The Effect of BPA-Treated Water on the Small Intestine via an In Vivo Study
Abstract
:1. Introduction
2. Methodology
2.1. Materials for In-Vivo Models
2.2. Photocatalytic Removal of BPA by N-Doped TiO2 DLHF Membrane
2.3. Animal Care, BPA Exposure and Dissection Procedure
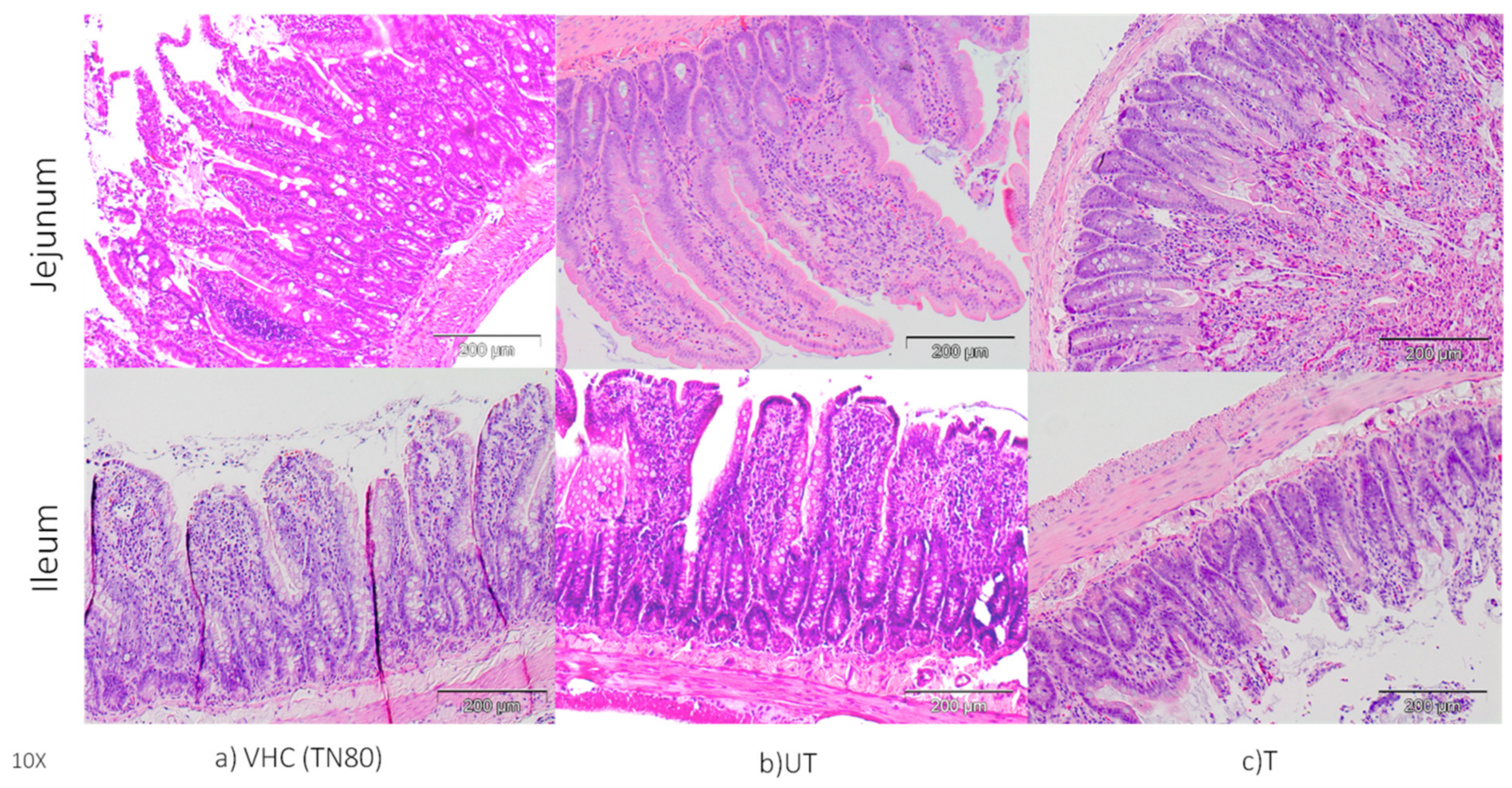
2.3.1. Hematoxylin & Eosin (H & E) Staining
2.3.2. Western Blot Analysis
2.4. Statistical Analysis
3. Results and Discussion
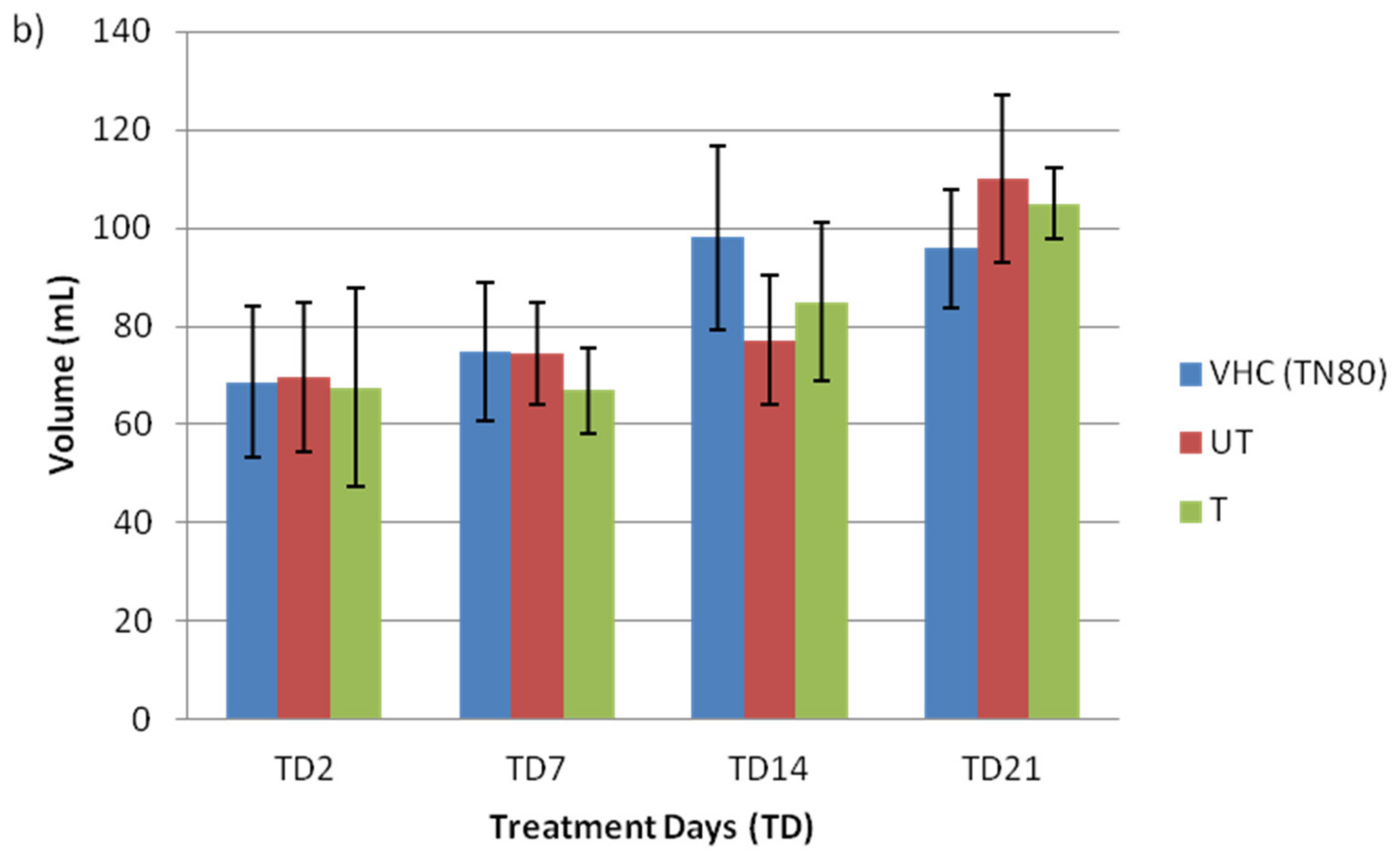
3.1. General Health of Rat Fetus and Mother
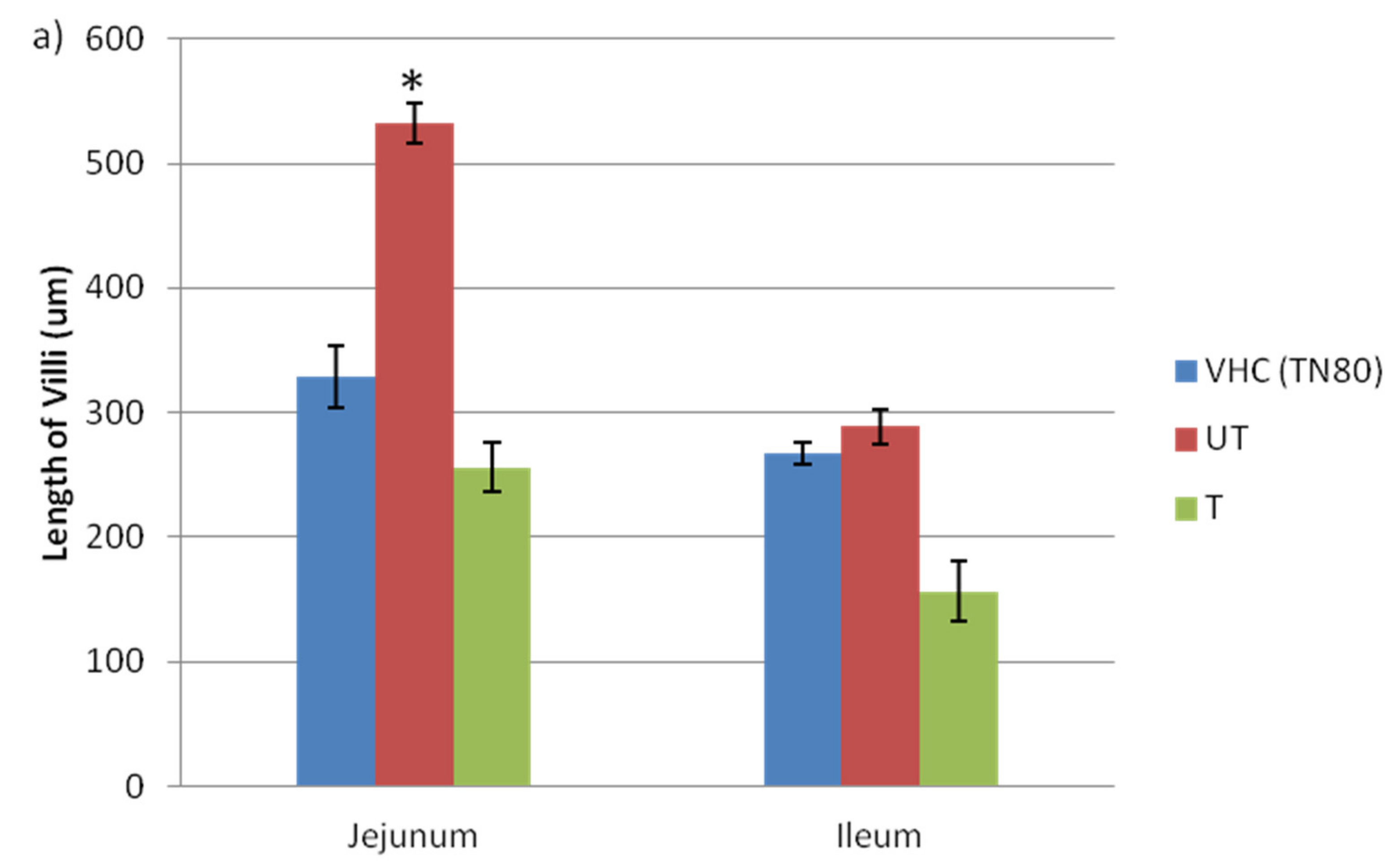
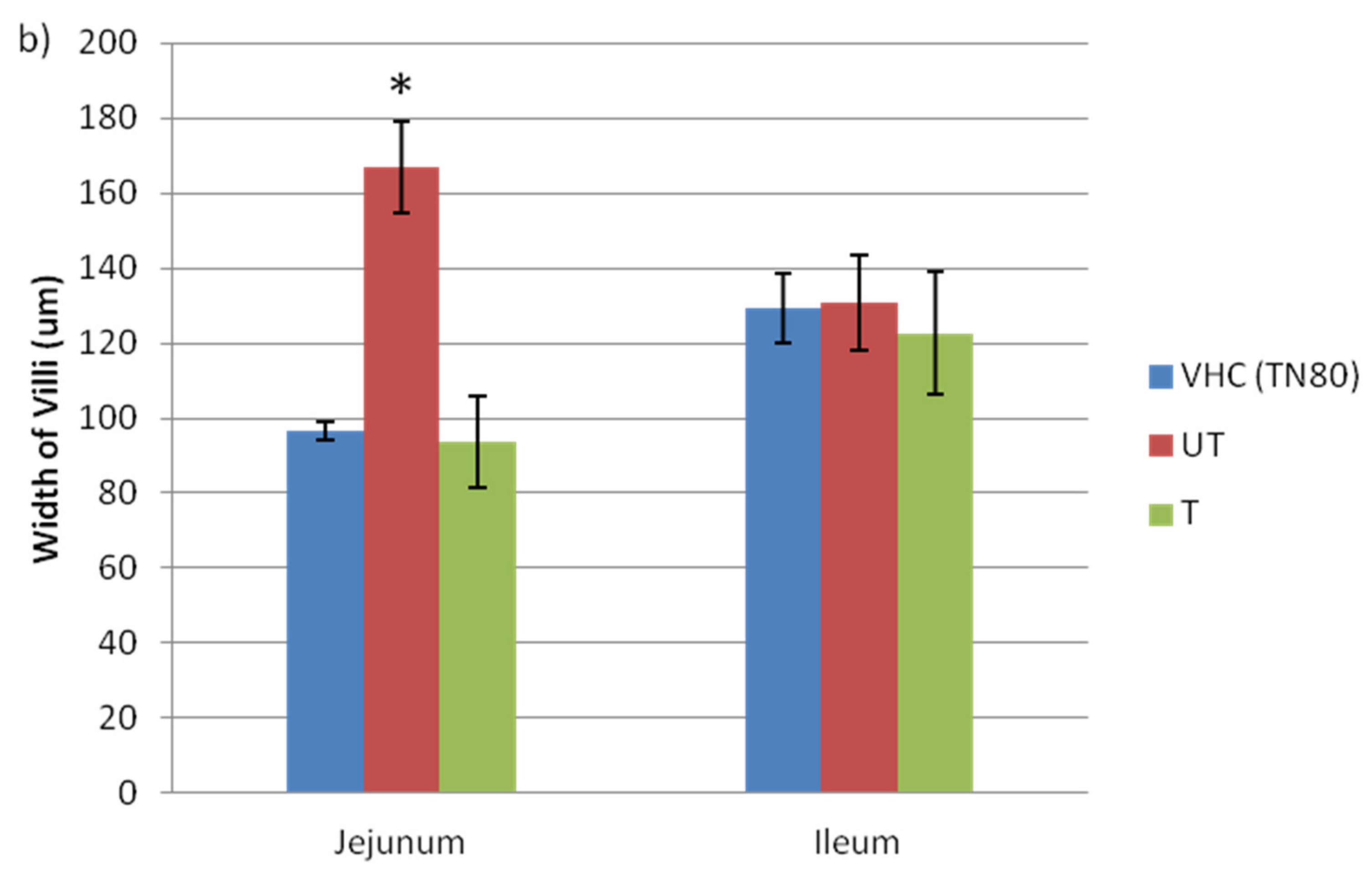
3.2. Changes in Morphology of Jejunum and Ileum and Claudin Protein Expression
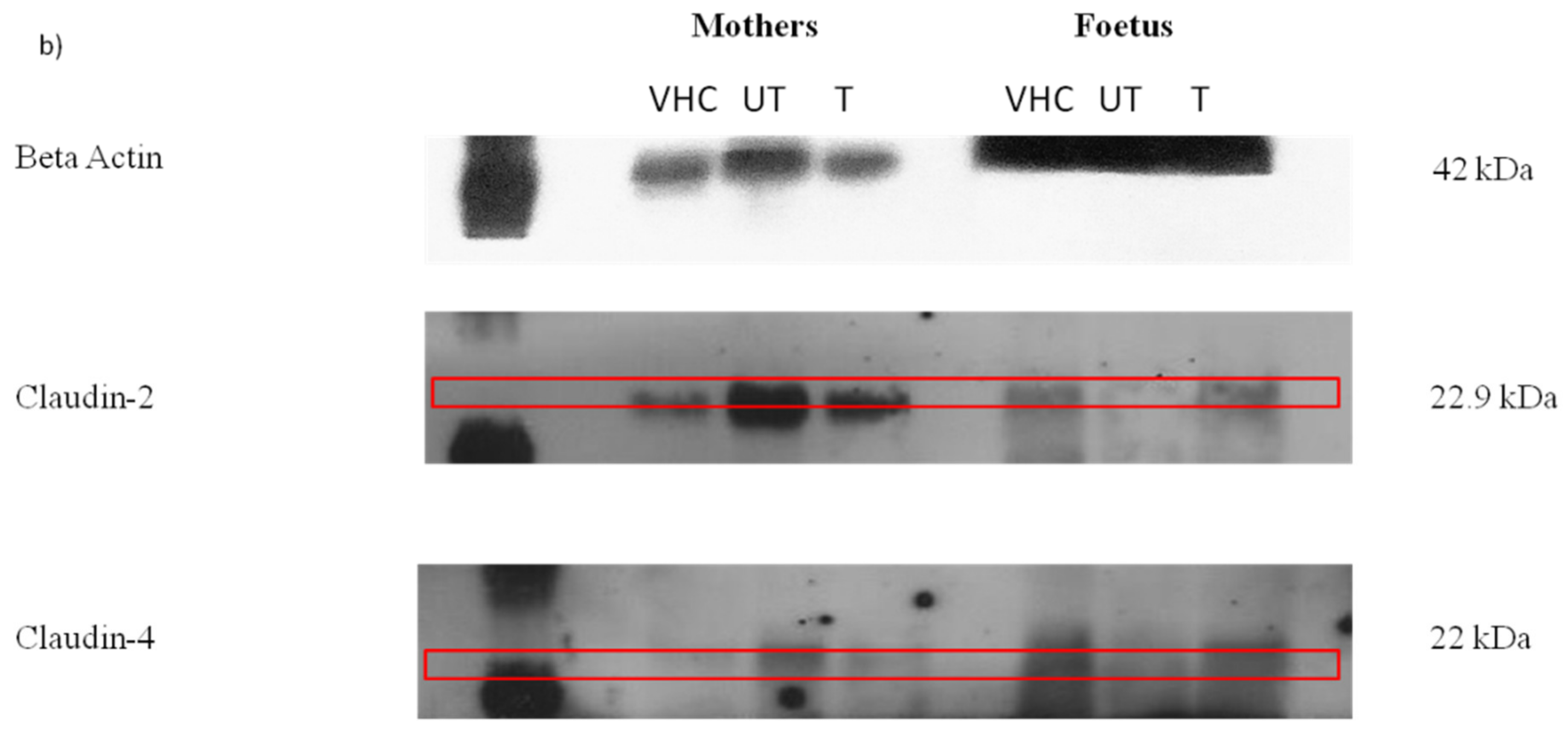
3.3. The Expression of Claudin Protein in Jejunum and Ileum
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Consent for Publication
References
- Braniste, V.; Leveque, M.; Buisson-Brenac, C.; Bueno, L.; Fioramonti, J.; Houdeau, E. Oestradiol decreases colonic permeability through oestrogen receptor β-mediated up-regulation of occludin and junctional adhesion molecule-A in epithelial cells. J. Physiol. 2009, 587, 3317–3328. [Google Scholar] [CrossRef] [PubMed]
- Javurek, A.B.; Spollen, W.G.; Johnson, S.A.; Bivens, N.J.; Bromert, K.H.; Givan, S.A.; Rosenfeld, C.S. Effects of exposure to bisphenol A and ethinyl estradiol on the gut microbiota of parents and their offspring in a rodent model. Gut Microbes 2016, 7, 471–485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szymanska, K.; Makowska, K.; Gonkowski, S. The Influence of High and Low Doses of Bisphenol A (BPA) on the Enteric Nervous System of the Porcine