RETRACTED: Environmental Risk Assessment of Dexamethasone Sodium Phosphate and Tocilizumab Mixture in Zebrafish Early Life Stage (Danio rerio)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Selection of Preliminary Concentrations
2.2. Solutions Preparation for ZFET Assay
2.3. Zebrafish Maintenance and Breeding
2.4. Zebrafish Embryo Toxicity (ZFET) Assay
- (a)
- Embryo coagulation—can also occur within a few hours of the start of exposure and indicates a generic acute toxic effect;
- (b)
- Lack of somite formation—somite should be visible 12 h after fertilization. If absent, the embryo will not develop further, thus causing its death;
- (c)
- Non-detachment of the tail—detachment of the tail from the yolk can be observed 24 h after fertilization, indicating a normal growth of the embryo;
- (d)
- Absence of heartbeat—the heartbeat is easily detectable 30 h after fertilization; its absence indicates the death of the embryo. Embryo coagulation and absence of heartbeat were considered as endpoints of mortality.
2.5. Western Blot
2.6. MDA, SOD, and CAT Measurements
2.7. Data Analysis
3. Results
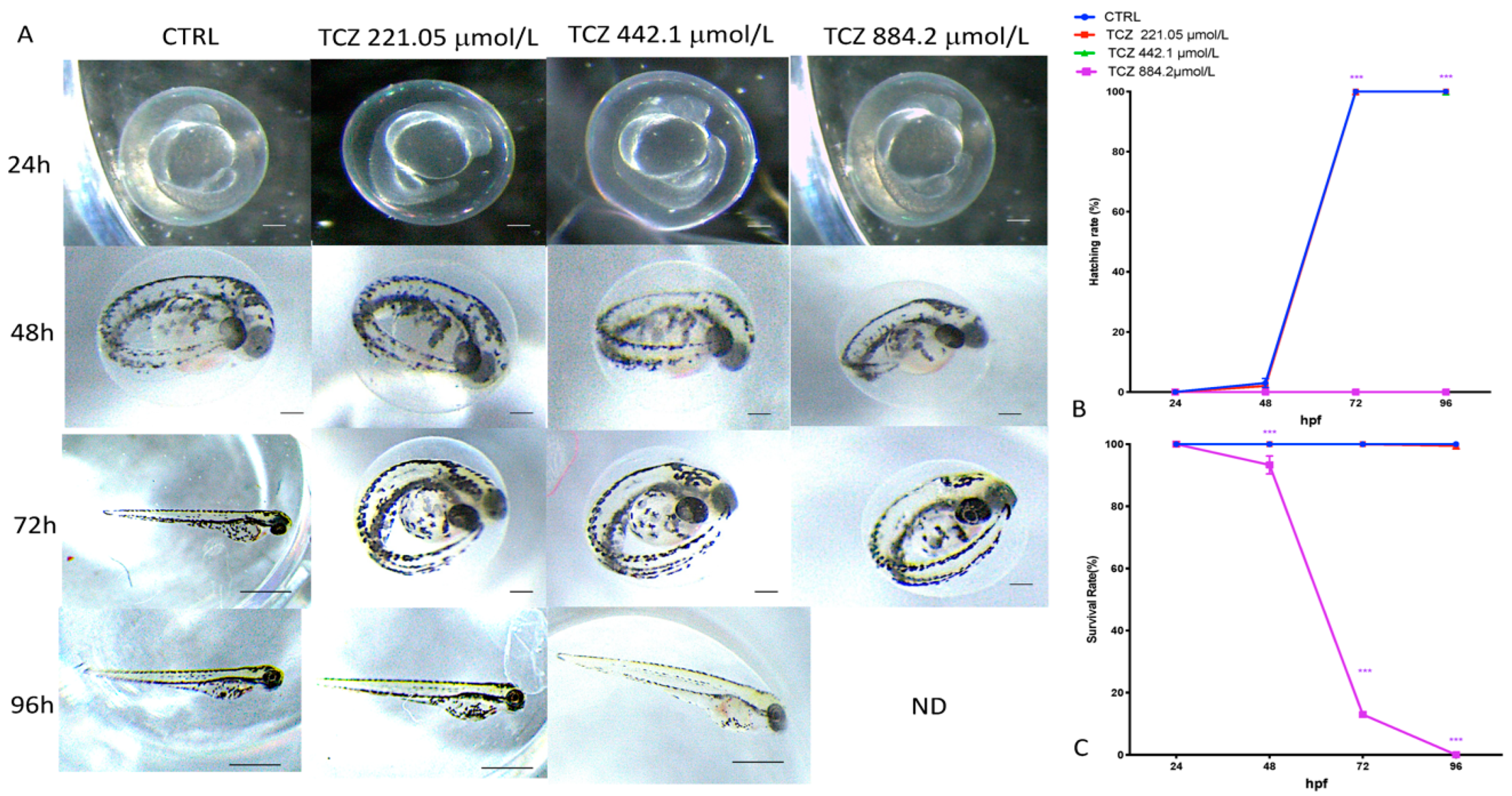
3.1. Viability and Morphology of Zebrafish Embryos after Preliminary DEX and TCZ Exposure
3.2. Mortality, Hatching Rate and Malformations after ZFET Assay
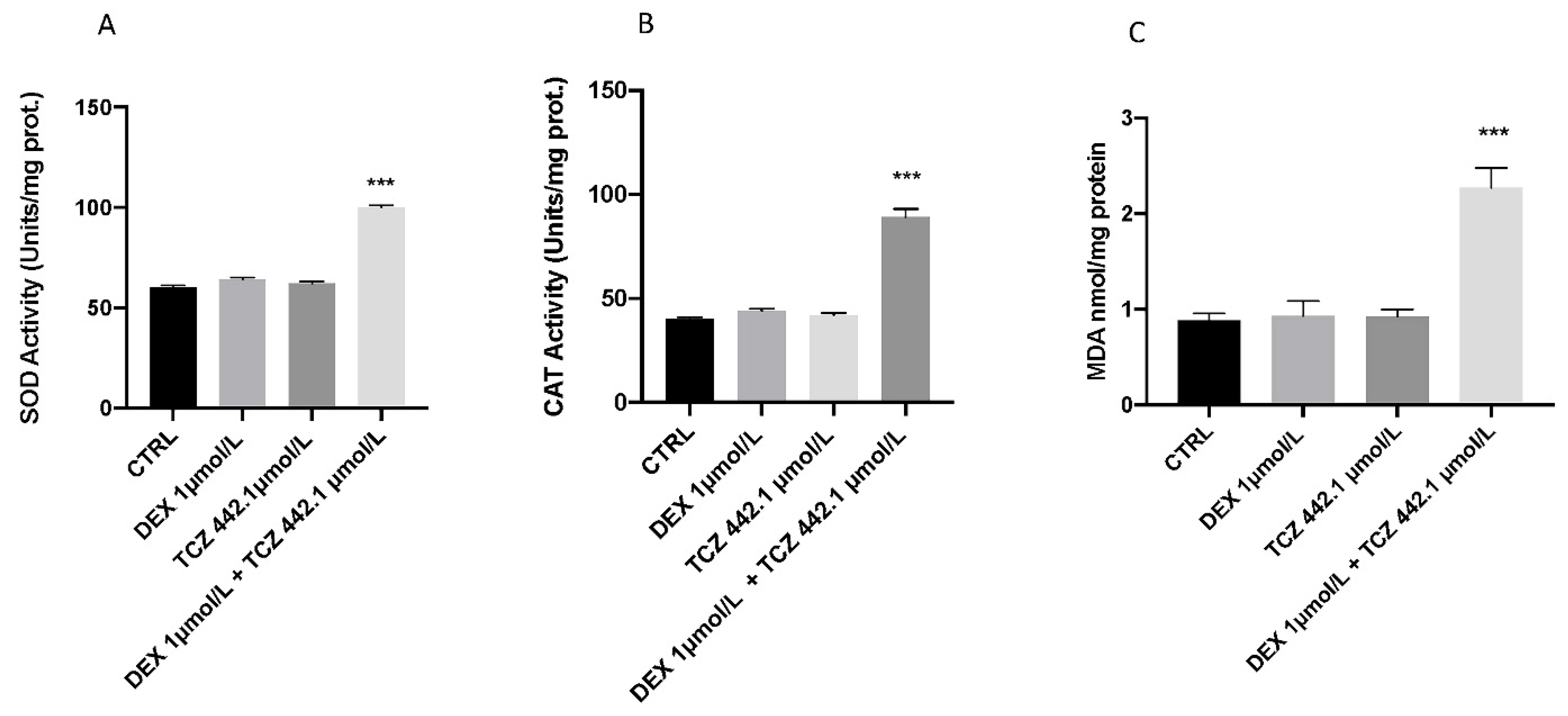
3.3. Effect of DEX and TCZ on Lipid Peroxidation and Stress Oxidative Pathway
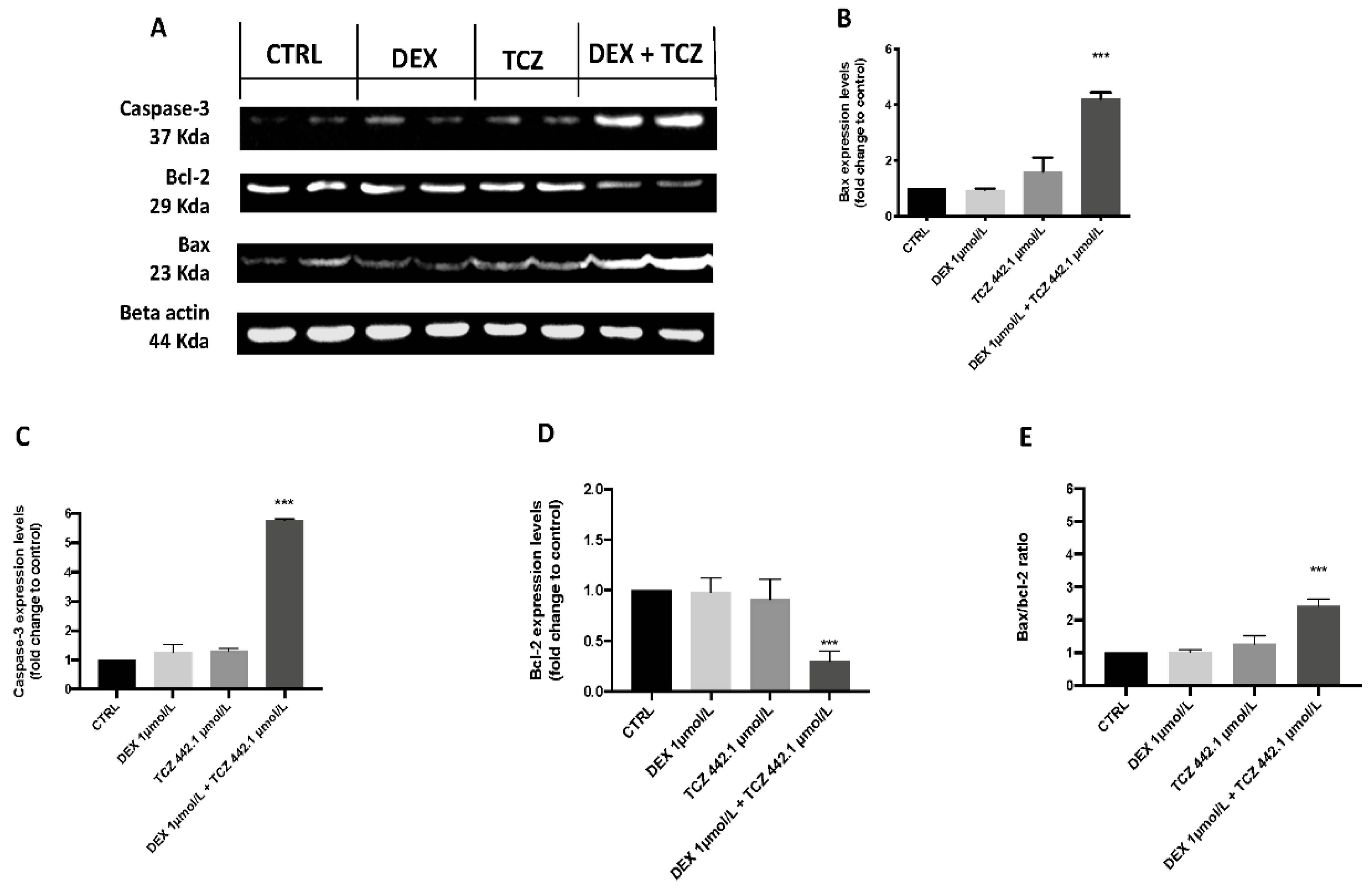
3.4. Apoptotic Process
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviation
| DEX | dexamethasone sodium phosphate |
| TCZ | tocilizumab |
| hpf | hours post fertilization |
| ZFET | zebrafish embryo toxicity |
| MDA | malondialdehyde |
| SOD | superoxide dismutase |
| CAT | catalase |
References
- Lu, R.; Zhao, X.; Li, J.; Niu, P.; Yang, B.; Wu, H.; Wang, W.; Song, H.; Huang, B.; Zhu, N. Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet 2020, 395, 565–574. [Google Scholar] [CrossRef]
