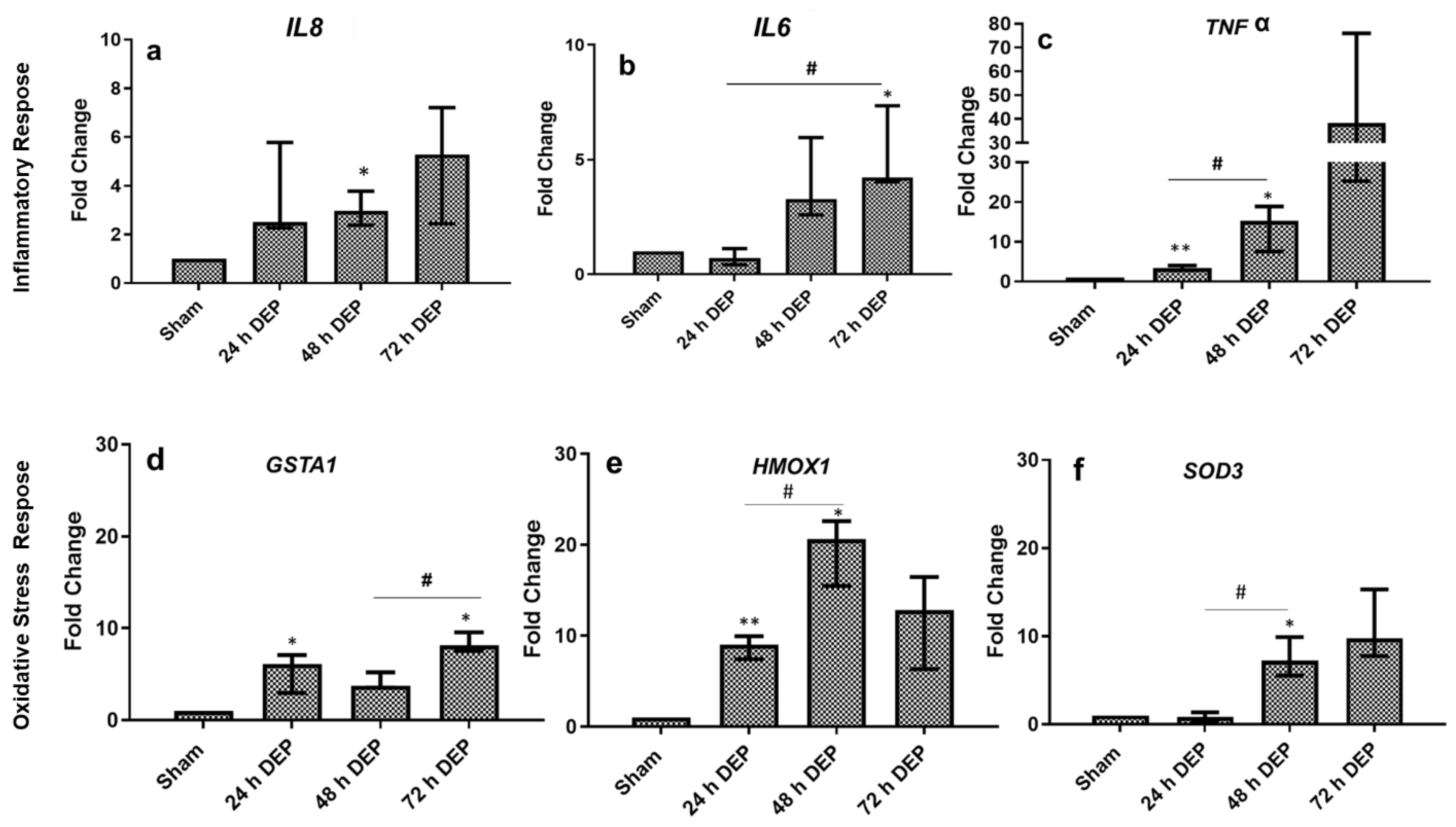
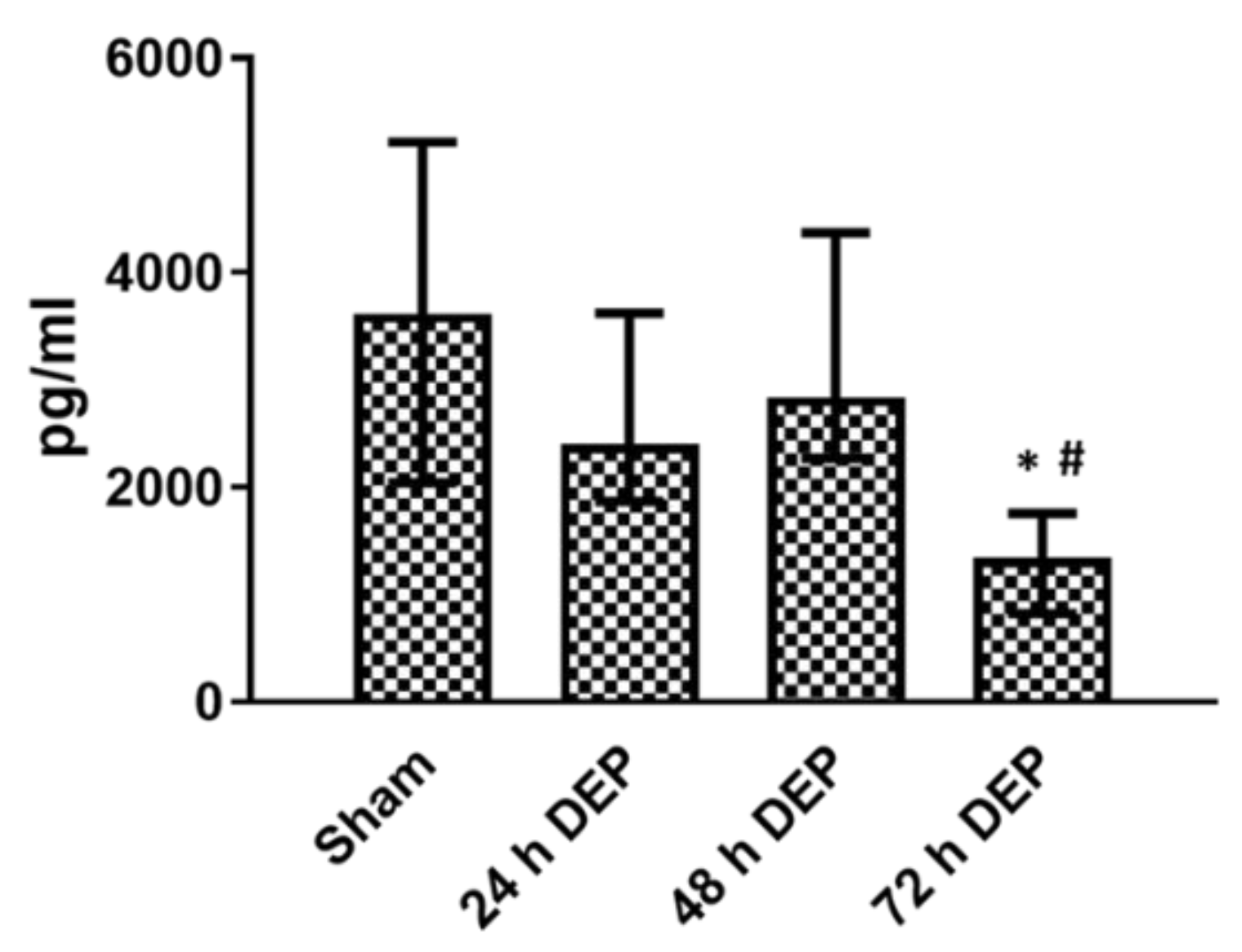
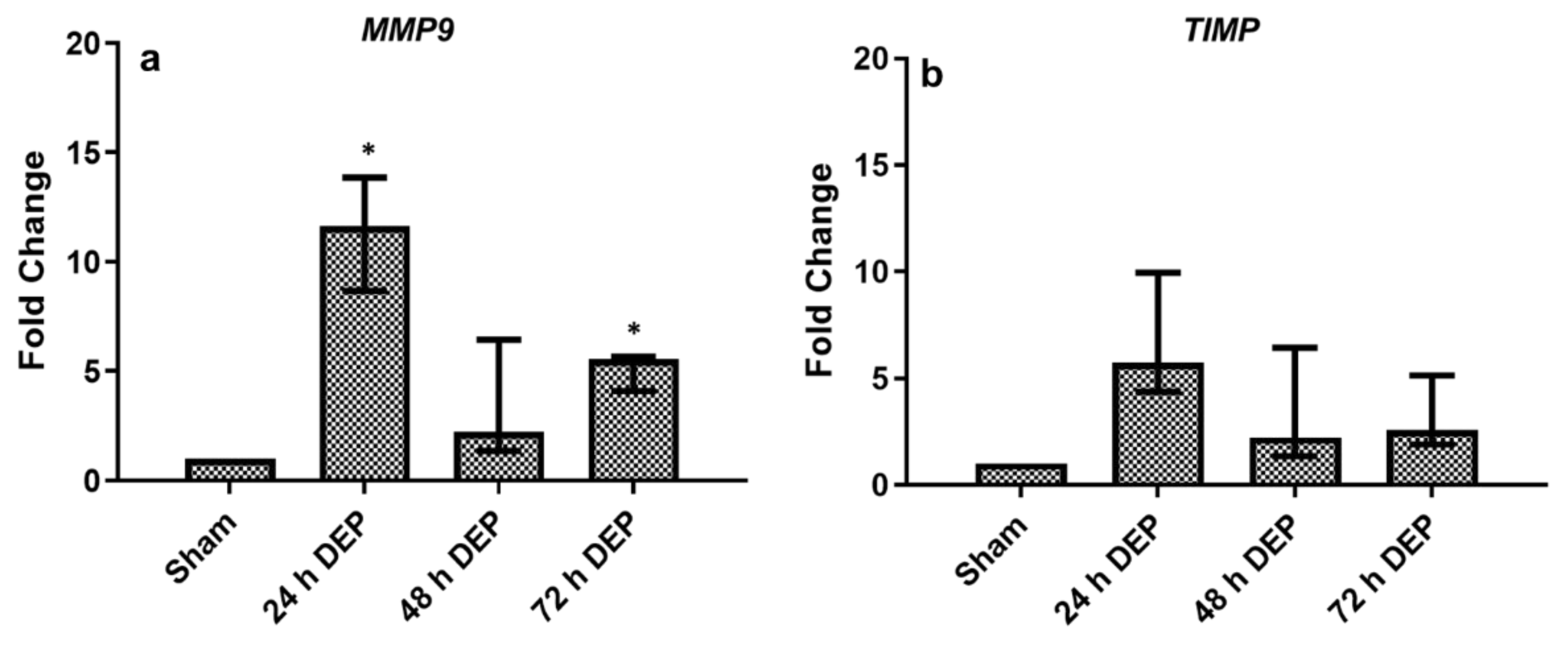
3.2. Repeated DEP Exposure Induced Pro-Inflammatory, Oxidative Stress and Tissue Injury/Repair Effect on Bro-ALI