Ileum. Int. J. Mol. Sci. 2018, 19, 917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarkar, K.; Tarafder, P.; Paul, G. Bisphenol A inhibits duodenal movementex vivoof rat through nitric oxide-mediated soluble guanylyl cyclase and α-adrenergic signaling pathways. J. Appl. Toxicol. 2015, 36, 131–139. [Google Scholar] [CrossRef]
- Braniste, V.; Jouault, A.; Gaultier, E.; Polizzi, A.; Buisson-Brenac, C.; Leveque, M.; Martin, P.G.; Theodorou, V.; Fioramonti, J.; Houdeau, E. Impact of oral bisphenol A at reference doses on intestinal barrier function and sex differences after perinatal exposure in rats. Proc. Natl. Acad. Sci. USA 2010, 107, 448–453. [Google Scholar] [CrossRef] [Green Version]
- Van Itallie, C.M.; Anderson, J.M. Claudins and Epithelial Paracellular Transport. Annu. Rev. Physiol. 2006, 68, 403–429. [Google Scholar] [CrossRef]
- Dörfel, M.J.; Huber, O. Modulation of Tight Junction Structure and Function by Kinases and Phosphatases Targeting Occludin. J. Biomed. Biotechnol. 2012, 2012, 807356. [Google Scholar] [CrossRef]
- Hartsock, A.; Nelson, W.J. Adherens and tight junctions: Structure, function and connections to the actin cytoskeleton. Biochim. et Biophys. Acta (BBA)-Biomembr. 2008, 1778, 660–669. [Google Scholar] [CrossRef] [Green Version]
- Weber, C.R.; Nalle, S.C.; Tretiakova, M.; Rubin, D.T.; Turner, J.R. Claudin-1 and claudin-2 expression is elevated in inflammatory bowel disease and may contribute to early neoplastic transformation. Lab. Investig. 2008, 88, 1110–1120. [Google Scholar] [CrossRef] [Green Version]
