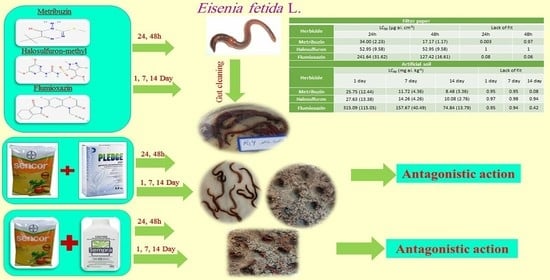
The Survival Response of Earthworm (Eisenia fetida L.) to Individual and Binary Mixtures of Herbicides
Abstract
:1. Introduction
2. Materials and Methods
2.1. Test Organism
2.2. Herbicides
2.3. Toxicity Test Methods
2.3.1. Filter Paper Test
2.3.2. Soil Toxicity Test
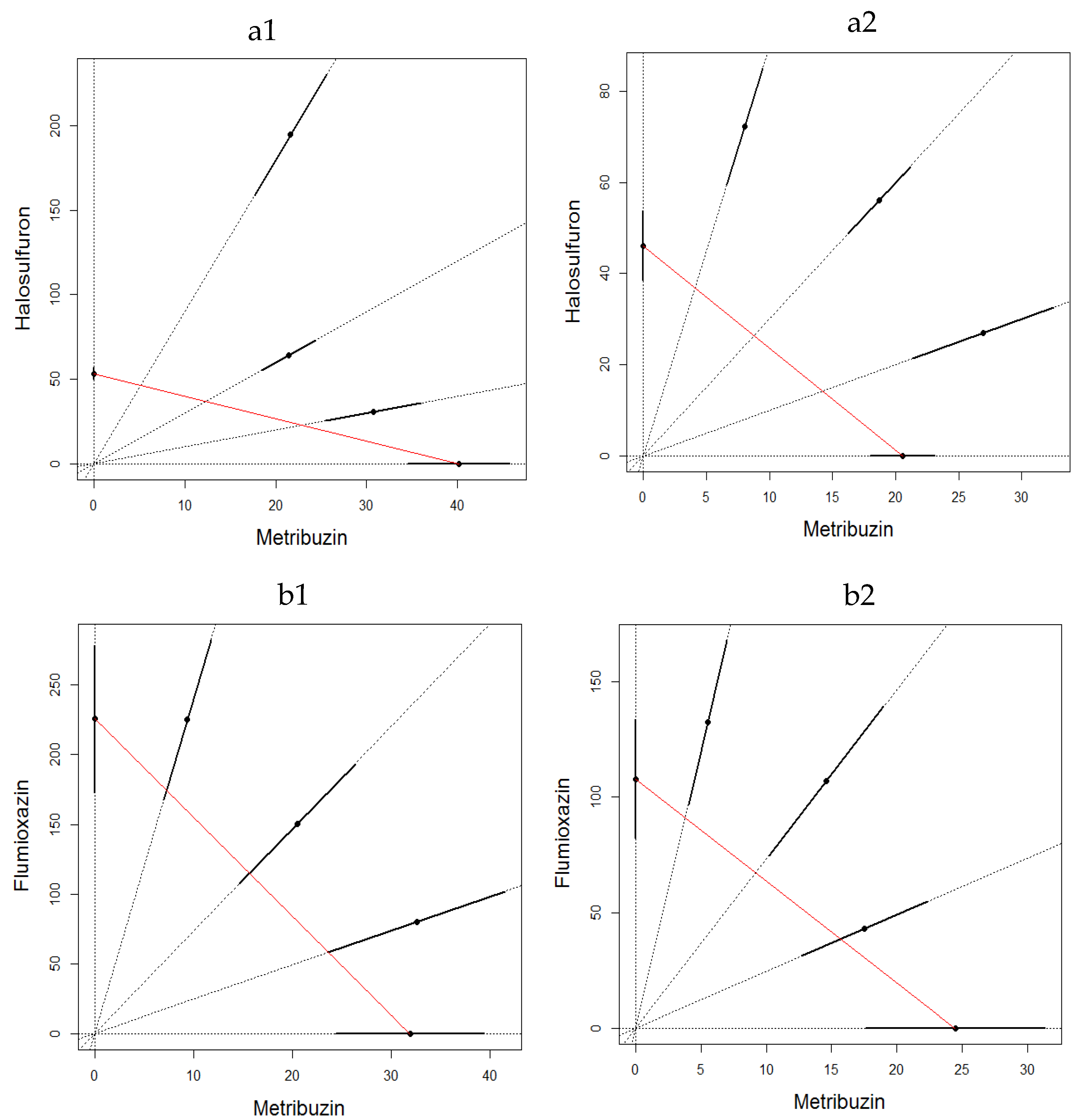
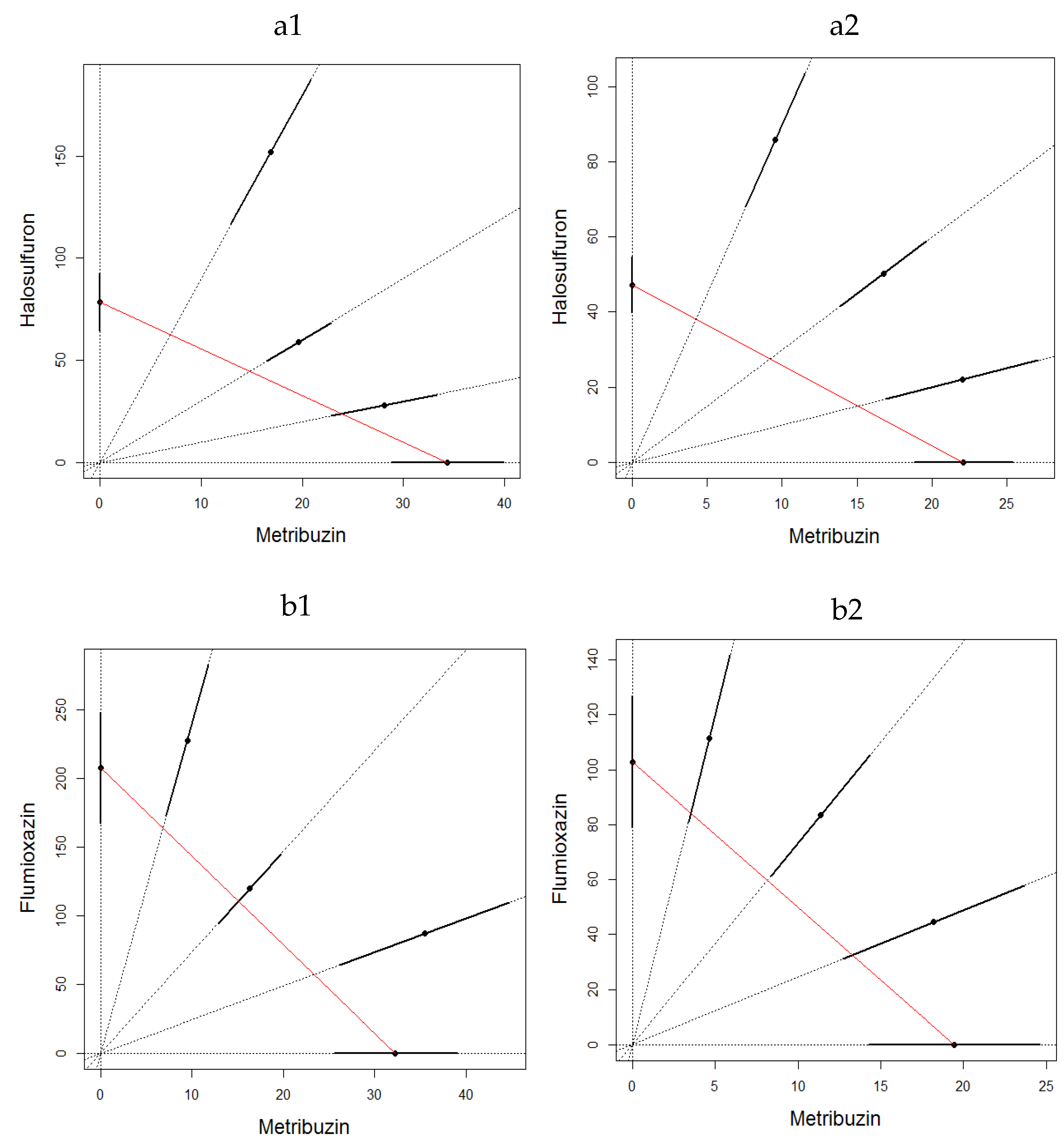
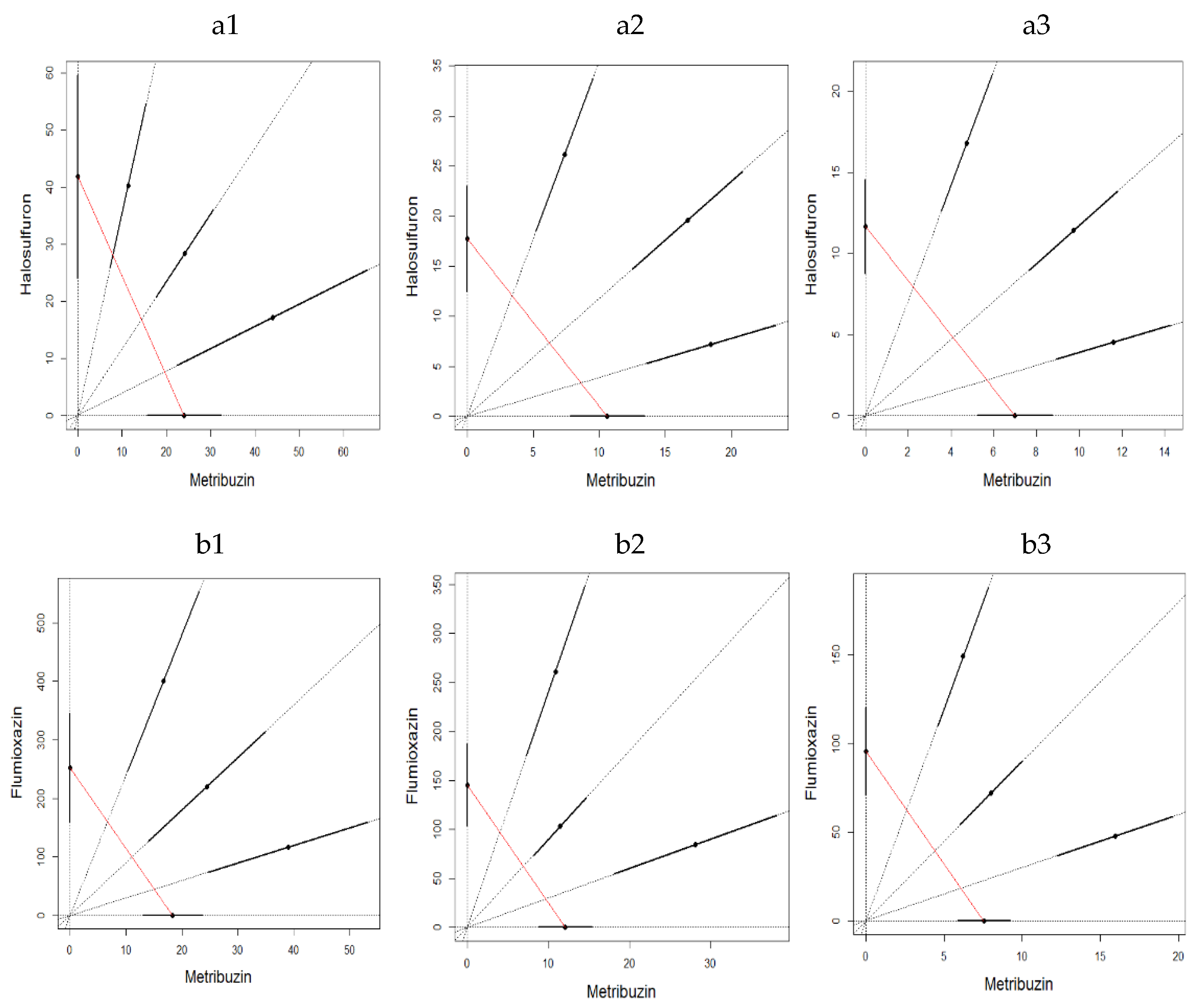
2.3.3. Mixture Toxicity
2.4. Statistical Analysis
3. Results
3.1. Filter Paper Test
3.2. Soil Toxicity Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zarea, M.J.; Karimi, N. Effect of herbicides on earthworms. Dyn. Soil Dyn. Plant 2012, 6, 5–13. [Google Scholar]
- Samadi Kalkhoran, E.; Alebrahim, M.T.; Mohammaddoust Chaman Abad, H.R.; Streibig, J.C.; Ghavidel, A. Investigation of relative toxicity of some combined herbicides on earthworm (Eisenia fetida L.) biomass. Iran. Water Soil Res. 2021, 52, 1661–1672. [Google Scholar] [CrossRef]
- Alebrahim, M.T.; Zangoueinejad, R.; Tseng, T.M.P. Biochemical and molecular knowledge about developing herbicides-resistant weeds. In Herbicides Resistance in Weeds and Crops; Chapter 5; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef] [Green Version]
- Khakzad, R.; Alebrahim, M.T.; Tobeh, A.; Oveisi, M.; Valiollahpor, R.; Tseng, T.M.P. Effects of different management practices on Portulaca oleracea emergence in soyabean. Weed Res. 2019, 59, 279–287. [Google Scholar] [CrossRef]
- Travlos, I.S.; Gkotsi, T.; Roussis, I.; Kontopoulou, C.K.; Kakabouki, I.; Bilalis, D.J. Effects of the herbicides benfluralin, metribuzin and propyzamide on the survival and weight of earthworms (Octodrilus complanatus). Plant. Soil Environ. 2017, 63, 117–124. [Google Scholar] [CrossRef] [Green Version]