- de Lucena, T.M.C.; da Silva Santos, A.F.; de Lima, B.R.; de Albuquerque Borborema, M.E.; de Azevêdo Silva, J. Mechanism of inflammatory response in associated comorbidities in COVID-19. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 597–600. [Google Scholar] [CrossRef] [PubMed]
- Ragab, D.; Salah Eldin, H.; Taeimah, M.; Khattab, R.; Salem, R. The COVID-19 cytokine storm; what we know so far. Front. Immunol. 2020, 11, 1446. [Google Scholar] [CrossRef] [PubMed]
- Salama, C.; Han, J.; Yau, L.; Reiss, W.G.; Kramer, B.; Neidhart, J.D.; Criner, G.J.; Kaplan-Lewis, E.; Baden, R.; Pandit, L. Tocilizumab in patients hospitalized with COVID-19 pneumonia. N. Engl. J. Med. 2021, 384, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Baño, J.; Pachón, J.; Carratalà, J.; Ryan, P.; Jarrín, I.; Yllescas, M.; Arribas, J.R.; Berenguer, J.; Muñoz, E.A.; Divasson, P.G. Treatment with tocilizumab or corticosteroids for COVID-19 patients with hyperinflammatory state: A multicentre cohort study (SAM-COVID-19). Clin. Microbiol. Infect. 2021, 27, 244–252. [Google Scholar] [CrossRef]
- Sinha, P.; Linas, B.P. Combination therapy with tocilizumab and dexamethasone cost-effectively reduces Coronavirus disease 2019 mortality. Clin. Infect. Dis. 2021, 73, 2116–2118. [Google Scholar] [CrossRef]
- Toolaram, A.P.; Kuemmerer, K.; Schneider, M. Environmental risk assessment of anti-cancer drugs and their transformation products: A focus on their genotoxicity characterization-state of knowledge and short comings. Mutat. Res. Rev. Mutat. Res. 2014, 760, 18–35. [Google Scholar] [CrossRef]
- Guichard, N.; Guillarme, D.; Bonnabry, P.; Fleury-Souverain, S. Antineoplastic drugs and their analysis: A state of the art review. Analyst 2017, 142, 2273–2321. [Google Scholar] [CrossRef]
- Herrero, P.; Borrull, F.; Marcé, R.; Pocurull, E. Pressurised liquid extraction and ultra-high performance liquid chromatography-tandem mass spectrometry to determine endogenous and synthetic glucocorticoids in sewage sludge. Talanta 2013, 103, 186–193. [Google Scholar] [CrossRef]