3.2.1. Pro-Inflammatory Effects
3.2.2. Tissue Injury/Repair
3.3. Repeated Gas (NO2 and SO2) Exposure Induced Pro-Inflammatory, Oxidative Stress and Tissue Injury/Repair Effect on Bro-ALI
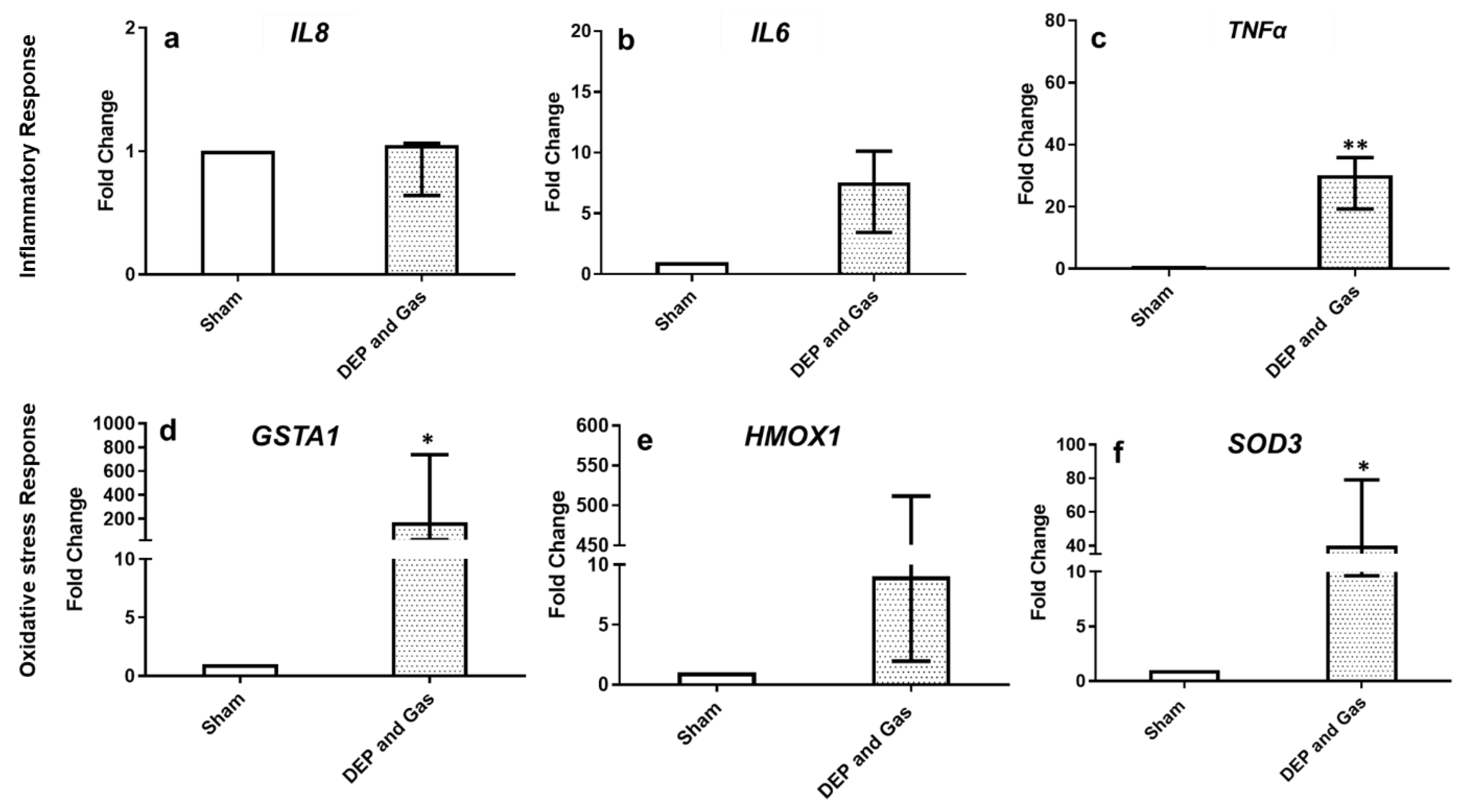
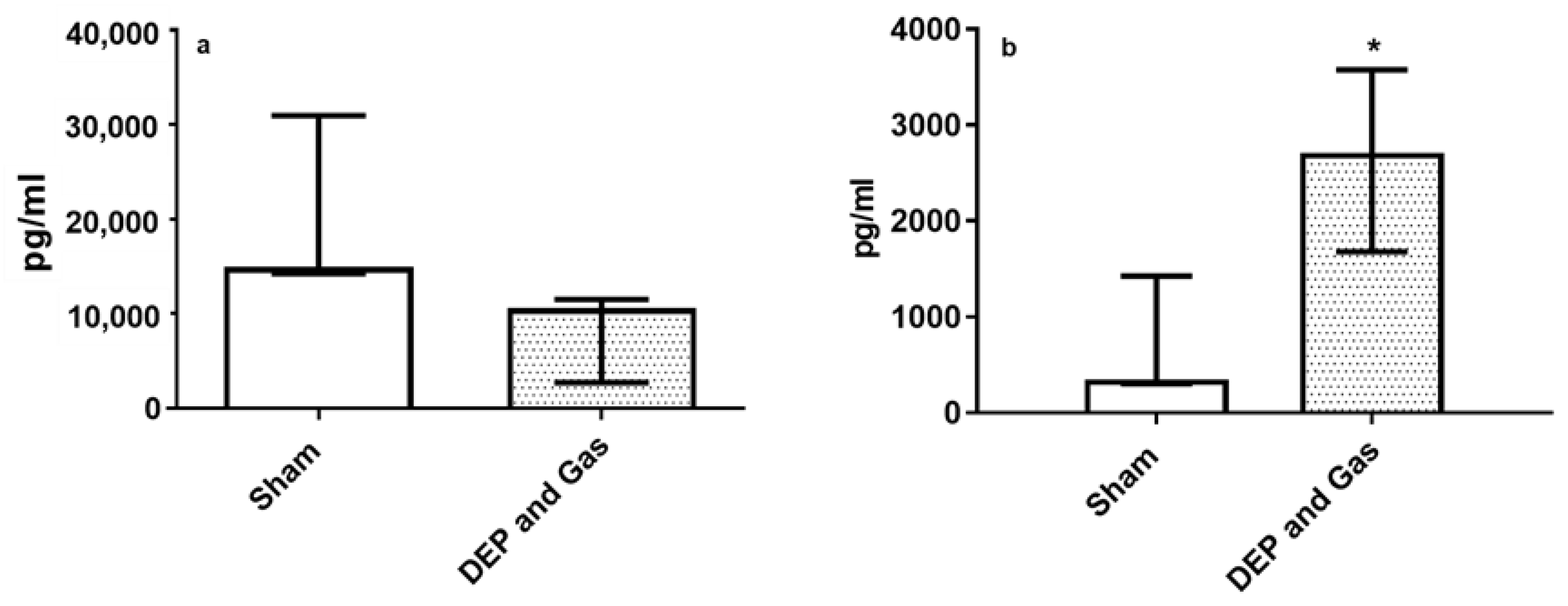
3.4. Single Combined Exposures (DEP with NO2 and SO2) Induced Pro-Inflammatory, Oxidative Stress and Tissue Injury/Repair Effect in Bro-AL
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- World Health Organization (WHO). WHO Global Air Quality Guidelines Aim to Save Millions of Lives from Air. 2021. Available online: https://www.who.int/news/item/22-09-2021-new-who-global-air-quality-guidelines-aim-to-save-millions-of-lives-from-air-pollution (accessed on 22 September 2021).