- Gill, H.K.; Garg, H. Pesticides-Toxic Aspects. In Environmental Impacts and Management Strategies; Chapter 8: Pesticides; IntechOpen: London, UK; Rijeka, Croatia, 2014. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Saleem, M.; Wang, C. Probiotic strain Stenotrophomonas acidaminiphila BJ1 degrades and reduces chlorothalonil toxicity to soil enzymes, microbial communities and plant roots. AMB Exp. 2017, 7, 227. [Google Scholar] [CrossRef]
- Xuzhi, L.; Wang, M.; Jiang, R.; Zheng, L.; Chen, W. Evaluation of joint toxicity of heavy metals and herbicide mixtures in soils to earthworms (Eisenia fetida). J. Environ. Sci. 2020, 94, 137–146. [Google Scholar] [CrossRef]
- Kumar, K.; Kumawat, P.A. Review of the effect of herbicides on the earthworms. Int. J. Zool. Stud. 2018, 3, 120–125. [Google Scholar]
- Wang, J.H.; Zhu, L.S.; Liu, W.; Wang, J.; Xie, H. Biochemical responses of earthworm (Eisenia fetida) to the pesticides chlorpyrifos and fenvalerate. Toxicol. Mech. Meth. 2012, 22, 236–241. [Google Scholar] [CrossRef]
- Chen, J.; Saleem, M.; Wang, C.; Liang, W.; Zhang, Q. Individual and combined effects of herbicide tribenuron-methyl and fungicide tebuconazole on soil earthworm Eisenia fetida. Sci. Rep. 2018, 8, 2967. [Google Scholar] [CrossRef]
- Hirano, T.; Tamae, K. Earthworms and soil pollutants. Sensors 2011, 11, 11157–11167. [Google Scholar] [CrossRef] [Green Version]
- Nahmani, J.; Hodson, M.E.; Black, S. Effects of metals on life cycle parameters of the earthworm Eisenia fetida exposed to field contaminated, metal-polluted soils. Environ. Pollut. 2007, 149, 44–58. [Google Scholar] [CrossRef] [PubMed]
- Nahmani, J.; Hodson, M.E.; Black, S.A. Review of studies performed to assess metal uptake by earthworms. Environ. Pollut. 2007, 145, 402–424. [Google Scholar] [CrossRef] [PubMed]
- Correia, F.V.; Moreira, J.C. Effects of glyphosate and 2,4-D on earthworms (Eisenia fetida) in laboratory tests. Bull. Environ. Contamin. Toxicol. 2010, 85, 264–268. [Google Scholar] [CrossRef]
