In Situ Measurements of Domestic Water Quality and Health Risks by Elevated Concentration of Heavy Metals and Metalloids Using Monte Carlo and MLGI Methods
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Water Samples Collection, Preparation, and Analysis
2.3. HM Pollution and HR Assessment
2.3.1. HMM Pollution Index (MPI)
2.3.2. Single-Factor Pollution Index (SFPI)
2.3.3. Nemerow’s Pollution Index (NPI)
2.3.4. Non-CR and CR Index
2.4. MCS and SA
2.5. Statistical Analysis
2.6. Spatial Concentration Mapping of Risk Indices Using Machine Learning Geostatistical Interpolation (MLGI) Method
3. Results
3.1. HMM Concentration in Water Samples
3.2. MPI and NPI Results
3.3. Human HR Assessment
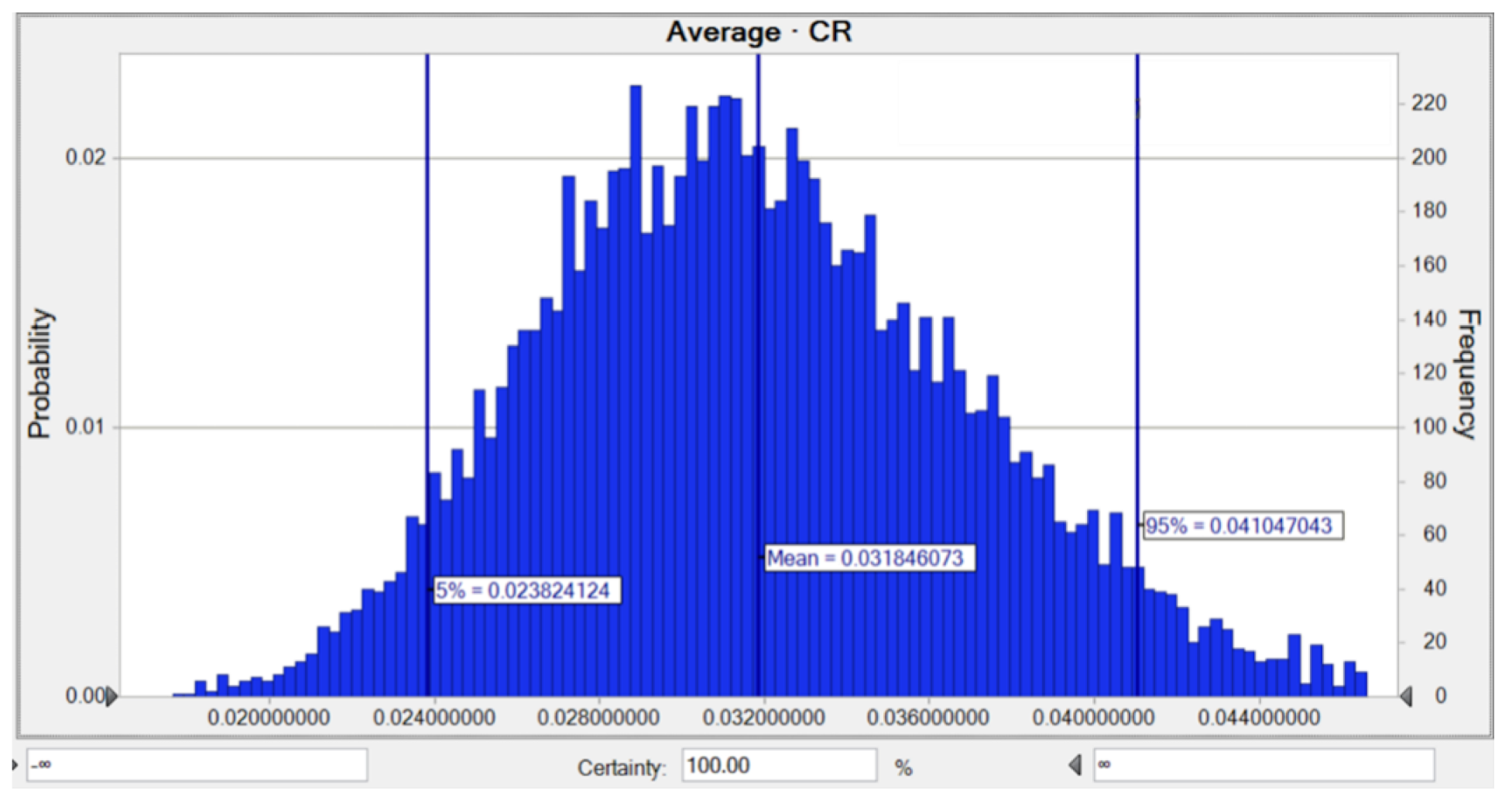
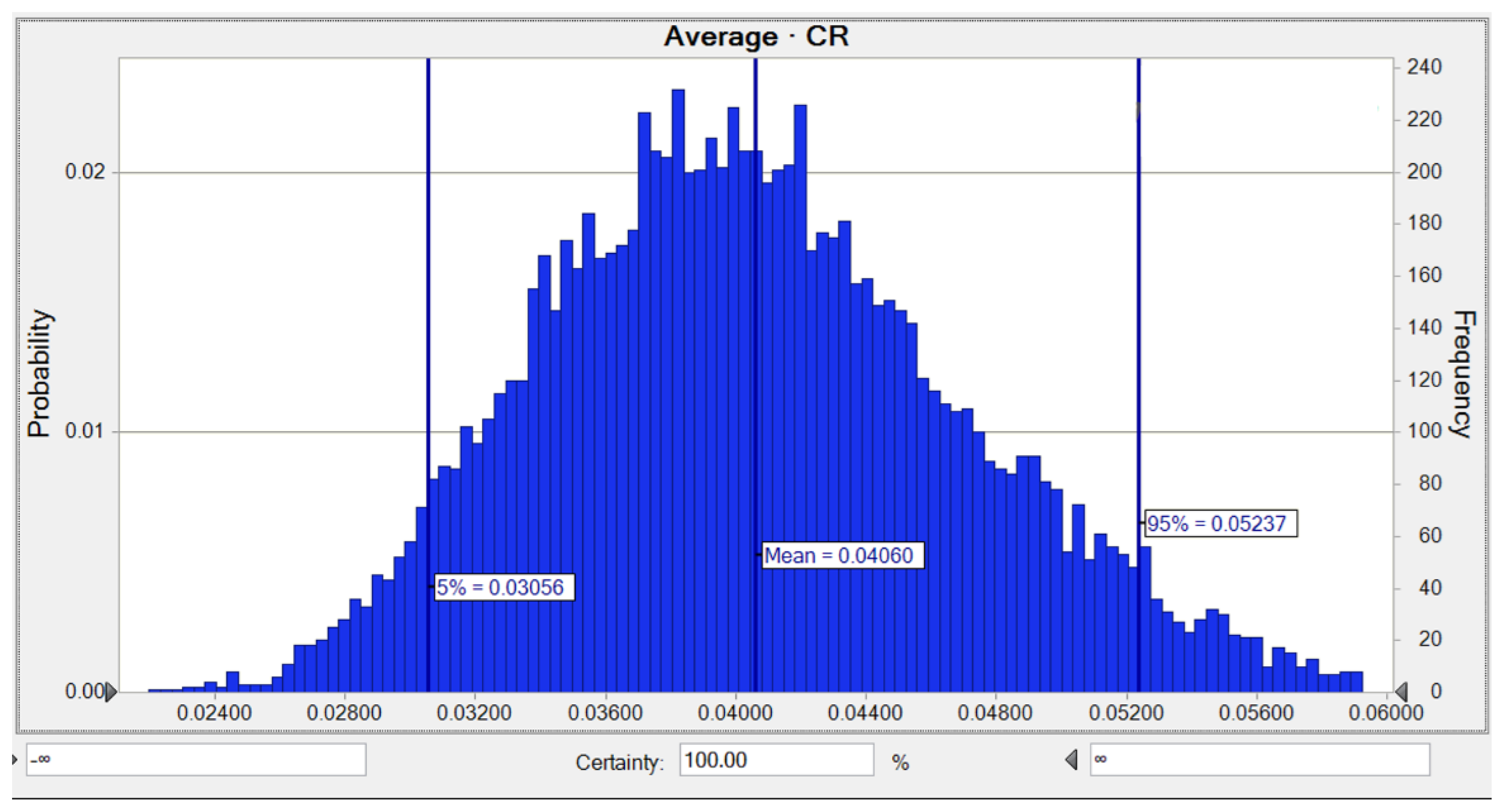
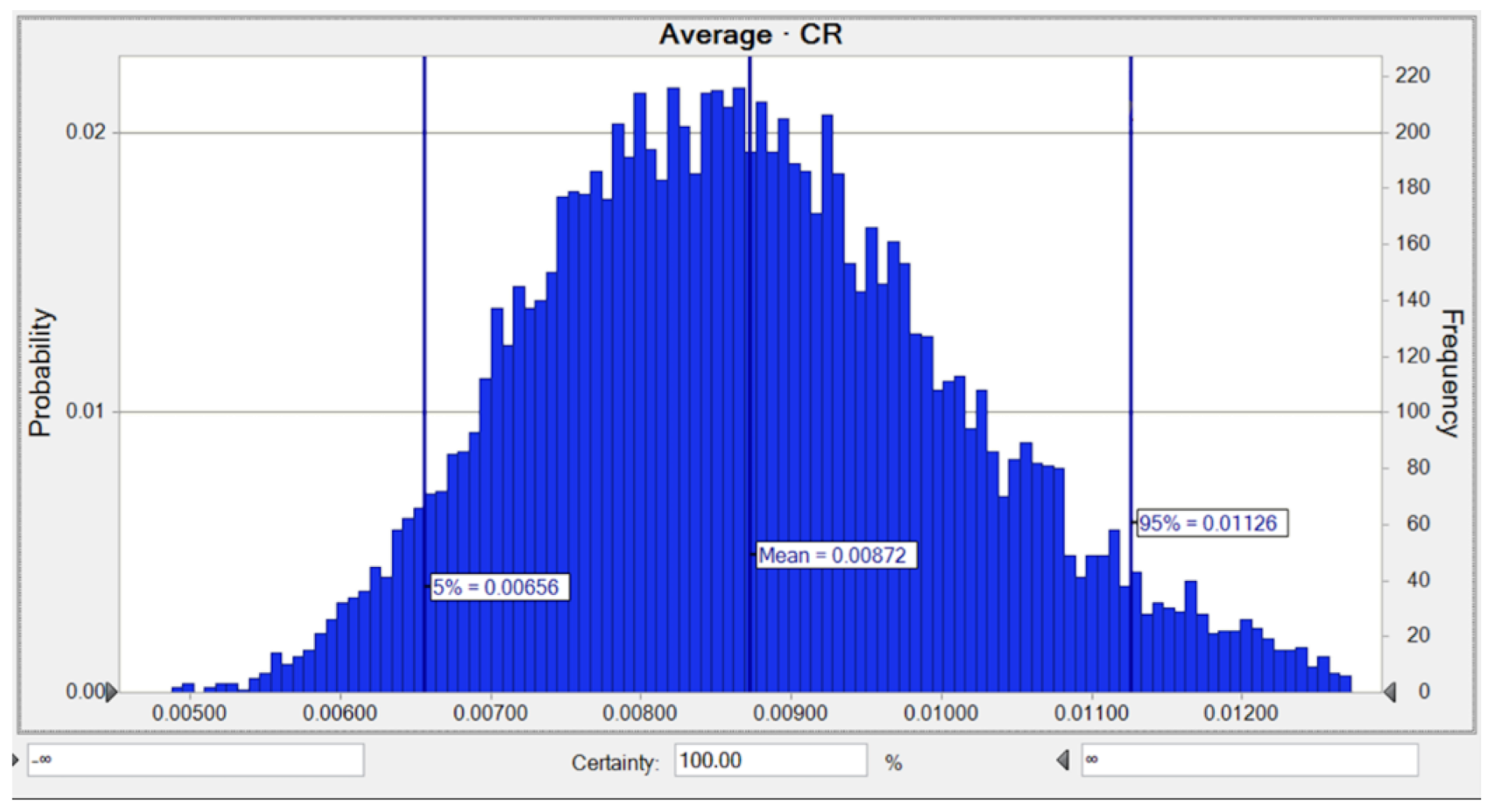
3.4. Monte Carlo Simulation and Sensitivity Analysis
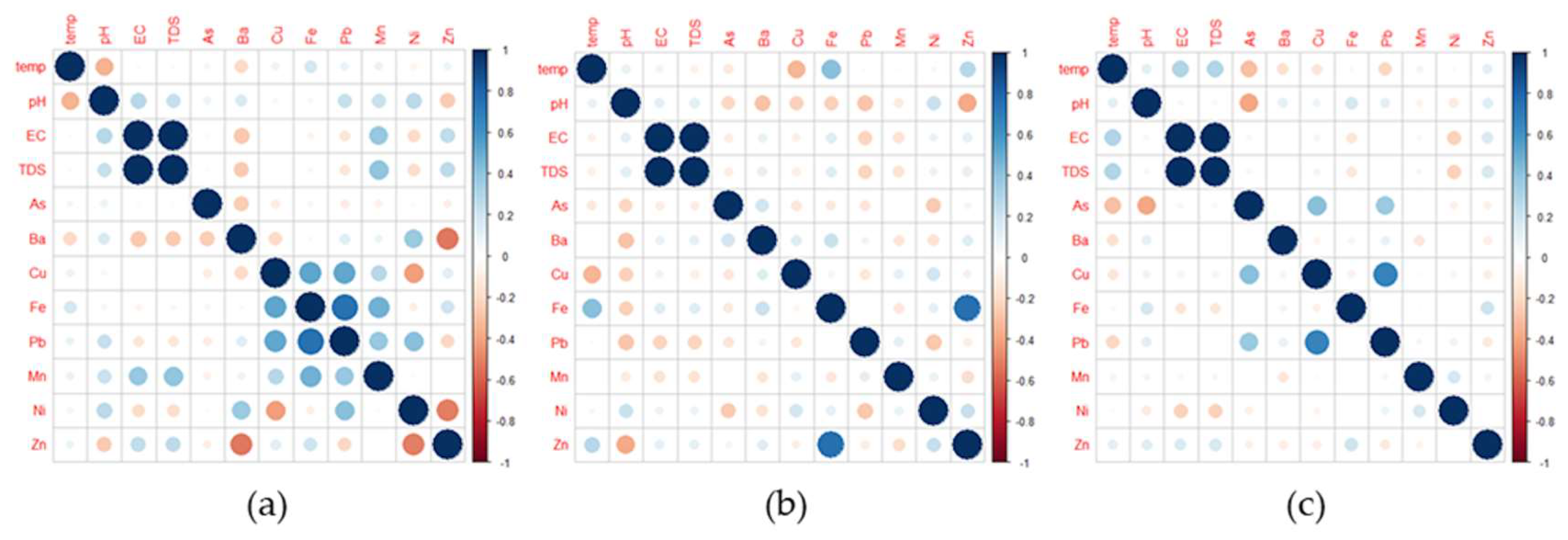
3.5. Relationship of WQ Parameters in the Domestic Water Samples
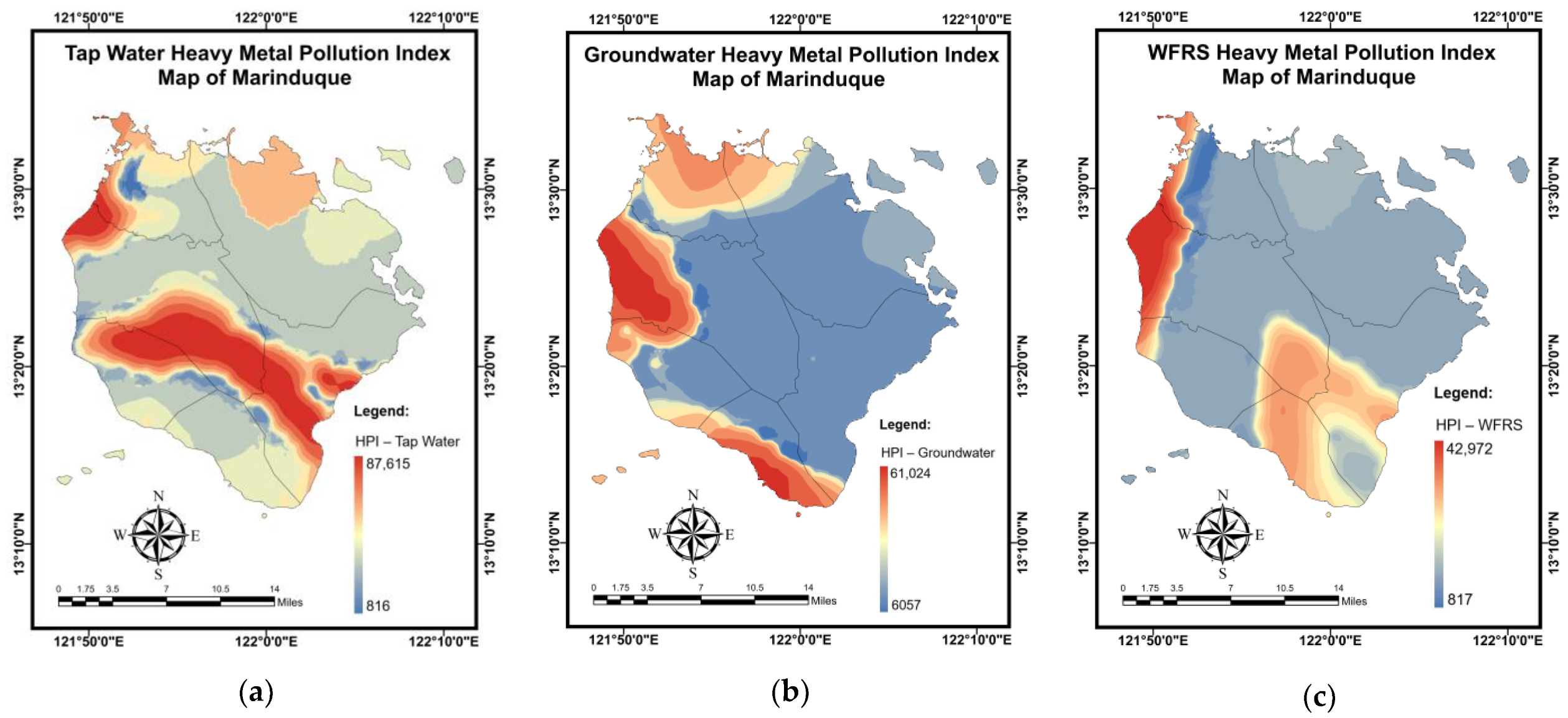
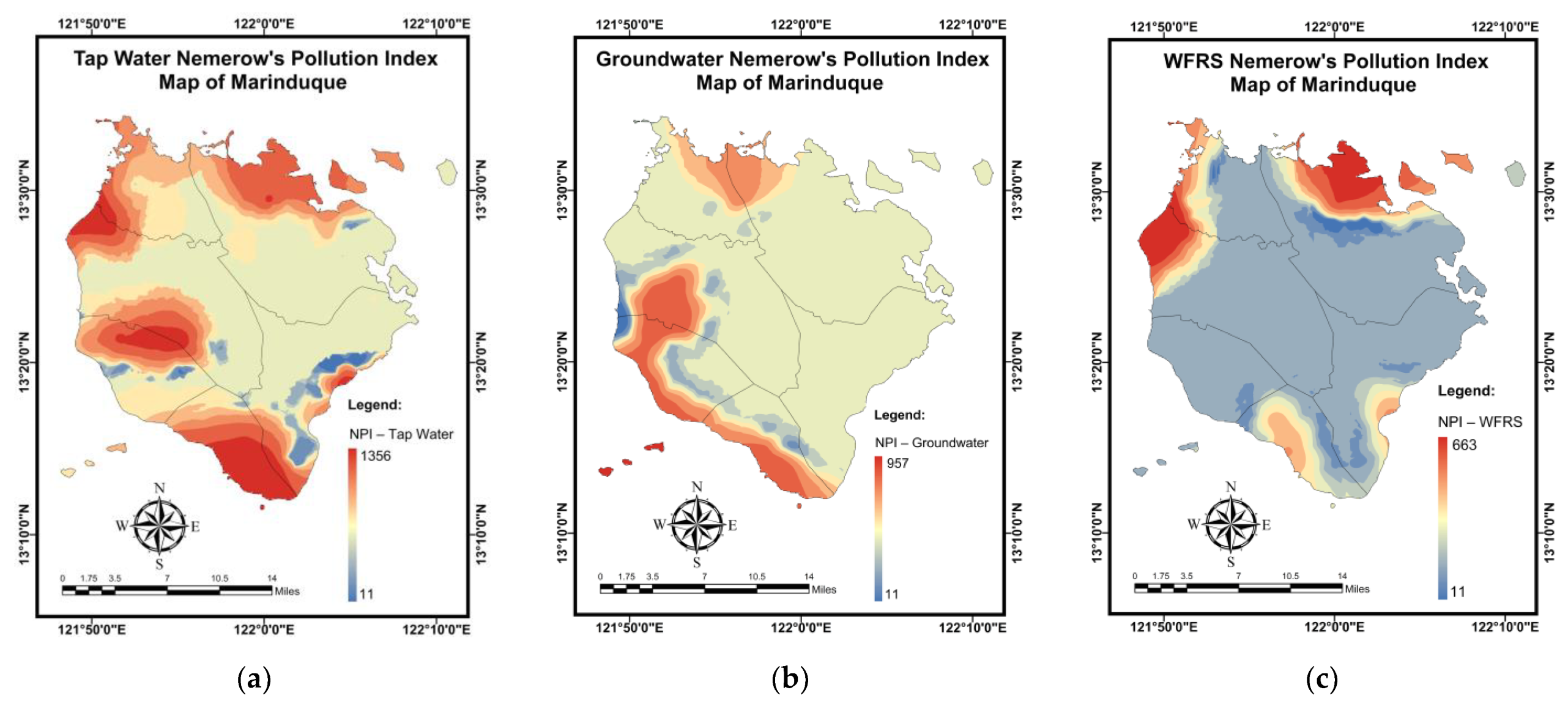
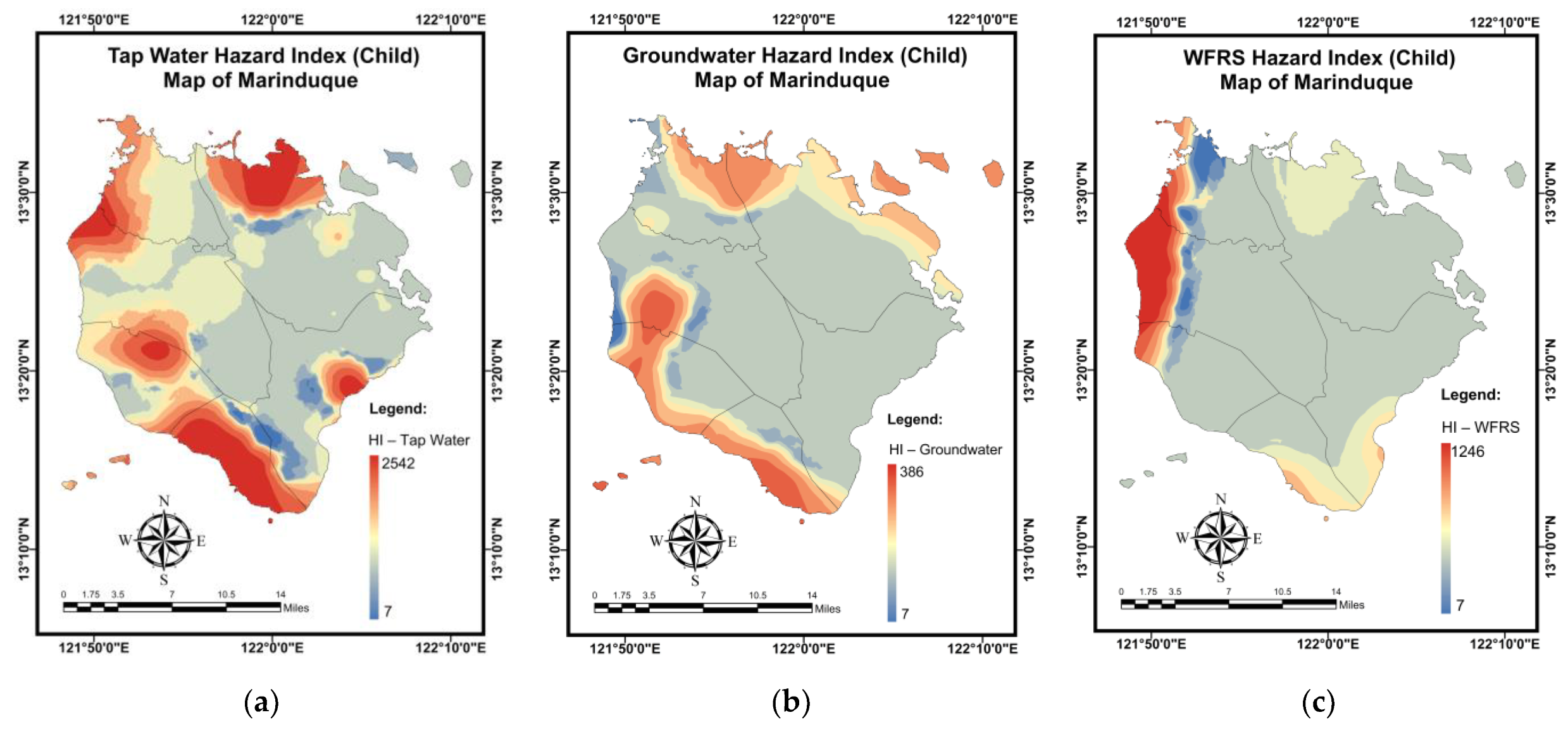
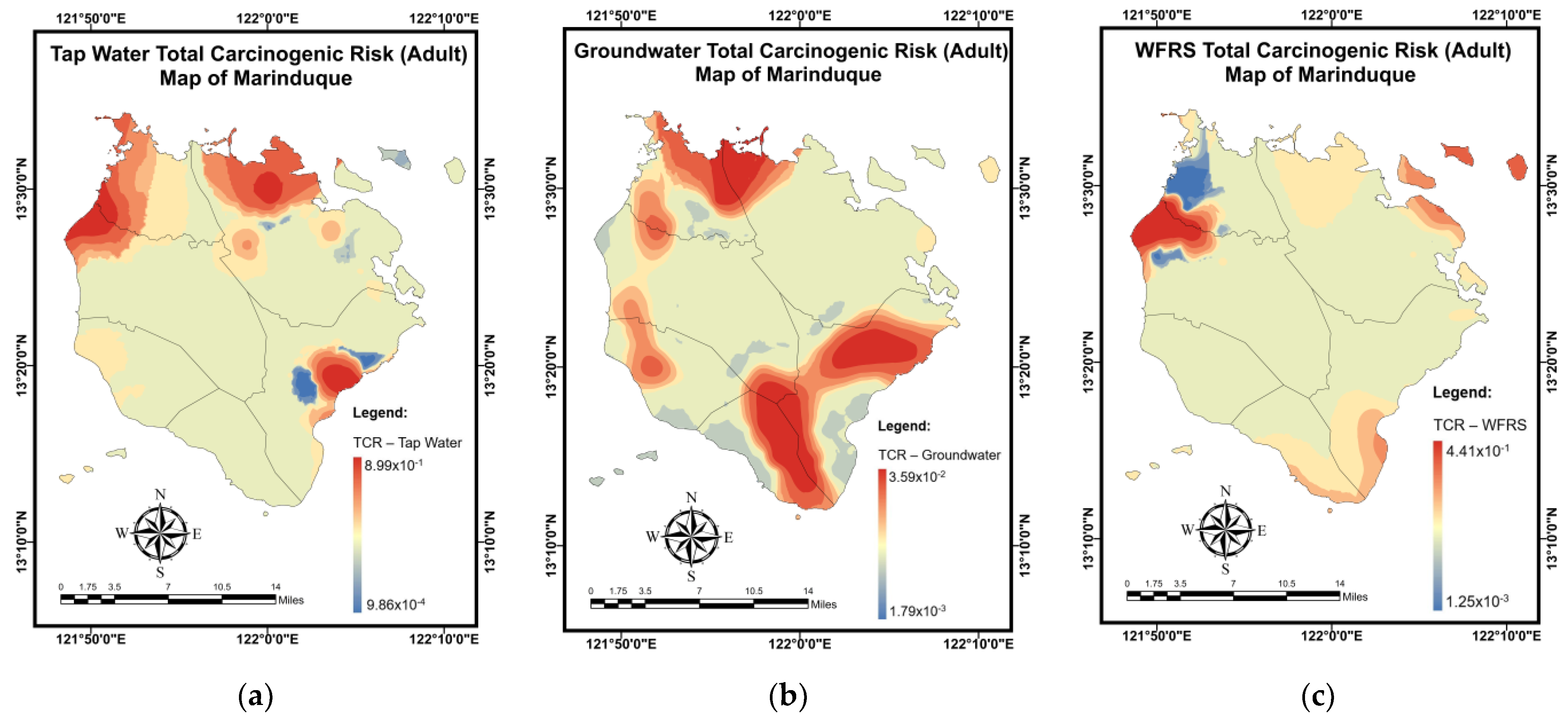