- European Economic Area (EEA). Health Impacts of Air Pollution in Europe. 2021. Available online: https://www.eea.europa.eu/publications/air-quality-in-europe-2021/health-impacts-of-air-pollution (accessed on 15 November 2021).
- Rogers, J.F.; Killough, G.G.; Thompson, S.J.; Addy, C.L.; McKeown, R.E.; Cowen, D.J. Estimating environmental exposures to sulfur dioxide from multiple industrial sources for a case–control study. J. Expo. Sci. Environ. Epidemiol. 1999, 9, 535–537. [Google Scholar] [CrossRef] [Green Version]
- Åström, S.; Källmark, L.; Yaramenka, K.; Grennfelt, P. European and Central Asian Actions on Air Quality: A Regional Summary of Emission Trends, Policies, and Programs to Reduce Air Pollution; Report C598; IVL Swedish Environmental Research Institute Ltd.: Stockholm, Sweden, 2021; ISBN 978-91-7883-300-9. [Google Scholar]
- Qian, X.; Xu, G.; Li, L.; Shen, Y.; He, T.; Liang, Y.; Yang, Z.; Zhou, W.W.; Xu, J. Knowledge and perceptions of air pollution in Ningbo, China. BMC Public Health 2016, 16, 1138. [Google Scholar] [CrossRef] [Green Version]
- Shi, L.; Steenland, K.; Li, H.; Liu, P.; Zhang, Y.; Lyles, R.H.; Requia, W.J.; Ilango, S.D.; Chang, H.H.; Wingo, T.; et al. A national cohort study (2000–2018) of long-term air pollution exposure and incident dementia in older adults in the United States. Nat. Commun. 2021, 12, 6754. [Google Scholar] [CrossRef]
- Cohen, A.J.; Brauer, M.; Burnett, R.; Anderson, H.R.; Frostad, J.; Estep, K.; Balakrishnan, K.; Brunekreef, B.; Dandona, L.; Dandona, R.; et al. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: An analysis of data from the Global Burden of Diseases Study 2015. Lancet 2017, 389, 1907–1918. [Google Scholar] [CrossRef] [Green Version]
- Hsu, H.T.; Wu, C.D.; Chung, M.C.; Shen, T.C.; Lai, T.J.; Chen, C.Y.; Wang, R.Y.; Chung, C.J. The effects of traffic-related air pollutants on chronic obstructive pulmonary disease in the community-based general population. Respir. Res. 2021, 22, 217. [Google Scholar] [CrossRef]
- Sinharay, R.; Gong, J.; Barratt, B.; Ohman-Strickland, P.; Ernst, S.; Kelly, F.J.; Zhang, J.; Collins, P.; Cullinan, P.; Chung, K.F. Respiratory and cardiovascular responses to walking down a traffic-polluted road compared with walking in a traffic-free area in participants aged 60 years and older with chronic lung or heart disease and age-matched healthy controls: A randomised, crossover study. Lancet 2018, 391, 339–349. [Google Scholar]
- Wongchang, T.; Sittichompoo, S.; Theinnoi, K.; Sawatmongkhon, B.; Jugjai, S. Impact of High-Voltage Discharge After-Treatment Technology on Diesel Engine Particulate Matter Composition and Gaseous Emissions. ACS Omega 2021, 6, 21181–21192. [Google Scholar] [CrossRef]
- Miller, M.R.; McLean, S.G.; Duffin, R.; Lawal, A.O.; Araujo, J.A.; Shaw, C.A.; Mills, N.L.; Donaldson, K.; Newby, D.E.; Hadoke, P.W. Diesel exhaust particulate increases the size and complexity of lesions in atherosclerotic mice. Part. Fibre Toxicol. 2013, 10, 61. [Google Scholar] [CrossRef] [Green Version]
- Nel, A.; Xia, T.; Mädler, L.; Li, N. Toxic potential of materials at the nano level. Science 2006, 311, 622–627. [Google Scholar] [CrossRef] [Green Version]
- Kyung, S.Y.; Jeong, S.H. Particulate-Matter Related Respiratory Diseases. Tuberc. Respir. Dis. 2020, 83, 116–121. [Google Scholar] [CrossRef]