- Samadi Kalkhoran, E.; Alebrahim, M.T.; Mohammaddoust Chaman Abad, H.R.; Streibig, J.C.; Ghavidel, A. The joint action of some broadleaf herbicides on potato (Solanum tuberosum L.) weeds and photosynthetic performance of potato. Agriculture 2021, 11, 1103. [Google Scholar] [CrossRef]
- Hanifezadeh Erdi, S.; Alebrahim, M.T.; Majd, R.; Samadi Kalkhoran, E. Effect of application ratio and rimsulfuron, oxadiargyl and metribuzin combination application time on weed biomass and tuber yield of potato (Solanum tuberosum). Iran. J. Weed Sci. 2019, 15, 79–92. [Google Scholar] [CrossRef]
- Mehler, W.T.; Schuler, L.J.; Lydy, M.J. Examining the joint toxicity of chlorpyrifos and atrazine in the aquatic species: Lepomis macrochirus, Pimephales promelas and Chironomus tentans. Environ. Pollut. 2008, 152, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Ohlesson, A.; Cedergreen, N.; Oskarsson, A.; Ulleras, E. Mixture effects of imadazole fungicides on cortisol and aldosterone secretion in human adrenocortical H295R cells. Toxicology 2010, 275, 21–28. [Google Scholar] [CrossRef]
- Wang, N.; Sun, R.; Ma, X.; Wang, X.; Zhou, J. Prediction of joint action of binary mixtures based on characteristic parameter k.Ecx from concentration-response curves. Ecotoxicol. Environ. Saf. 2021, 215, 112155. [Google Scholar] [CrossRef]
- Cedergreen, N.; Christensen, A.M.; Kamper, A.; Kudsk, P.; Mathiassen, S.K.; Streibig, J.C.; Sørensen, H. A review of independent action compared to concentration addition as reference models for mixtures of compounds with different molecular target sites. Environ. Toxicol. Chem. 2008, 27, 1621. [Google Scholar] [CrossRef]
- Streibig, J.C.; Jensen, J.E. Action of herbicides in mixtures. In Herbicides and their Mechanisms of Action; Cobb, A.H., Kirkwood, R.C., Eds.; Sheffield Academic Press: Sheffield, UK, 2000; pp. 153–180. [Google Scholar]
- Wang, Y.; An, X.; Shen, W.; Chen, L.; Jiang, J.; Wang, Q.; Cai, L. Individual and combined toxic effects of herbicide atrazine and three insecticides on the earthworm, Eisenia fetida. Ecotoxicology 2016, 25, 991–999. [Google Scholar] [CrossRef]