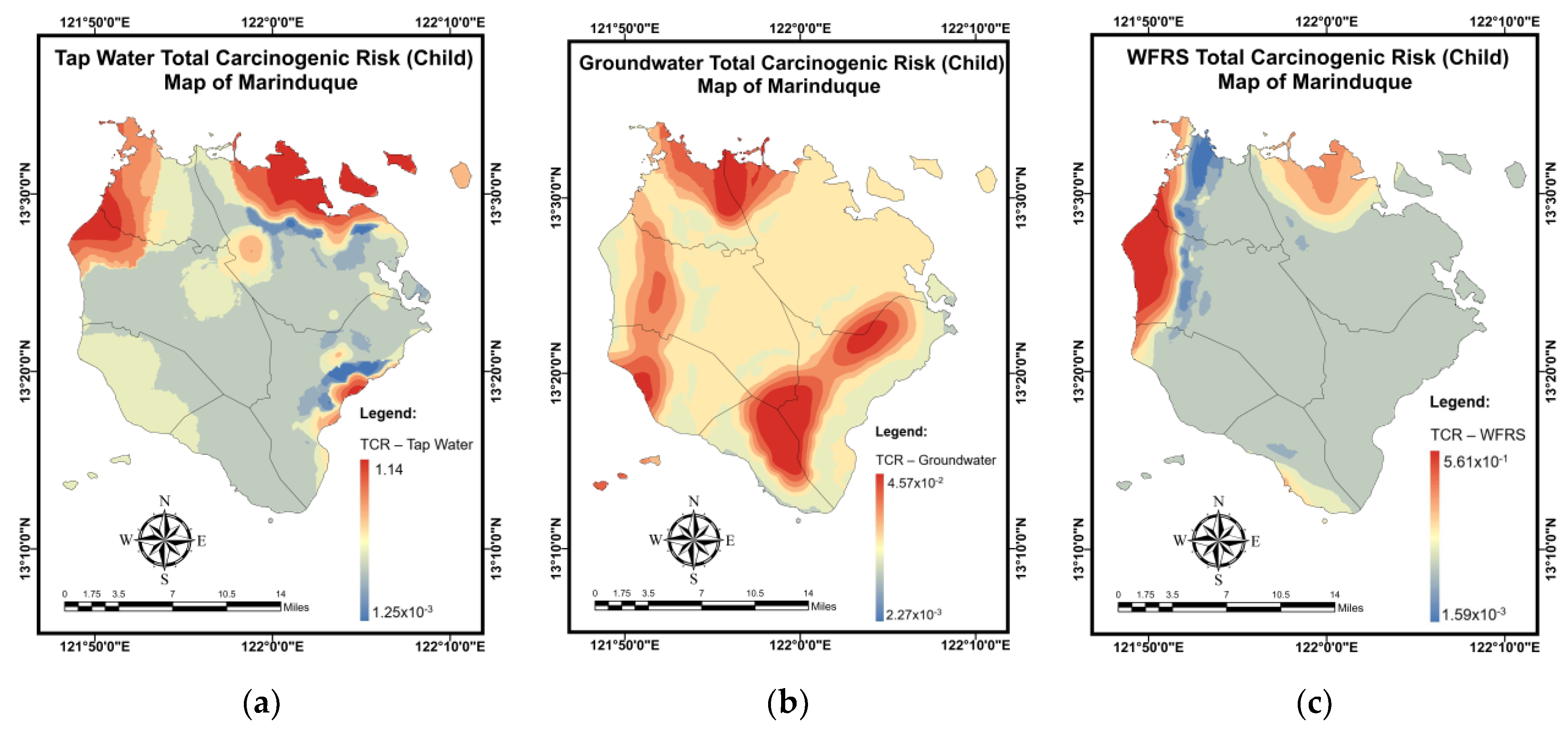
3.6. Pollution and Health Risk Mapping Using the MLGI Method
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

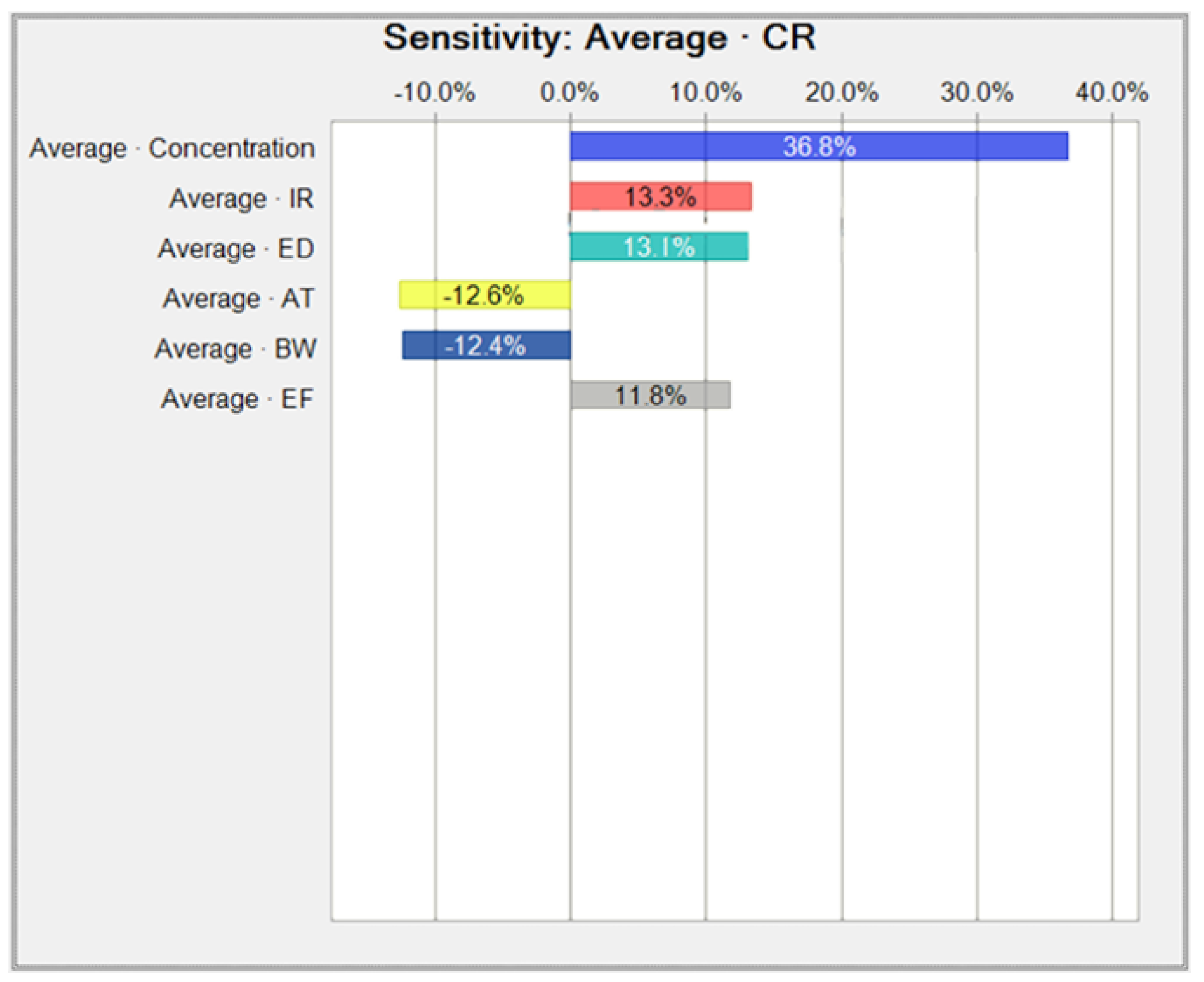
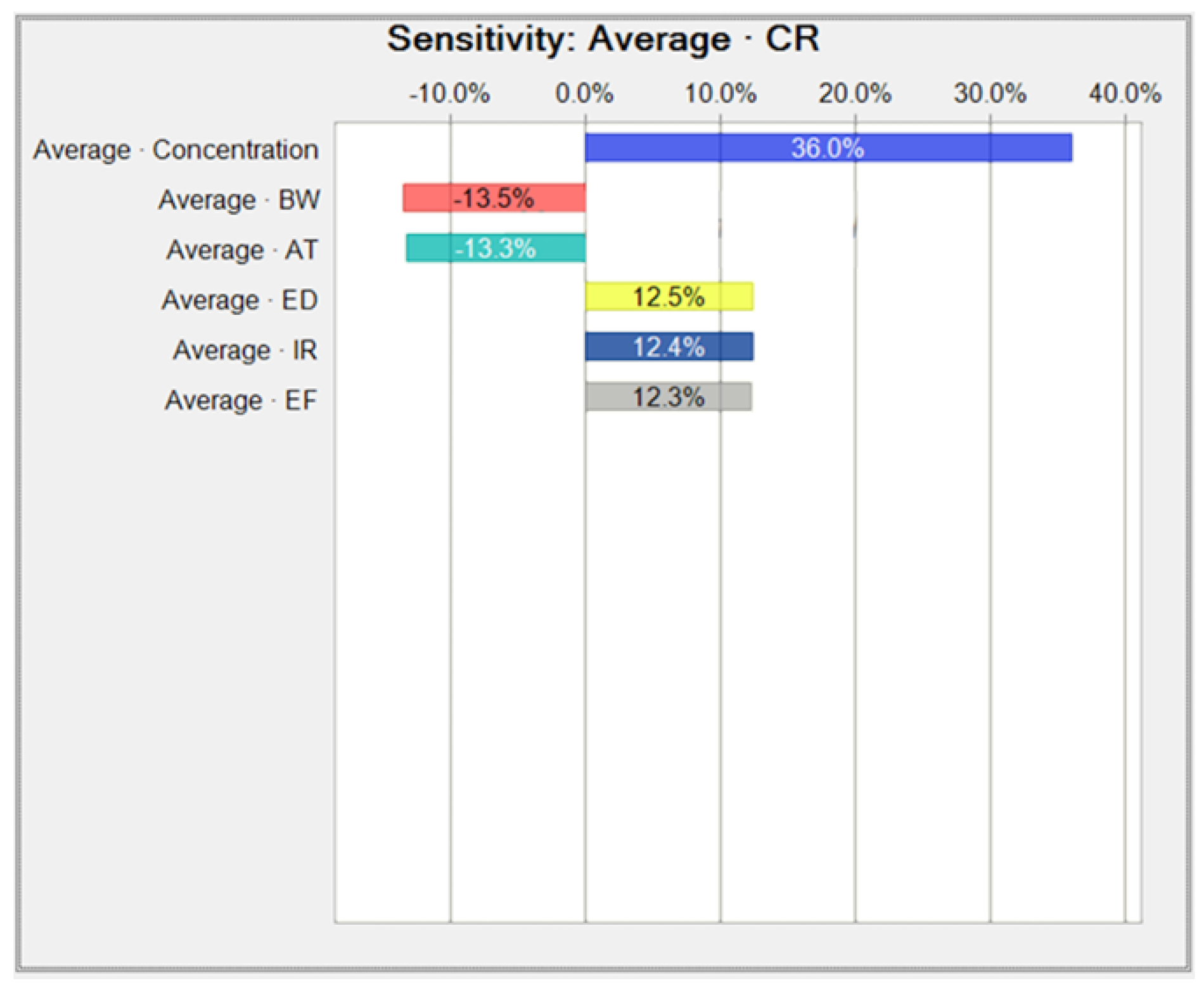
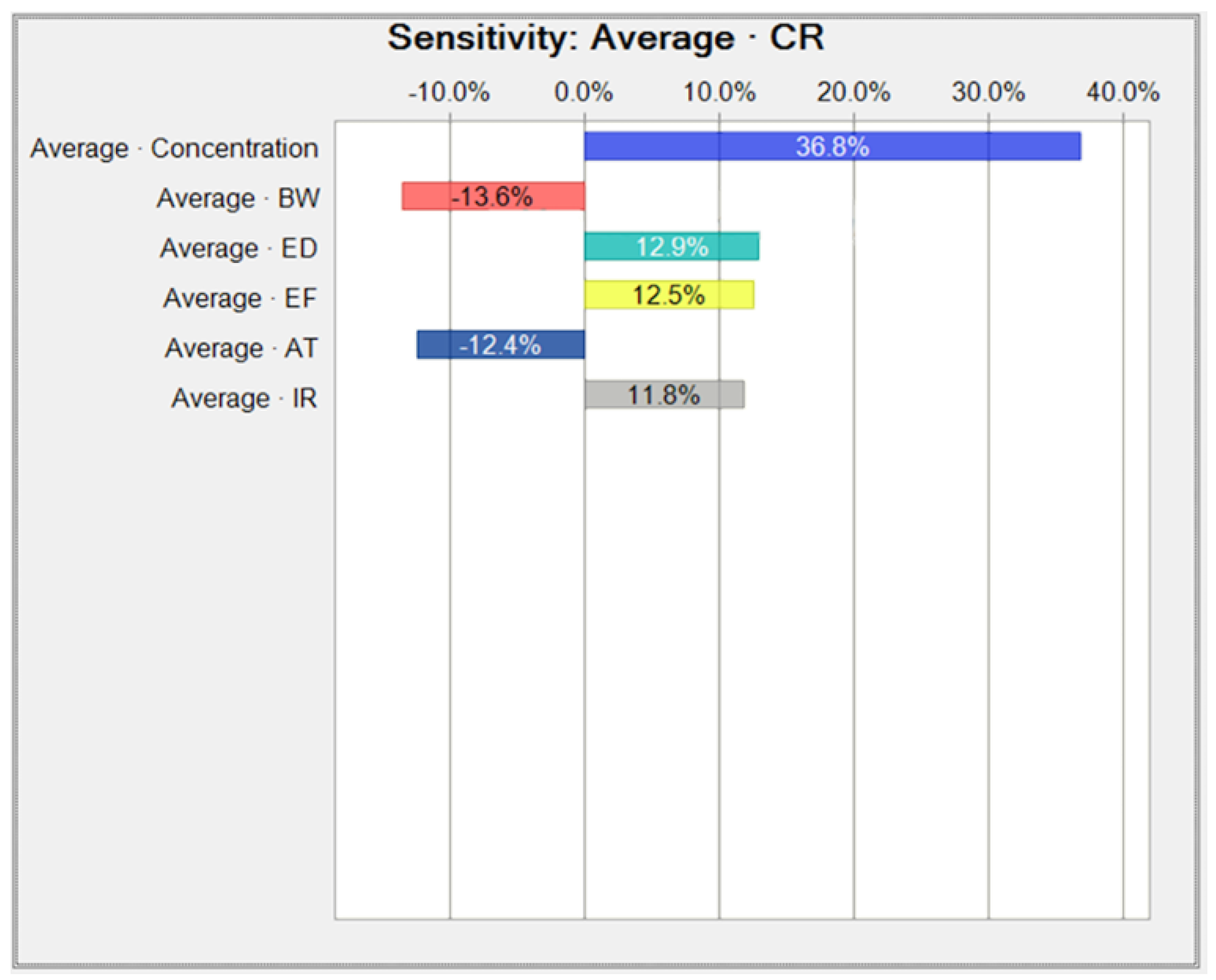
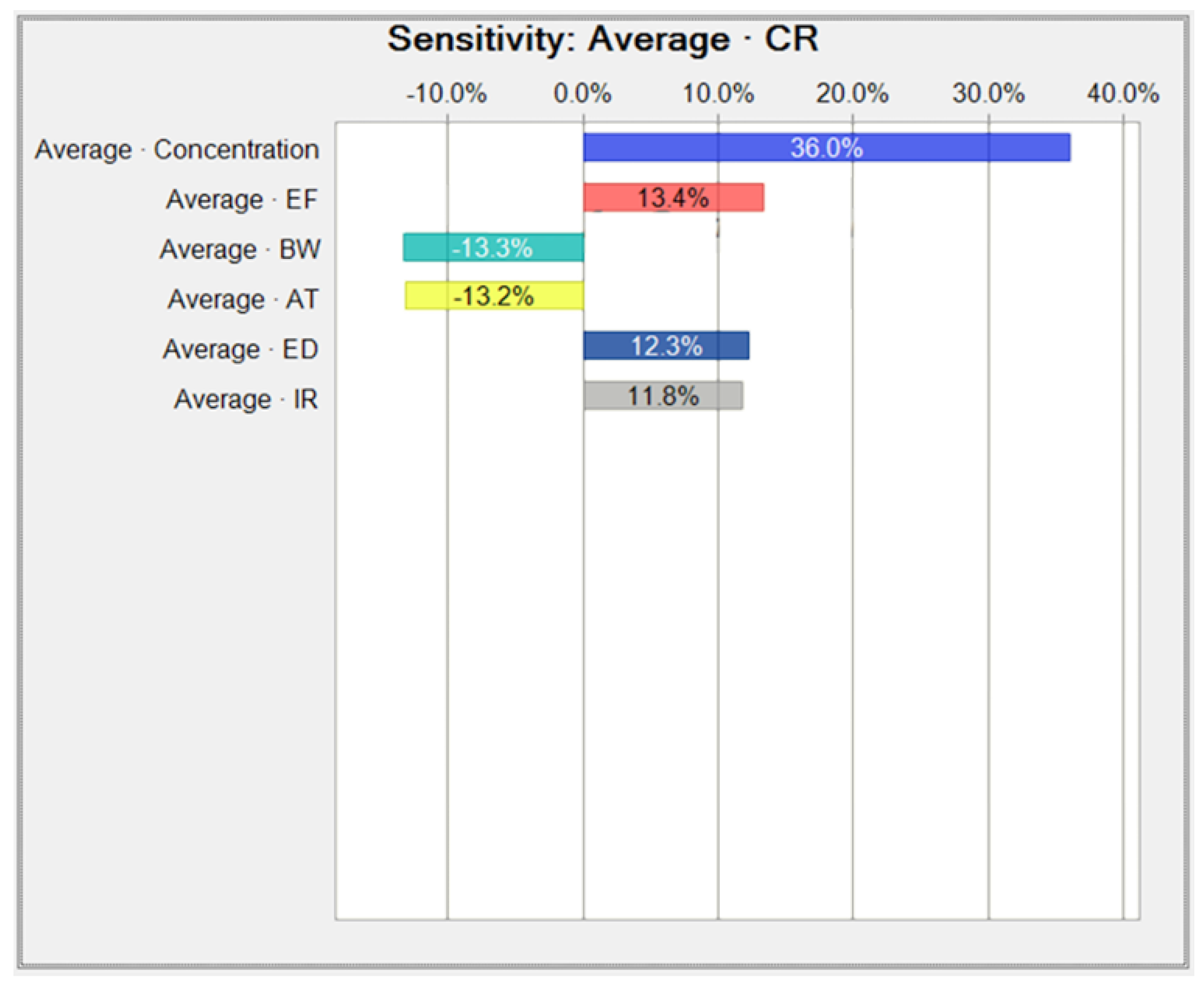

Appendix B


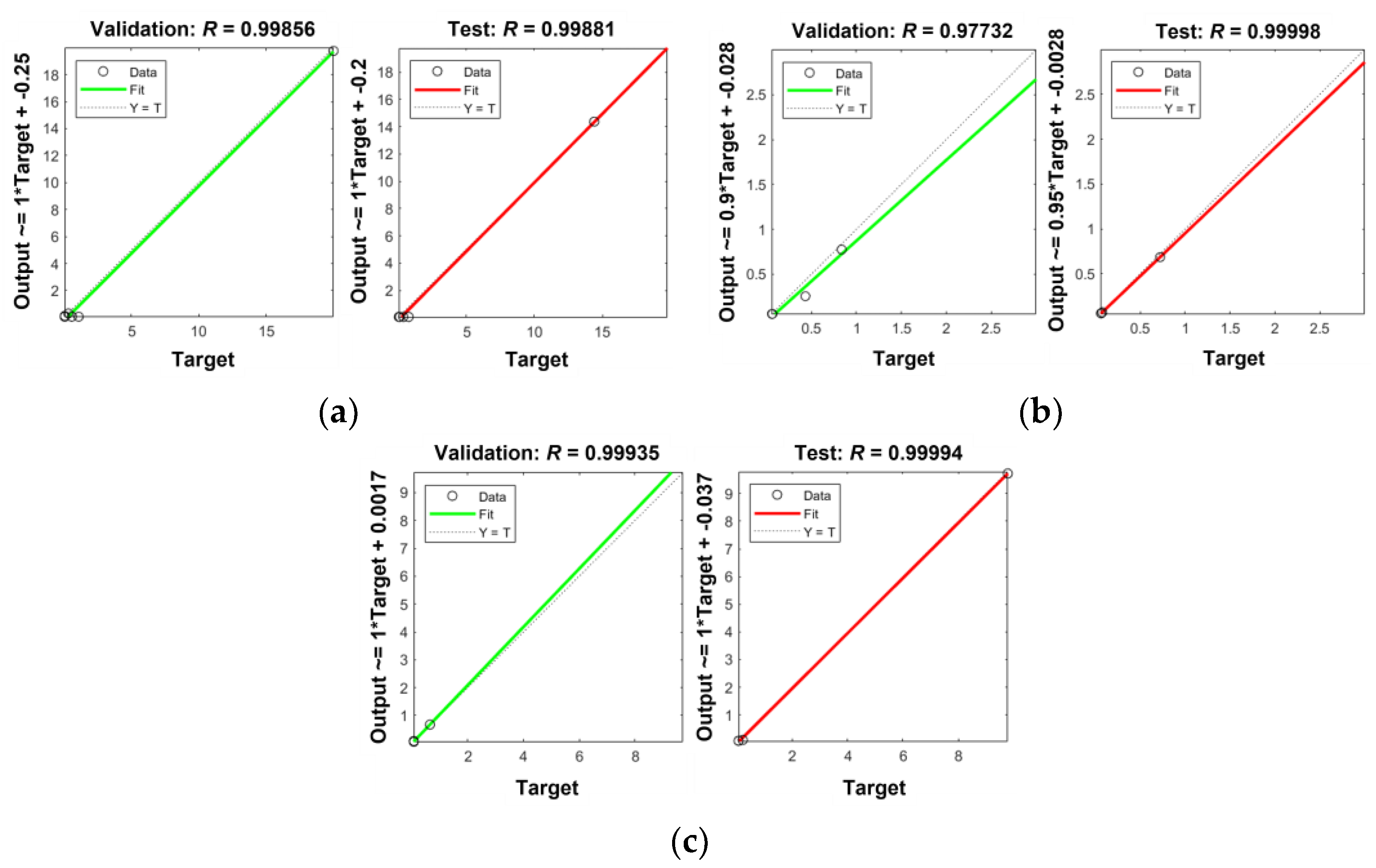

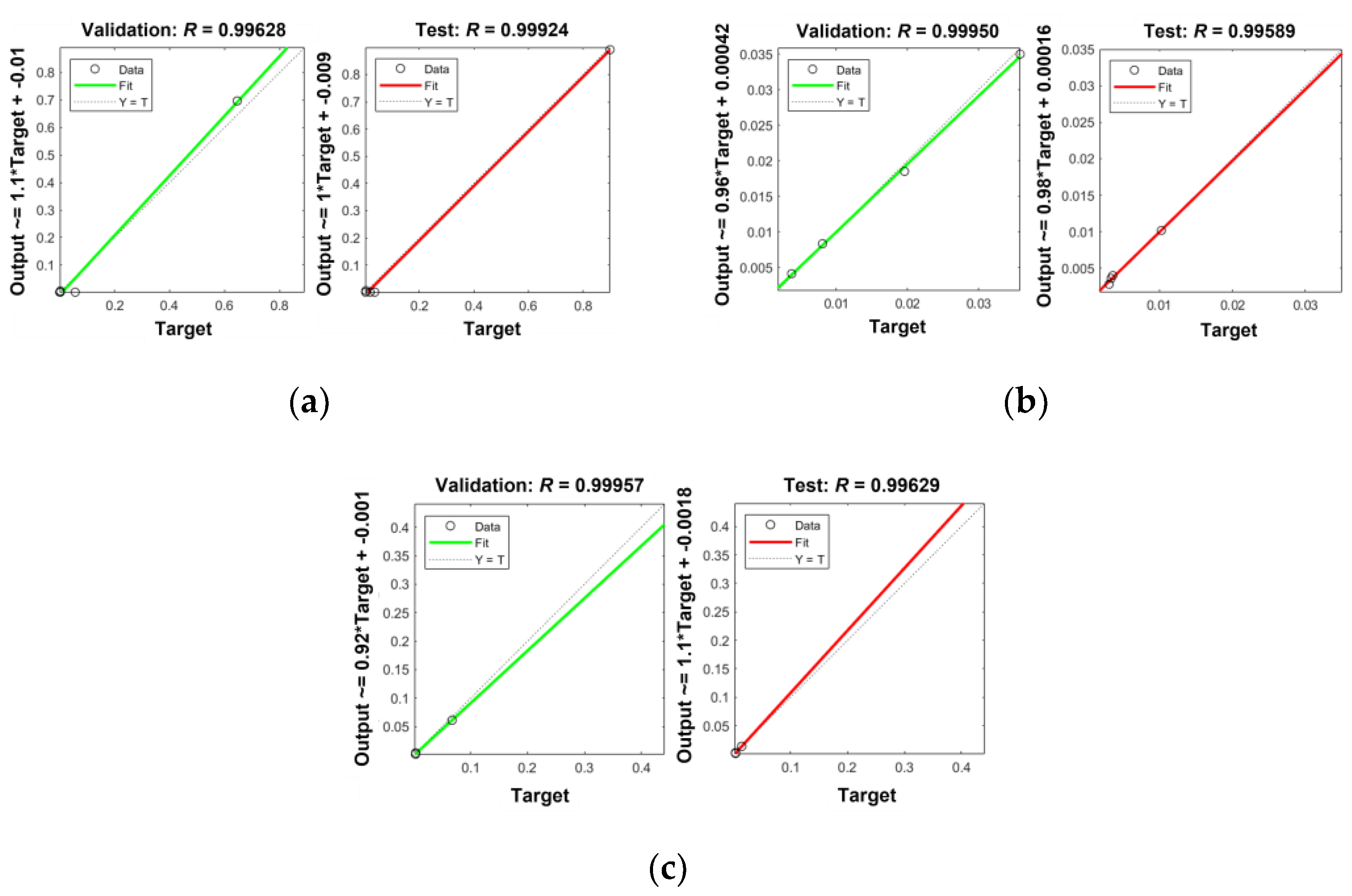
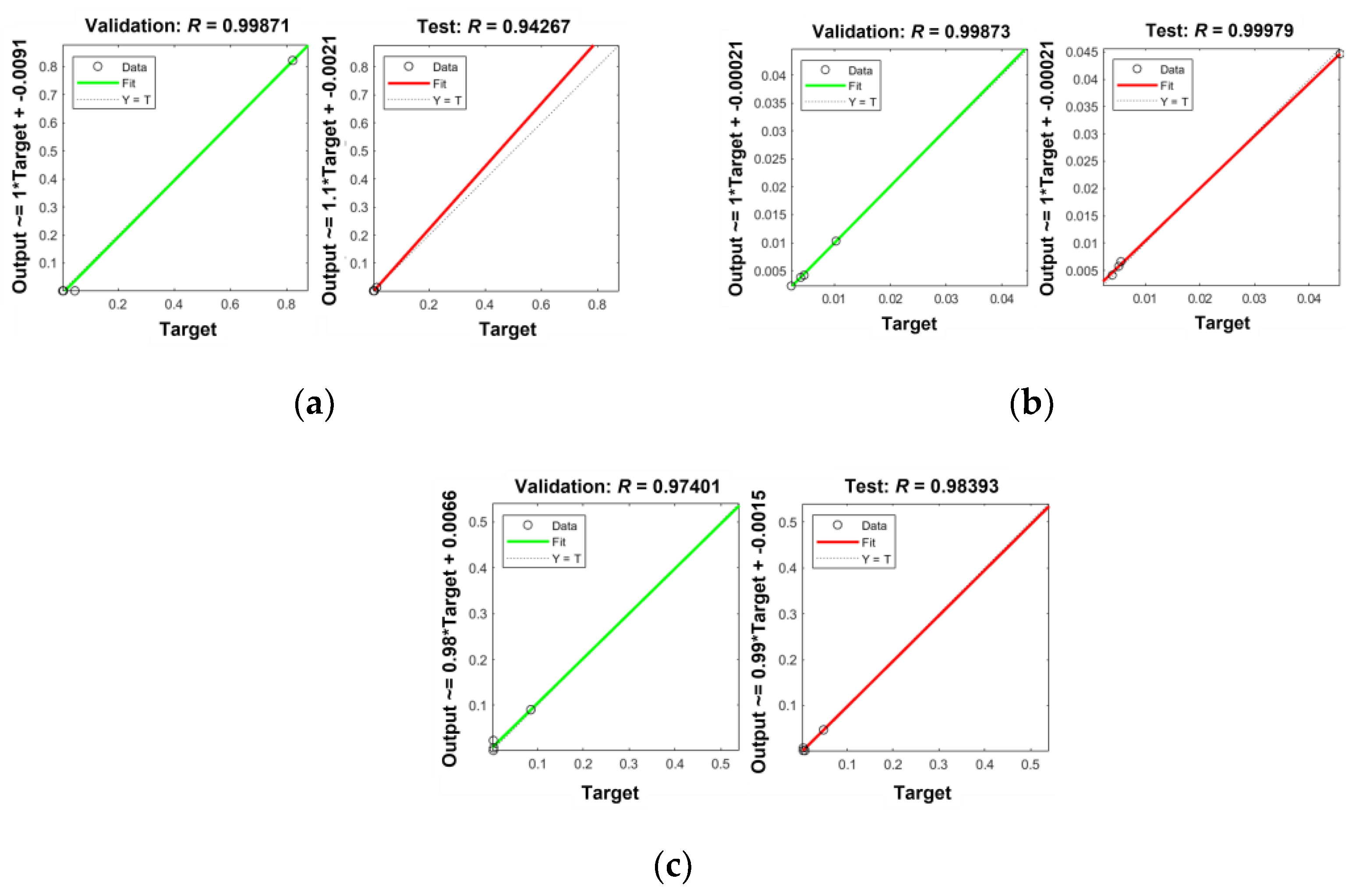
References
- World Health Organization. Drinking Water. Available online: https://www.who.int/news-room/fact-sheets/detail/drinking-water (accessed on 15 January 2022).