- Mees, S.T.; Mennigen, R.; Spieker, T.; Rijcken, E.; Senninger, N.; Haier, J.; Bruewer, M. Expression of tight and adherens junction proteins in ulcerative colitis associated colorectal carcinoma: Upregulation of claudin-1, claudin-3, claudin-4, and β-catenin. Int. J. Color. Dis. 2009, 24, 361–368. [Google Scholar] [CrossRef]
- Kamaludin, R.; Othman, M.H.D.; Kadir, S.H.S.A.; Ismail, A.F.; Rahman, M.A.; Jaafar, J. Visible-Light-Driven Photocatalytic N-Doped TiO2 for Degradation of Bisphenol A (BPA) and Reactive Black 5 (RB5) Dye. Water Air Soil Pollut. 2018, 229, 363. [Google Scholar] [CrossRef]
- Kamaludin, R.; Puad, A.S.M.; Othman, M.H.D.; Kadir, S.H.S.A.; Harun, Z. Incorporation of N-doped TiO2 into dual layer hollow fiber (DLHF) membrane for visible light-driven photocatalytic removal of reactive black 5. Polym. Test. 2019, 78, 105939. [Google Scholar] [CrossRef]
- Doll, T.E.; Frimmel, F.H. Removal of selected persistent organic pollutants by heterogeneous photocatalysis in water. Catal. Today 2005, 101, 195–202. [Google Scholar] [CrossRef]
- Kamaludin, R.; Rasdi, Z.; Othman, M.H.D.; Kadir, S.H.S.A.; Nor, N.S.M.; Khan, J.; Zain, W.N.I.W.M.; Ismail, A.F.; A Rahman, M.; Jaafar, J. Visible-Light Active Photocatalytic Dual Layer Hollow Fiber (DLHF) Membrane and Its Potential in Mitigating the Detrimental Effects of Bisphenol A in Water. Membranes 2020, 10, 32. [Google Scholar] [CrossRef] [Green Version]
- Dzinun, H.; Othman, M.H.D.; Ismail, A.F.; Puteh, M.H.; Rahman, M.A.; Jaafar, J. Morphological study of co-extruded dual-layer hollow fiber membranes incorporated with different TiO2 loadings. J. Membr. Sci. 2015, 479, 123–131. [Google Scholar] [CrossRef]