- Sanchez, W.; Sremski, W.; Piccini, B.; Palluel, O.; Maillot-Marechal, E.; Betoulle, S.; Jaffal, A.; Ait-Aissa, S.; Brion, F.; Thybaud, E. Adverse effects in wild fish living downstream from pharmaceutical manufacture discharges. Environ. Int. 2011, 37, 1342–1348. [Google Scholar] [CrossRef]
- Chang, H.; Hu, J.; Shao, B. Occurrence of natural and synthetic glucocorticoids in sewage treatment plants and receiving river waters. Environ. Sci. Technol. 2007, 41, 3462–3468. [Google Scholar] [CrossRef] [PubMed]
- Musee, N.; Kebaabetswe, L.P.; Tichapondwa, S.; Tubatsi, G.; Mahaye, N.; Leareng, S.K.; Nomngongo, P.N. Occurrence, Fate, Effects, and Risks of Dexamethasone: Ecological Implications Post-COVID-19. Int. J. Environ. Res. Public Health 2021, 18, 11291. [Google Scholar] [CrossRef]
- MacRae, C.A.; Peterson, R.T. Zebrafish as tools for drug discovery. Nat. Rev. Drug Discov. 2015, 14, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Zon, L.I.; Peterson, R.T. In vivo drug discovery in the zebrafish. Nat. Rev. Drug Discov. 2005, 4, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Peterson, R.T. Discovery of therapeutic targets by phenotype-based zebrafish screens. Drug Discov. Today Technol. 2004, 1, 49–54. [Google Scholar] [CrossRef]
- Guiloski, I.C.; Ribas, J.L.C.; da Silva Pereira, L.; Neves, A.P.P.; de Assis, H.C.S. Effects of trophic exposure to dexamethasone and diclofenac in freshwater fish. Ecotoxicol. Environ. Saf. 2015, 114, 204–211. [Google Scholar] [CrossRef]
- DellaGreca, M.; Fiorentino, A.; Isidori, M.; Lavorgna, M.; Previtera, L.; Rubino, M.; Temussi, F. Toxicity of prednisolone, dexamethasone and their photochemical derivatives on aquatic organisms. Chemosphere 2004, 54, 629–637. [Google Scholar] [CrossRef]
- Bal, N.; Kumar, A.; Du, J.; Nugegoda, D. Multigenerational effects of two glucocorticoids (prednisolone and dexamethasone) on life-history parameters of crustacean Ceriodaphnia dubia (Cladocera). Environ. Pollut. 2017, 225, 569–578. [Google Scholar] [CrossRef]