- Jansen, K.L.; Larson, T.V.; Koenig, J.Q.; Mar, T.F.; Fields, C.; Stewart, J.; Lippmann, M. Associations between Health Effects and Particulate Matter and Black Carbon in Subjects with Respiratory Disease. Environ. Health Perspect. 2005, 113, 1741–1746. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, Y.; An, Z.; Li, W.; Zeng, X.; Li, H.; Jiang, J.; Song, J.; Wu, W. Seasonal Variations in PM2.5-induced Oxidative Stress and Up-regulation of Pro-inflammatory Mediators. Aerosol Air Qual. Res. 2020, 20, 1686–1694. [Google Scholar] [CrossRef] [Green Version]
- Mudway, I.S.; Kelly, F.J.; Holgatec, S.T. Oxidative stress in air pollution research. Free Radic. Biol. Med. 2020, 151, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Wang, W.; Liu, Q.; Li, Z.; Lei, L.; Ren, L.; Deng, F.; Guo, X.; Wu, S. Association between gaseous air pollutants and biomarkers of systemic inflammation: A systematic review and meta-analysis. Environ. Pollut. 2022, 292, 118336. [Google Scholar] [CrossRef]
- Kurt, O.K.; Zhang, J.; Pinkerton, K.E. Pulmonary Health Effects of Air Pollution. Curr. Opin. Pulm. Med. 2016, 22, 138–143. [Google Scholar] [CrossRef]
- Upadhyay, S.; Palmberg, L. Air-Liquid Interface: Relevant In Vitro Models for Investigating Air Pollutant-Induced Pulmonary Toxicity. Toxicol. Sci. 2018, 164, 21–30. [Google Scholar] [CrossRef] [Green Version]
- Drasler, B.; Sayre, P.; Steinhäuser, K.G.; Fink, A.; Rothen-Rutishauser, B. Corrigendum to “In vitro approaches to assess the hazard of nanomaterials”. NanoImpact 2018, 8, 99–116. [Google Scholar] [CrossRef]
- Lenz, A.-G.; Stoeger, T.; Cei, D.; Schmidmeir, M.; Semren, N.; Burgstaller, G.; Lentner, B.; Eickelberg, O.; Meiners, S.; Schmid, O. Efficient Bioactive Delivery of Aerosolized Drugs to Human Pulmonary Epithelial Cells Cultured in Air–Liquid Interface Conditions. Am. J. Respir. Cell Mol. Biol. 2014, 51, 526–535. [Google Scholar] [CrossRef]
- Ji, J.; Upadhyay, S.; Xiong, X.; Malmlöf, M.; Sandström, T.; Gerde, P.; Palmberg, L. Multi-cellular human bronchial models exposed to diesel exhaust particles: Assessment of inflammation, oxidative stress and macrophage polarization. Part. Fibre Toxicol. 2018, 15, 19. [Google Scholar] [CrossRef] [Green Version]
- Ji, J.; Ganguly, K.; Mihai, X.; Sun, J.; Malmlöf, M.; Gerde, P.; Upadhyay, S.; Palmberg, L. Exposure of normal and chronic bronchitis-like mucosa models to aerosolized carbon nanoparticles: Comparison of pro-inflammatory oxidative stress and tissue injury/repair responses. Nanotoxicology 2019, 13, 1362–1379. [Google Scholar] [CrossRef]
- Thimraj, T.A.; Sompa, S.I.; Ganguly, K.; Ernstgård, L.; Johanson, G.; Palmberg, L.; Upadhyay, S. Evaluation of diacetyl mediated pulmonary effects in physiologically relevant air-liquid interface models of human primary bronchial epithelial cells. Toxicol. Vitr. 2019, 61, 104617. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, A.M.; Upadhyay, S.; Johanson, G.; Ernstgård, L.; Palmberg, L. Inflammatory effects of acrolein, crotonaldehyde and hexanal vapors on human primary bronchial epithelial cells cultured at air-liquid interface. Toxicol. Vitr. 2018, 46, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Air Quality in Europe—2017 Report. 2017, p. 42. Available online: https://www.eea.europa.eu/publications/air-quality-in-europe-2017 (accessed on 25 August 2017).
- ENVIS centre CPB on control of pollution Water, Air & Noise. In Air Pollution in Delhi An Analysis; ENVIS Centre CPCB: Delhi, India, 2015; pp. 11–14.