- Dzinun, H.; Othman, M.H.D.; Ismail, A.F.; Puteh, M.H.; Rahman, M.A.; Jaafar, J. Fabrication of Dual Layer Hollow Fibre Membranes for Photocatalytic Degradation of Organic Pollutants. Int. J. Chem. Eng. Appl. 2015, 6, 289–292. [Google Scholar] [CrossRef] [Green Version]
- Hengstler, J.G.; Foth, H.; Gebel, T.; Kramer, P.-J.; Lilienblum, W.; Schweinfurth, H.; Völkel, W.; Wollin, K.-M.; Gundert-Remy, U. Critical evaluation of key evidence on the human health hazards of exposure to bisphenol A. Crit. Rev. Toxicol. 2011, 41, 263–291. [Google Scholar] [CrossRef] [Green Version]
- Desai, M.; Ferrini, M.G.; Han, G.; Jellyman, J.K.; Ross, M.G. In vivo maternal and in vitro BPA exposure effects on hypothalamic neurogenesis and appetite regulators. Environ. Res. 2018, 164, 45–52. [Google Scholar] [CrossRef]
- Somm, E.; Schwitzgebel, V.M.; Toulotte, A.; Cederroth, C.R.; Combescure, C.; Nef, S.; Aubert, M.L.; Hüppi, P.S. Perinatal Exposure to Bisphenol A Alters Early Adipogenesis in the Rat. Environ. Health Perspect. 2009, 117, 1549–1555. [Google Scholar] [CrossRef] [Green Version]
- Matuszczak, E.; Komarowska, M.D.; Debek, W.; Hermanowicz, A. The Impact of Bisphenol A on Fertility, Reproductive System, and Development: A Review of the Literature. Int. J. Endocrinol. 2019, 2019, 4068717. [Google Scholar] [CrossRef] [Green Version]
- Christiansen, S.; Axelstad, M.; Boberg, J.; Vinggaard, A.M.; Pedersen, G.A.; Hass, U. Low-dose effects of bisphenol A on early sexual development in male and female rats. Reproduction 2013, 147, 477–487. [Google Scholar] [CrossRef] [Green Version]