- OECD. Guidelines for the Testing of Chemicals No. 222. Earthworm Reproduction Test (Eisenia fetida/Eisenia andrei); Organisation for Economic Co-Operation and Development: Paris, France, 2004; pp. 1–9. [Google Scholar]
- Grichar, W.J.; Besler, B.A.; Brewer, K.D. Purple nutsedge control and potato (Solanum tuberosum) tolerance to sulfentrazone and halosulfuron. Weed Technol. 2003, 17, 485–490. [Google Scholar] [CrossRef]
- Hutchinson, P.J.S. A comparison of flumioxazin and rimsulfuron tank mixtures for weed control in potato. Weed Technol. 2007, 21, 1023–1028. [Google Scholar] [CrossRef]
- Alebrahim, M.T.; Majd, R.; Rashed Mohassel, M.H.; Wilkakson, S.; Baghestani, M.A.; Ghorbani, R.; Kudsk, P. Evaluation the efficacy of pre- and post-emergence herbicides for controlling Amaranthus retroflexus and Chenopodium album in potato. Crop Protect. 2012, 42, 346–350. [Google Scholar] [CrossRef]
- Chand, M.; Singh, S.; Bir, D.; Singh, N.; Kumar, V. Halosulfuron methyl: A new post emergence herbicide in India for effective control of Cyperus rotundus in sugarcane and its residual effects on the succeeding crops. Sugar Tech 2014, 16, 67–74. [Google Scholar] [CrossRef]
- Vencill, W.K. Herbicide Handbook, 8th ed.; Weed Science Society of America: Lawrence, KS, USA, 2002; p. 477. [Google Scholar]
- Mossler, M.A.; Langeland, K.A. Florida Crop/Pest Management Profile: Aquatic Weeds; University of Florida IFAS Extension: Gainesville, FL, USA, 2006; Available online: http://edis.ifas.ufl.edu/pdffiles/PI/PI17500.pdf (accessed on 8 January 2008).
- Vasilakoglou, I.; Dhima, K.; Paschalidis, K.; Gatsis, T.; Zacharis, K.; Galanis, M. Field bindweed (Convolvulus arvensis L.) and redroot pigweed (Amaranthus retroflexus L.) control in potato by pre- or post-emergence applied flumioxazin and sulfosulfuron. Chil. J. Agric. Res. 2013, 73, 24–30. [Google Scholar] [CrossRef] [Green Version]