- Chen, B.; Lu, S.; Li, S.; Wang, B. Impact of fine particulate fluctuation and other variables on Beijing’s air quality index. Environ. Sci. Pollut. Res. 2015, 22, 5139–5151. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Hedelin, A.; Malmlöf, M.; Kessler, V.; Seisenbaeva, G.; Gerde, P.; Palmberg, L. Development of Combining of Human Bronchial Mucosa Models with XposeALI® for Exposure of Air Pollution Nanoparticles. PLoS ONE 2017, 12, e0170428. [Google Scholar] [CrossRef]
- Ganguly, K.; Nordström, A.; Thimraj, T.A.; Rahman, M.; Ramström, M.; Sompa, S.I.; Lin, E.Z.; O’Brien, F.; Koelmel, J.; Ernstgård, L.; et al. Addressing the challenges of E-cigarette safety profiling by assessment of pulmonary toxicological response in bronchial and alveolar mucosa models. Sci. Rep. 2020, 10, 20460. [Google Scholar] [CrossRef]
- Lelieveld, J.; Evans, J.S.; Fnais, M.; Giannadaki, D.; Pozzer, A. The contribution of outdoor air pollution sources to premature mortality on a global scale. Nature 2015, 525, 367–371. [Google Scholar] [CrossRef]
- Kaur, R.; Pandey, P. Air Pollution, Climate Change, and Human Health in Indian Cities: A Brief Review. Front. Sustain. Cities 2021, 3, 77. [Google Scholar] [CrossRef]
- Orach, J.; Rider, C.F.; Carlsten, C. Concentration-dependent health effects of air pollution in controlled human exposures. Environ. Int. 2021, 150, 106424. [Google Scholar] [CrossRef]
- Brook, R.D.; Franklin, B.; Cascio, W.; Hong, Y.; Howard, G.; Lipsett, M.; Luepker, R.; Mittleman, M.; Samet, J.; Smith, S.C., Jr.; et al. Air Pollution and Cardiovascular Disease. Circulation 2004, 109, 2655–2671. [Google Scholar] [CrossRef]
- Upadhyay, S.; Stoeger, T.; George, L.; Schladweiler, M.C.; Kodavanti, U.; Ganguly, K.; Schulz, H. Ultrafine carbon particle mediated cardiovascular impairment of aged spontaneously hypertensive rats. Part. Fibre Toxicol. 2014, 11, 36. [Google Scholar] [CrossRef] [Green Version]
- Nemmar, A.; Holme, J.A.; Rosas, I.; Schwarze, P.E.; Alfaro-Moreno, E. Recent advances in particulate matter and nanoparticle toxicology: A review of the in vivo and in vitro studies. Biomed. Res. Int. 2013, 2013, 279371. [Google Scholar] [CrossRef] [Green Version]
- Manisalidis, I.; Stavropoulou, E.; Stavropoulos, A.; Bezirtzoglou, E. Environmental and Health Impacts of Air Pollution: A Review. Front. Public Health 2020, 8, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, S.O.; Hong, S.H.; Han, Y.-J.; Bak, S.H.; Kim, J.; Lee, M.K.; London, S.J.; Kim, W.J.; Kim, S.-Y. Long-term exposure to PM10 and NO2 in relation to lung function and imaging phenotypes in a COPD cohort. Respir. Res. 2020, 21, 247. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Xu, X.; Chu, M.; Guo, Y.; Wang, J. Air particulate matter and cardiovascular disease: The epidemiological, biomedical and clinical evidence. J. Thorac. Dis. 2016, 8, E8–E19. [Google Scholar] [PubMed]
- Souza, M.B.; Saldiva, P.H.; Pope III, C.A.; Capelozzi, V.L. Respiratory Changes due to Long-term Exposure to Urban Levels of Air Pollution: A Histopathologic Study in Humans. Chest 1998, 113, 1312–1318. [Google Scholar] [CrossRef] [PubMed]
- Fehrenbach, H.; Wagner, C.; Wegmann, M. Airway remodeling in asthma: What really matters. Cell Tissue Res. 2017, 367, 551–569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Auger, F.; Gendron, M.-C.; Chamot, C.; Marano, F.; Dazy, A.-C. Responses of well-differentiated nasal epithelial cells exposed to particles: Role of the epithelium in airway inflammation. Toxicol. Appl. Pharmacol. 2006, 215, 285–294. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, M.; Wang, L.; Chen, C.; Song, Y. Amphiregulin potentiates airway inflammation and mucus hypersecretion induced by urban particulate matter via the EGFR-PI3Kα-AKT/ERK pathway. Cell Signal. 2018, 53, 122–131. [Google Scholar] [CrossRef]
- Veronesi, B.; Oortgiesen, M.; Carter, J.; Devlin, R. Particulate Matter Initiates Inflammatory Cytokine Release by Activation of Capsaicin and Acid Receptors in a Human Bronchial Epithelial Cell Line. Toxicol. Appl. Pharmacol. 1999, 154, 106–115. [Google Scholar] [CrossRef]
- Paplinska-Goryca, M.; Misiukiewicz-Stepien, P.; Proboszcz, M.; Nejman-Gryz, P.; Gorska, K.; Zajusz-Zubek, E.; Krenke, R. Interactions of nasal epithelium with macrophages and dendritic cells variously alter urban PM-induced inflammation in healthy, asthma and COPD. Sci. Rep. 2021, 11, 13259. [Google Scholar] [CrossRef]