- Caserta, D.; Di Segni, N.I.; Mallozzi, M.; Giovanale, V.; Mantovani, A.; Marci, R.; Moscarini, M. Bisphenol a and the female reproductive tract: An overview of recent laboratory evidence and epidemiological studies. Reprod. Biol. Endocrinol. 2014, 12, 37. [Google Scholar] [CrossRef] [Green Version]
- Reddivari, L.; Veeramachaneni, D.N.R.; Walters, W.A.; Lozupone, C.; Palmer, J.; Hewage, M.K.K.; Bhatnagar, R.; Amir, A.; Kennett, M.J.; Knight, R.; et al. Perinatal Bisphenol A Exposure Induces Chronic Inflammation in Rabbit Offspring via Modulation of Gut Bacteria and Their Metabolites. mSystems 2017, 2, e00093-17. [Google Scholar] [CrossRef] [Green Version]
- Apaydin, F.G.; Uzunhisarcikli, M.; Aslantürk, A.; Kalender, S. Bisphenol A-Induced Histopathological Alterations on Small Intestine Tissues of Rats : The Protective Role of Taurine and Curcumin. Igdir Univ. J. Inst. Sci. Technol. 2018, 8, 43–47. [Google Scholar] [CrossRef] [Green Version]
- Mahmoudi, A.; Ghorbel, H.; Bouallagui, Z.; Marrekchi, R.; Isoda, H.; Sayadi, S. Oleuropein and hydroxytyrosol protect from bisphenol A effects in livers and kidneys of lactating mother rats and their pups’. Exp. Toxicol. Pathol. 2015, 67, 413–425. [Google Scholar] [CrossRef] [Green Version]
- Umar, S. Intestinal Stem Cells. Curr. Gastroenterol. Rep. 2010, 12, 340–348. [Google Scholar] [CrossRef]
- Clevers, H. The Intestinal Crypt, A Prototype Stem Cell Compartment. Cell 2013, 154, 274–284. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Wang, J.; Mou, D.; Che, L.; Fang, Z.; Feng, B.; Lin, Y.; Xu, S.; Li, J.; Wu, D. Maternal Methyl Donor Supplementation during Gestation Counteracts the Bisphenol A-Induced Impairment of Intestinal Morphology, Disaccharidase Activity, and Nutrient Transporters Gene Expression