- Xue, Y.G.; Gu, X.Y.; Wang, X.R.; Sun, C.; Xu, X.H.; Sun, J.; Zhang, B.G. The hydroxyl radical generation and oxidative stress for the earthworm Eisenia fetida exposed totetrabromobisphenol A. Ecotoxicology 2009, 18, 693–699. [Google Scholar] [CrossRef]
- Ritz, C.; Baty, F.; Streibig, J.C.; Gerhard, D. Dose-response analysis using R. PLoS ONE 2015, 10, e146021. [Google Scholar] [CrossRef] [Green Version]
- Ritz, C.; Streibig, J.C.; Kniss, A. How to use statistics to claim antagonism and synergism from inary mixture expriments. Pest. Manag. Sci. 2020, 77, 3890–3899. [Google Scholar] [CrossRef]
- Gessner, P.K. Isobolographic analysis of interactions: An update on applications and utility. Toxicology 1995, 105, 161–179. [Google Scholar] [CrossRef]
- Streibig, J.C.; Kudsk, P.; Jensen, J.E. A general joint action model for herbicide mixtures. Pestic. Sci. 1998, 53, 21–28. [Google Scholar] [CrossRef]
- Iordache, M.; Borza, I. Study of the acute toxicity of some pesticides on earthworms Eisenia fetida (Savigny, 1826). Res. J. Agric. Sci. 2011, 43, 95–100. [Google Scholar]
- Pelosi, C.; Barot, S.; Capowiez, Y.; Hedde, M.; Vandenbulcke, F. Pesticides and earthworms. A review. Agron. Sustain. Dev. 2014, 34, 199–228. [Google Scholar] [CrossRef] [Green Version]
- Pertile, M.; Antunes, J.E.L.; Araujo, F.F.E.; Mendes, L.W.; Van den Brink, P.J.; Araujo, A.S.F. Responses of soil microbial biomass and enzyme activity to herbicides imazethapyr and flumioxazin. Sci. Rep. 2020, 10, 7694. [Google Scholar] [CrossRef]
- Landrum, M.; Can˜as, J.E.; Coimbatore, G.; Cobb, G.P.; Jackson, W.A.; Zhang, B.; Anderson, T.A. Effects of perchlorate on earthworm (Eisenia fetida) survival and reproductive success. Sci. Total Environ. 2006, 363, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Burrows, L.A.; Edwards, C.A. The use of integrated soil microcosms to predict effects of pesticides on soil ecosystems. Eur. J. Soil Biol. 2002, 38, 245–249. [Google Scholar] [CrossRef]