- Hiraiwa, K.; van Eeden, S.F. Contribution of lung macrophages to the inflammatory responses induced by exposure to air pollutants. Mediat. Inflamm. 2013, 2013, 619523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lodovici, M.; Bigagli, E. Oxidative Stress and Air Pollution Exposure. J. Toxicol. 2011, 2011, 487074. [Google Scholar] [CrossRef] [PubMed]
- Michael, S.; Montag, M.; Dott, W. Pro-inflammatory effects and oxidative stress in lung macrophages and epithelial cells induced by ambient particulate matter. Environ. Pollut. 2013, 183, 19–29. [Google Scholar] [CrossRef]
- Liu, C.W.; Lee, T.L.; Chen, Y.C.; Liang, C.J.; Wang, S.H.; Lue, J.H.; Tsai, J.S.; Lee, S.W.; Chen, S.H.; Yang, Y.F.; et al. PM2.5-induced oxidative stress increases intercellular adhesion molecule-1 expression in lung epithelial cells through the IL-6/AKT/STAT3/NF-κB-dependent pathway. Part. Fibre Toxicol. 2018, 15, 4. [Google Scholar] [CrossRef] [PubMed]
- Cabral-Pacheco, G.A.; Garza-Veloz, I.; Castruita-De la Rosa, C.; Ramirez-Acuna, J.M.; Perez-Romero, B.A.; Guerrero-Rodriguez, J.F.; Martinez-Avila, N.; Martinez-Fierro, M.L. The Roles of Matrix Metalloproteinases and Their Inhibitors in Human Diseases. Int. J. Mol. Sci. 2020, 21, 9739. [Google Scholar] [CrossRef]
- Bakand, S. Development of In Vitro Methods for Toxicity Assessment of Workplace Air Contaminants. Ph.D. Thesis, University of New South Wales, Sydney, Australia, 2006. [Google Scholar] [CrossRef]
- Knorst, M.M.; Kienast, K.; Müller-Quernheim, J.; Ferlinz, R. Effect of Sulfur Dioxide on Cytokine Production of Human Alveolar Macrophages in Vitro. Arch. Environ. Health Int. J. 1996, 51, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Jia, Y.; Pan, X.; Liu, L.; Wichmann, H.-E. The association between fine particulate air pollution and hospital emergency room visits for cardiovascular diseases in Beijing, China. Sci. Total Environ. 2009, 407, 4826–4830. [Google Scholar] [CrossRef]
- Tomašek, I.; Horwell, C.J.; Damby, D.E.; Barošová, H.; Geers, C.; Petri-Fink, A.; Rothen-Rutishauser, B.; Clift, M.J.D. Combined exposure of diesel exhaust particles and respirable Soufrière Hills volcanic ash causes a (pro-)inflammatory response in an in vitro multicellular epithelial tissue barrier model. Part. Fibre Toxicol. 2016, 13, 67. [Google Scholar] [CrossRef] [Green Version]
- Silins, I.; Hogberg, J. Combined Toxic Exposures and Human Health: Biomarkers of Exposure and Effect. Int. J. Environ. Res. Public Health 2011, 8, 629–647. [Google Scholar] [CrossRef] [Green Version]
- Zheng, D.; Wang, N.; Wang, X.; Tang, Y.; Zhu, L.; Huang, Z.; Tang, H.; Shi, Y.; Wu, Y.; Zhang, M.; et al. Effects of the interaction of TiO2 nanoparticles with bisphenol A on their physicochemical properties and in vitro toxicity. J. Hazard. Mater. 2012, 199, 426–432. [Google Scholar] [CrossRef]
- Forest, V. Combined effects of nanoparticles and other environmental contaminants on human health—An issue often overlooked. NanoImpact 2021, 23, 100344. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Upadhyay, S.; Chakraborty, A.; Thimraj, T.A.; Baldi, M.; Steneholm, A.; Ganguly, K.; Gerde, P.; Ernstgård, L.; Palmberg, L. Establishment of Repeated In Vitro Exposure System for Evaluating Pulmonary Toxicity of Representative Criteria Air Pollutants Using Advanced Bronchial Mucosa Models. Toxics 2022, 10, 277. https://doi.org/10.3390/toxics10060277
Upadhyay S, Chakraborty A, Thimraj TA, Baldi M, Steneholm A, Ganguly K, Gerde P, Ernstgård L, Palmberg L. Establishment of Repeated In Vitro Exposure System for Evaluating Pulmonary Toxicity of Representative Criteria Air Pollutants Using Advanced Bronchial Mucosa Models. Toxics. 2022; 10(6):277. https://doi.org/10.3390/toxics10060277
Chicago/Turabian StyleUpadhyay, Swapna, Ashesh Chakraborty, Tania A. Thimraj, Marialuisa Baldi, Anna Steneholm, Koustav Ganguly, Per Gerde, Lena Ernstgård, and Lena Palmberg. 2022. "Establishment of Repeated In Vitro Exposure System for Evaluating Pulmonary Toxicity of Representative Criteria Air Pollutants Using Advanced Bronchial Mucosa Models" Toxics 10, no. 6: 277. https://doi.org/10.3390/toxics10060277