- Yin, G.; Cao, L.; Du, J.; Jia, R.; Kitazawa, T.; Kubota, A.; Teraoka, H. Dexamethasone-induced hepatomegaly and steatosis in larval zebrafish. J. Toxicol. Sci. 2017, 42, 455–459. [Google Scholar] [CrossRef]
- Yin, H.; Wang, J.; Wu, M.; Ma, Y.; Wang, S.; Su, Q. Preventive effects of evodiamine on dexamethasone-induced osteoporosis in zebrafish. BioMed Res. Int. 2019, 2019, 5859641. [Google Scholar] [CrossRef]
- OECD. OECD Annual Report 2001; OECD: Paris, France, 2001. [Google Scholar]
- Boni, P. Acute toxicity and elimination of phenol injected into fish (Carassius auratus L.). Experientia 1965, 21, 222–223. [Google Scholar] [CrossRef] [PubMed]
- OECD. Test No. 236: Fish Embryo Acute Toxicity (FET) Test; OECD: Paris, France, 2013. [Google Scholar]
- Di Paola, D.; Capparucci, F.; Lanteri, G.; Cordaro, M.; Crupi, R.; Siracusa, R.; D’Amico, R.; Fusco, R.; Impellizzeri, D.; Cuzzocrea, S.; et al. Combined Toxicity of Xenobiotics Bisphenol A and Heavy Metals on Zebrafish Embryos (Danio rerio). Toxics 2021, 9, 344. [Google Scholar] [CrossRef] [PubMed]
- Di Paola, D.; Capparucci, F.; Abbate, J.M.; Cordaro, M.; Crupi, R.; Siracusa, R.; D’Amico, R.; Fusco, R.; Genovese, T.; Impellizzeri, D.; et al. Environmental Risk Assessment of Oxaliplatin Exposure on Early Life Stages of Zebrafish (Danio rerio). Toxics 2022, 10, 81. [Google Scholar] [CrossRef] [PubMed]
- Parenti, C.C.; Ghilardi, A.; Della Torre, C.; Magni, S.; Del Giacco, L.; Binelli, A. Evaluation of the infiltration of polystyrene nanobeads in zebrafish embryo tissues after short-term exposure and the related biochemical and behavioural effects. Environ. Pollut. 2019, 254, 112947. [Google Scholar] [CrossRef]
- Di Paola, D.; Natale, S.; Gugliandolo, E.; Cordaro, M.; Crupi, R.; Siracusa, R.; D’Amico, R.; Fusco, R.; Impellizzeri, D.; Cuzzocrea, S.; et al. Assessment of 2-Pentadecyl-2-oxazoline Role on Lipopolysaccharide-Induced Inflammation on Early Stage Development of Zebrafish (Danio rerio). Life 2022, 12, 128. [Google Scholar] [CrossRef]