in Newborn and Weaning Pigs. Nutrients 2017, 9, 423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pluske, J.; Thompson, M.; Atwood, C.S.; Bird, P.H.; Williams, I.H.; Hartmann, P. Maintenance of villus height and crypt depth, and enhancement of disaccharide digestion and monosaccharide absorption, in piglets fed on cows’ whole milk after weaning. Br. J. Nutr. 1996, 76, 409–422. [Google Scholar] [CrossRef] [Green Version]
- Dixit, D.; Singh, S.; Tiwari, A.; Mandal, M. Effects of chronic ingestion of Bisphenol A on gut contractility in rats. Natl. J. Physiol. Pharm. Pharmacol. 2017, 7, 1109–1115. [Google Scholar] [CrossRef]
- Hwang, I.; Yang, H.; Kang, H.-S.; Ahn, C.-H.; Lee, G.-S.; Hong, E.-J.; An, B.-S.; Jeung, E.-B. Spatial expression of claudin family members in various organs of mice. Mol. Med. Rep. 2014, 9, 1806–1812. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Peña, A.A.; Rivera-Baños, J.; Méndez-Carrillo, L.L.; Ramírez-Solano, M.I.; Galindo-Bustamante, A.; Páez-Franco, J.C.; Morimoto, S.; González-Mariscal, L.; Cruz, M.E.; Mendoza-Rodríguez, C.A. Perinatal administration of bisphenol A alters the expression of tight junction proteins in the uterus and reduces the implantation rate. Reprod. Toxicol. 2017, 69, 106–120. [Google Scholar] [CrossRef] [PubMed]
- Kozieł, M.J.; Kowalska, K.; Piastowska-Ciesielska, A.W. Claudins: New Players in Human Fertility and Reproductive System Cancers. Cancers 2020, 12, 711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, A.B.; Sharma, A.; Dhawan, P. Claudin Family of Proteins and Cancer: An Overview. J. Oncol. 2010, 2010, 541957. [Google Scholar] [CrossRef]