- Chinye-Ikejiunor, N.; Iloegbunam, G.O.; Chukwuka, A.; Ogbeide, O. Groundwater contamination and health risk assessment across an urban gradient: Case study of Onitcha metropolis, south-eastern Nigeria. Groundw. Sustain. Dev. 2021, 14, 100642. [Google Scholar] [CrossRef]
- Adeyemi, A.A.; Ojekunle, Z.O. Concentrations and health risk assessment of industrial heavy metals pollution in groundwater in Ogun state, Nigeria. Sci. Afr. 2021, 11, e00666. [Google Scholar] [CrossRef]
- Long, X.; Liu, F.; Zhou, X.; Pi, J.; Yin, W.; Li, F.; Huang, S.; Ma, F. Estimation of spatial distribution and health risk by arsenic and heavy metals in shallow groundwater around Dongting Lake plain using GIS mapping. Chemosphere 2021, 269, 128698. [Google Scholar] [CrossRef] [PubMed]
- Lapong, E.; Fujihara, M. Water Resources in the Philippines: An Overview of its Uses, Management, Problems and Prospects. J. Rainwater Catchment Syst. 2008, 14, 57–67. [Google Scholar] [CrossRef]
- Magtibay, B.B. Water Refilling Station: An alternative source of drinking water supply in the Philippines. In Proceedings of the 30th WEDC