- Peritore, A.F.; Crupi, R.; Scuto, M.; Gugliandolo, E.; Siracusa, R.; Impellizzeri, D.; Cordaro, M.; D’amico, R.; Fusco, R.; Di Paola, R.; et al. The Role of Annexin A1 and Formyl Peptide Receptor 2/3 Signaling in Chronic Corticosterone-Induced Depression-Like behaviors and Impairment in Hippocampal-Dependent Memory. CNS Neurol. Disord. Drug Targets 2020, 19, 27–43. [Google Scholar] [CrossRef]
- Lubrano, V.; Balzan, S. Enzymatic antioxidant system in vascular inflammation and coronary artery disease. World J. Exp. Med. 2015, 5, 218. [Google Scholar] [CrossRef]
- Yasui, K.; Baba, A. Therapeutic potential of superoxide dismutase (SOD) for resolution of inflammation. Inflamm. Res. 2006, 55, 359–363. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.; Wei, Y.; Zhang, H.; Xu, M.; Dai, J. Induction of time-dependent oxidative stress and related transcriptional effects of perfluorododecanoic acid in zebrafish liver. Aquat. Toxicol. 2008, 89, 242–250. [Google Scholar] [CrossRef]
- Di Paola, D.; Iaria, C.; Capparucci, F.; Cordaro, M.; Crupi, R.; Siracusa, R.; D’Amico, R.; Fusco, R.; Impellizzeri, D.; Cuzzocrea, S.; et al. Aflatoxin B1 Toxicity in Zebrafish Larva (Danio rerio): Protective Role of Hericium erinaceus. Toxins 2021, 13, 710. [Google Scholar] [CrossRef]
- Fusco, R.; Cordaro, M.; Siracusa, R.; Peritore, A.F.; Gugliandolo, E.; Genovese, T.; D’Amico, R.; Crupi, R.; Smeriglio, A.; Mandalari, G.; et al. Consumption of Anacardium occidentale L. (Cashew Nuts) Inhibits Oxidative Stress through Modulation of the Nrf2/HO−1 and NF-kB Pathways. Molecules 2020, 25, 4426. [Google Scholar] [CrossRef] [PubMed]
- Kooistra, E.J.; van Berkel, M.; van Kempen, N.F.; van Latum, C.R.; Bruse, N.; Frenzel, T.; van den Berg, M.J.; Schouten, J.A.; Kox, M.; Pickkers, P. Dexamethasone and tocilizumab treatment considerably reduces the value of C-reactive protein and procalcitonin to detect secondary bacterial infections in COVID-19 patients. Crit. Care 2021, 25, 281. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.T. Human Pharmaceuticals: Assessing the Impacts on Aquatic Ecosystems; Allen Press/ACG Publishing: Lawrence, KS, USA, 2005. [Google Scholar]