- Shan, J.; Wang, Y.; Wang, L.; Yan, X.; Ji, R. Effects of the geophagous earthworm Metaphire guillelmi on sorption, mineralization, and bound-residue formation of 4-nonylphenol in an agricultural soil. Environ. Pollut. 2014, 189, 202–207. [Google Scholar] [CrossRef]
- Abdollahi, F.; Alebrahim, M.T.; Ngov, C.H.; Lallemand, E.; Zheng, y.; Villette, C.; Zumsteg, J.; André, F.; Navrot, N.; Werck-Reichhart, D.; et al. Innate promiscuity of the CYP706 family of P450 enzymes provides a suitable context for the evolution of dinitroaniline resistance in weed. New Phytol. 2021, 229, 3253–3268. [Google Scholar] [CrossRef] [PubMed]
- Alebrahim, M.T.; Majd, R.; Abdollahi, F.; Zangoueinejad, R.; Dayan, F.E.; Mathiassen, S.K.; Kudsk, P. Absorption and Metabolism of Foliar-Applied Rimsulfuron in Potato (Solanum tuberosum L.), Common Lambsquarters (Chenopodium album L.) and Redroot Pigweed (Amaranthus retroflexus L.). Potato Res. 2021, 64, 635–648. [Google Scholar] [CrossRef]
- Hyzak, D.L.; Zimdahl, R.L. Rate of degradation of metribu zin and two analogs in soil. Weed Sci. 1974, 22, 75–79. [Google Scholar] [CrossRef]
- Teuschler, L.K. Deciding which chemical mixtures risk assessment methods work best for what mixtures. Toxicol. Appl. Pharma 2007, 223, 139–147. [Google Scholar] [CrossRef]
- Chou, T.C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharma Rev. 2006, 58, 621–681. [Google Scholar] [CrossRef] [PubMed]
- Sigurnjak Bures, M.; Cvetnic, M.; Miloloza, M.; Kucic Grgic, D.; Markic, M.; Kusic, H.; Bolanca, T.; Rogosic, M. Modeling the toxicity of pollutants mixtures for risk assessment: A review. Environ. Chem. Lett. 2021, 19, 1629–1655. [Google Scholar] [CrossRef]