- Muto, S.; Hata, M.; Taniguchi, J.; Tsuruoka, S.; Moriwaki, K.; Saitou, M.; Furuse, K.; Sasaki, H.; Fujimura, A.; Imai, M.; et al. Claudin-2–deficient mice are defective in the leaky and cation-selective paracellular permeability properties of renal proximal tubules. Proc. Natl. Acad. Sci. USA 2010, 107, 8011–8016. [Google Scholar] [CrossRef] [Green Version]
- Oshima, T.; Miwa, H.; Joh, T. Changes in the expression of claudins in active ulcerative colitis. J. Gastroenterol. Hepatol. 2008, 23 (Suppl. S2), S146–S150. [Google Scholar] [CrossRef]
- Lameris, A.L.; Huybers, S.; Kaukinen, K.; Mäkelä, T.H.; Bindels, R.J.; Hoenderop, J.G.; Nevalainen, P.I. Expression profiling of claudins in the human gastrointestinal tract in health and during inflammatory bowel disease. Scand. J. Gastroenterol. 2013, 48, 58–69. [Google Scholar] [CrossRef]
- Prasad, S.; Mingrino, R.; Kaukinen, K.; Hayes, K.L.; Powell, R.M.; Macdonald, T.T.; Collins, J. Inflammatory processes have differential effects on claudins 2, 3 and 4 in colonic epithelial cells. Lab. Investig. 2005, 85, 1139–1162. [Google Scholar] [CrossRef]
- Pan, X.Y.; Wang, B.; Che, Y.C.; Weng, Z.P.; Dai, H.Y.; Peng, W. Expression of claudin-3 and claudin-4 in normal, hyperplastic, and malignant endometrial tissue. Int. J. Gynecol. Cancer 2007, 17, 233–241. [Google Scholar] [CrossRef]
- Michl, P.; Buchholz, M.; Rolke, M.; Kunsch, S.; Löhr, M.; McClane, B.; Tsukita, S.; Leder, G.; Adler, G.; Gress, T.M. Claudin-4: A new target for pancreatic cancer treatment using Clostridium perfringens enterotoxin. Gastroenterology 2001, 121, 678–684. [Google Scholar] [CrossRef]