- Rosi-Marshall, E.J.; Royer, T.V. Pharmaceutical compounds and ecosystem function: An emerging research challenge for aquatic ecologists. Ecosystems 2012, 15, 867–880. [Google Scholar] [CrossRef]
- Luo, S.; Yang, Y.; Chen, J.; Zhong, Z.; Huang, H.; Zhang, J.; Cui, L. Tanshinol stimulates bone formation and attenuates dexamethasone-induced inhibition of osteogenesis in larval zebrafish. J. Orthop. Transl. 2016, 4, 35–45. [Google Scholar] [CrossRef]
- Samaee, S.-M.; Rabbani, S.; Jovanović, B.; Mohajeri-Tehrani, M.R.; Haghpanah, V. Efficacy of the hatching event in assessing the embryo toxicity of the nano-sized TiO2 particles in zebrafish: A comparison between two different classes of hatching-derived variables. Ecotoxicol. Environ. Saf. 2015, 116, 121–128. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, Y.; Luo, G.-Z.; Wang, X.; Yue, Y.; Wang, X.; Zong, X.; Chen, K.; Yin, H.; Fu, Y. Abundant DNA 6mA methylation during early embryogenesis of zebrafish and pig. Nat. Commun. 2016, 7, 13052. [Google Scholar] [CrossRef]
- Papiya, S.; Kanamadi, R. Effect of mercurial fungicide Emisan®-6 on the embryonic developmental stages of zebrafish, Brachydanio (Danio) rerio. J. Adv. Zool. 2000, 21, 12–18. [Google Scholar]
- Ismail, A.; Yusof, S. Effect of mercury and cadmium on early life stages of Java medaka (Oryzias javanicus): A potential tropical test fish. Mar. Pollut. Bull. 2011, 63, 347–349. [Google Scholar] [CrossRef]
- Mutsaers, H.A.; Tofighi, R. Dexamethasone enhances oxidative stress-induced cell death in murine neural stem cells. Neurotox. Res. 2012, 22, 127–137. [Google Scholar] [CrossRef]
- Lv, Z.P.; Peng, Y.Z.; Zhang, B.B.; Fan, H.; Liu, D.; Guo, Y.M. Glucose and lipid metabolism disorders in the chickens with dexamethasone-induced oxidative stress. J. Anim. Physiol. Anim. Nutr. 2018, 102, e706–e717. [Google Scholar] [CrossRef]