- Stepić, S.; Hackenberger, B.K.; Velki, M.; Lončarić, Ž.; Hackenberger, D.K. Effects of individual and binary-combined commercial insecticides endosulfan, temephos, malathion and pirimiphos-methyl on biomarker responses in earthworm Eisenia andrei. Environ. Toxicol. Pharma 2013, 36, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Lowe, C.N.; Butt, K.R. Earthworm culture, maintenance and species selection in chronic ecotoxicological studies: A critical review. Eur. J. Soil Biol. 2007, 43, S281–S288. [Google Scholar] [CrossRef]
- Svendsen, C.; Weeks, J.M. A simple low-cost field mesocosm for ecotoxicological studies on earthworms. Comp. Biochem. Physiol. Part C: Pharmacol. Toxicol. Endocrinol. 1997, 117, 31–40. [Google Scholar] [CrossRef]
- Heimbach, F. Correlations between three methods for determining the toxicity of chemicals to earthworms. Pestic. Sci. 1984, 15, 605–611. [Google Scholar] [CrossRef]
- Culy, M.D.; Berry, E.C. Toxicity of soil-applied granular insecticides to earthworm populations in cornfields. Down Earth 1995, 50, 20–25. [Google Scholar]
- Holmstrup, M. Field assessment of toxic effects on reproduction in the earthworms Aporrectodea longa and Aporrectodea rosea. Environ. Toxicol. Chem. 2000, 19, 1781–1787. [Google Scholar] [CrossRef]
- Svendsen, T.S.; Hansen, P.E.; Sommer, C.; Martinussen, T.; Gronvold, J.; Holter, P. Life history characteristics of Lumbricus terrestris and effects of the veterinary antiparasitic compounds ivermectin and fenbendazole. Soil Biol. Biochem. 2005, 37, 927–936. [Google Scholar] [CrossRef]

| Herbicide | Concentration for Filter Paper Test (µg ai. cm−2) |
| Metribuzin | 0, 0.156, 0.312, 0.625, 1.25, 2.5, 3.5, 5, 7, 10, 14, 28, 56, 112, 224 |
| Halosulfuron | 0, 0.0156, 0.0312, 0.0625, 0.125, 0.250, 0.500, 1, 2, 4, 8, 16, 32, 64, 128, 256 |
| Flumioxazin | 0, 10, 20, 40, 80, 160, 320, 640 |
| Concentration for artificial soil test (mg ai. kg−1) | |
| Metribuzin | 0, 0.359, 0.718, 1.436, 2.872, 5.744, 11.488, 22.976 |
| Halosulfuron | 0, 0.2051, 0.410, 0.820, 1.640, 3.281, 6.563, 13.126 |
| Flumioxazin | 0, 4.1026, 8.205, 16.41, 32.82, 65.64, 131, 262 |
| Filter paper | ||||||||||||