| Group | No. Pregnant Rats | No. of Fetuses | Total No. of Foetus | Gender Distribution | |||||
|---|---|---|---|---|---|---|---|---|---|
| Mum 1 | Mum 2 | Mum 3 | Mum 4 | Mum 5 | Male | Female | |||
| VHC (TN80) | 3 | 8 | 10 | 6 | Nil | Nil | 24 | 11 | 13 |
| UT | 5 | 8 | 5 | 7 | 7 | 5 | 32 | 14 | 18 |
| T | 4 | 10 | 10 | 8 | 10 | Nil | 38 | 14 | 24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamaludin, R.; Rasdi, Z.; Othman, M.H.D.; Sheikh Abdul Kadir, S.H.; Idorus, M.Y.; Khan, J.; Wan Mohamad Zain, W.N.I.; Ismail, A.F.; Rahman, M.A.; Jaafar, J. The Effect of BPA-Treated Water on the Small Intestine via an In Vivo Study. Toxics 2022, 10, 296. https://doi.org/10.3390/toxics10060296
Kamaludin R, Rasdi Z, Othman MHD, Sheikh Abdul Kadir SH, Idorus MY, Khan J, Wan Mohamad Zain WNI, Ismail AF, Rahman MA, Jaafar J. The Effect of BPA-Treated Water on the Small Intestine via an In Vivo Study. Toxics. 2022; 10(6):296. https://doi.org/10.3390/toxics10060296
Chicago/Turabian StyleKamaludin, Roziana, Zatilfarihiah Rasdi, Mohd Hafiz Dzarfan Othman, Siti Hamimah Sheikh Abdul Kadir, Mohd Yusri Idorus, Jesmine Khan, Wan Nor I’zzah Wan Mohamad Zain, Ahmad Fauzi Ismail, Mukhlis A. Rahman, and Juhana Jaafar. 2022. "The Effect of BPA-Treated Water on the Small Intestine via an In Vivo Study" Toxics 10, no. 6: 296. https://doi.org/10.3390/toxics10060296
APA StyleKamaludin, R., Rasdi, Z., Othman, M. H. D., Sheikh Abdul Kadir, S. H., Idorus, M. Y., Khan, J., Wan Mohamad Zain, W. N. I., Ismail, A. F., Rahman, M. A., & Jaafar, J. (2022). The Effect of BPA-Treated Water on the Small Intestine via an In Vivo Study. Toxics, 10(6), 296. https://doi.org/10.3390/toxics10060296