- Steiling, H.; Munz, B.; Werner, S.; Brauchle, M. Different types of ROS-scavenging enzymes are expressed during cutaneous wound repair. Exp. Cell Res. 1999, 247, 484–494. [Google Scholar] [CrossRef] [PubMed]
- Kanzaki, H.; Wada, S.; Narimiya, T.; Yamaguchi, Y.; Katsumata, Y.; Itohiya, K.; Fukaya, S.; Miyamoto, Y.; Nakamura, Y. Pathways that regulate ROS scavenging enzymes, and their role in defense against tissue destruction in periodontitis. Front. Physiol. 2017, 8, 351. [Google Scholar] [CrossRef] [PubMed]
- Ighodaro, O.; Akinloye, O. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Negre-Salvayre, A.; Coatrieux, C.; Ingueneau, C.; Salvayre, R. Advanced lipid peroxidation end products in oxidative damage to proteins. Potential role in diseases and therapeutic prospects for the inhibitors. Br. J. Pharmacol. 2008, 153, 6–20. [Google Scholar] [CrossRef]
- Niki, E. Lipid peroxidation products as oxidative stress biomarkers. Biofactors 2008, 34, 171–180. [Google Scholar] [CrossRef]
- Talas, D.U.; Nayci, A.; Polat, G.; Atis, S.; Comelekoglu, U.; Bagdatoglu, O.T.; Bagdatoglu, C. The effects of dexamethasone on lipid peroxidation and nitric oxide levels on the healing of tracheal anastomoses: An experimental study in rats. Pharmacol. Res. 2002, 46, 265–271. [Google Scholar] [CrossRef]
- Yi, J.; Zhu, R.; Wu, J.; Wu, J.; Xia, W.; Zhu, L.; Jiang, W.; Xiang, S.; Tan, Z. In vivo protective effect of betulinic acid on dexamethasone induced thymocyte apoptosis by reducing oxidative stress. Pharmacol. Rep. 2016, 68, 95–100. [Google Scholar] [CrossRef]
- Franco, R.; Sánchez-Olea, R.; Reyes-Reyes, E.M.; Panayiotidis, M.I. Environmental toxicity, oxidative stress and apoptosis: Menage a trois. Mutat. Res. Genet. Toxicol. Environ. Mutagenes. 2009, 674, 3–22. [Google Scholar] [CrossRef]
- Ozben, T. Oxidative stress and apoptosis: Impact on cancer therapy. J. Pharm. Sci. 2007, 96, 2181–2196. [Google Scholar] [CrossRef]