| Herbicide | LC50 (µg ai. cm−2) | Lack of fit | ||||||||||
| 24 h | 48 h | 24 h | 48 h | |||||||||
| Metribuzin | 34.00 (2.23) | 17.17 (1.17) | 0.003 | 0.97 | ||||||||
| Halosulfuron | 52.95 (9.58) | 52.95 (9.58) | 1 | 1 | ||||||||
| Flumioxazin | 241.64 (31.62) | 127.42 (16.61) | 0.08 | 0.06 | ||||||||
| Artificial soil | ||||||||||||
| Herbicide | LC50 (mg ai. kg−1) | Lack of fit | ||||||||||
| 1 day | 7 days | 14 days | 1 day | 7 days | 14 days | |||||||
| Metribuzin | 25.75 (12.44) | 11.72 (4.36) | 8.48 (3.36) | 0.95 | 0.95 | 0.08 | ||||||
| Halosulfuron | 27.63 (13.38) | 14.26 (4.26) | 10.08 (2.76) | 0.97 | 0.98 | 0.94 | ||||||
| Flumioxazin | 315.09 (115.05) | 157.67 (40.49) | 74.84 (13.79) | 0.95 | 0.94 | 0.42 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samadi Kalkhoran, E.; Alebrahim, M.T.; Mohammaddoust Chamn Abad, H.R.; Streibig, J.C.; Ghavidel, A.; Tseng, T.-M.P. The Survival Response of Earthworm (Eisenia fetida L.) to Individual and Binary Mixtures of Herbicides. Toxics 2022, 10, 320. https://doi.org/10.3390/toxics10060320
Samadi Kalkhoran E, Alebrahim MT, Mohammaddoust Chamn Abad HR, Streibig JC, Ghavidel A, Tseng T-MP. The Survival Response of Earthworm (Eisenia fetida L.) to Individual and Binary Mixtures of Herbicides. Toxics. 2022; 10(6):320. https://doi.org/10.3390/toxics10060320
Chicago/Turabian StyleSamadi Kalkhoran, Elham, Mohammad Taghi Alebrahim, Hamid Reza Mohammaddoust Chamn Abad, Jens Carl Streibig, Akbar Ghavidel, and Te-Ming Paul Tseng. 2022. "The Survival Response of Earthworm (Eisenia fetida L.) to Individual and Binary Mixtures of Herbicides" Toxics 10, no. 6: 320. https://doi.org/10.3390/toxics10060320
APA StyleSamadi Kalkhoran, E., Alebrahim, M. T., Mohammaddoust Chamn Abad, H. R., Streibig, J. C., Ghavidel, A., & Tseng, T.-M. P. (2022). The Survival Response of Earthworm (Eisenia fetida L.) to Individual and Binary Mixtures of Herbicides. Toxics, 10(6), 320. https://doi.org/10.3390/toxics10060320