- Choi, J.E.; Kim, S.; Ahn, J.H.; Youn, P.; Kang, J.S.; Park, K.; Yi, J.; Ryu, D.-Y. Induction of oxidative stress and apoptosis by silver nanoparticles in the liver of adult zebrafish. Aquat. Toxicol. 2010, 100, 151–159. [Google Scholar] [CrossRef]
- Xia, Q.; Wei, L.; Zhang, Y.; Kong, H.; Shi, Y.; Wang, X.; Chen, X.; Han, L.; Liu, K. Psoralen induces developmental toxicity in zebrafish embryos/larvae through oxidative stress, apoptosis, and energy metabolism disorder. Front. Pharmacol. 2018, 9, 1457. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Paola, D.; Abbate, J.M.; Iaria, C.; Cordaro, M.; Crupi, R.; Siracusa, R.; D’Amico, R.; Fusco, R.; Impellizzeri, D.; Cuzzocrea, S.; et al. RETRACTED: Environmental Risk Assessment of Dexamethasone Sodium Phosphate and Tocilizumab Mixture in Zebrafish Early Life Stage (Danio rerio). Toxics 2022, 10, 279. https://doi.org/10.3390/toxics10060279
Di Paola D, Abbate JM, Iaria C, Cordaro M, Crupi R, Siracusa R, D’Amico R, Fusco R, Impellizzeri D, Cuzzocrea S, et al. RETRACTED: Environmental Risk Assessment of Dexamethasone Sodium Phosphate and Tocilizumab Mixture in Zebrafish Early Life Stage (Danio rerio). Toxics. 2022; 10(6):279. https://doi.org/10.3390/toxics10060279
Chicago/Turabian StyleDi Paola, Davide, Jessica Maria Abbate, Carmelo Iaria, Marika Cordaro, Rosalia Crupi, Rosalba Siracusa, Ramona D’Amico, Roberta Fusco, Daniela Impellizzeri, Salvatore Cuzzocrea, and et al. 2022. "RETRACTED: Environmental Risk Assessment of Dexamethasone Sodium Phosphate and Tocilizumab Mixture in Zebrafish Early Life Stage (Danio rerio)" Toxics 10, no. 6: 279. https://doi.org/10.3390/toxics10060279
APA StyleDi Paola, D., Abbate, J. M., Iaria, C., Cordaro, M., Crupi, R., Siracusa, R., D’Amico, R., Fusco, R., Impellizzeri, D., Cuzzocrea, S., Spanò, N., Gugliandolo, E., & Peritore, A. F. (2022). RETRACTED: Environmental Risk Assessment of Dexamethasone Sodium Phosphate and Tocilizumab Mixture in Zebrafish Early Life Stage (Danio rerio). Toxics, 10(6), 279. https://doi.org/10.3390/toxics10060279