International Conference, Vientiane, Laos, 25–29 October 2004; Loughborough University: Loughborough, UK, 2004; pp. 590–593. [Google Scholar]
- Ochieng, G.M.; Seanego, E.S.; Nkwonta, O.I. Impacts of mining on water resources in South Africa: A review. Sci. Res. Essays 2010, 5, 3351–3357. [Google Scholar]
- Lee, J.S.; Chon, H.T.; Kim, K.W. Human risk assessment of As, Cd, Cu and Zn in the abandoned metal mine site. Environ. Geochem. Health 2005, 27, 185–191. [Google Scholar] [CrossRef]
- Monjardin, C.E.F.; Senoro, D.B.; Magbanlac, J.J.M.; de Jesus, K.L.M.; Tabelin, C.B.; Natal, P.M. Geo-Accumulation Index of Manganese in Soils Due to Flooding in Boac and Mogpog Rivers, Marinduque, Philippines with Mining Disaster Exposure. Appl. Sci. 2022, 12, 3527. [Google Scholar] [CrossRef]
- Senoro, D.B.; de Jesus, K.L.M.; Yanuaria, C.A.; Bonifacio, P.B.; Manuel, M.T.; Wang, B.N.; Kao, C.C.; Wu, T.N.; Ney, F.P.; Natal, P. Rapid site assessment in a small island of the Philippines contaminated with mine tailings using ground and areal technique: The environmental quality after twenty years. IOP Conf. Ser. Earth Environ. Sci. 2019, 351, 012022. [Google Scholar] [CrossRef]
- Senoro, D.B.; Bonifacio, P.B.; Mascareñas, D.R.; Tabelin, C.B.; Ney, F.P.; Lamac, M.R.L.; Tan, F.J. Spatial distribution of agricultural yields with elevated metal concentration of the island exposed to acid mine drainage. J. Degrad. Min. Lands Manag. 2020, 8, 2551–2558. [Google Scholar] [CrossRef]
- Marges, M.; Su, G.S.; Ragragio, E. Assessing heavy metals in the waters and soils of Calancan Bay, Marinduque Island, Philippines. J. Appl. Sci. Environ. Sanit. 2011, 6, 45–49. [Google Scholar]
- Gigantone, C.B.; Sobremisana, M.J.; Trinidad, L.C.; Migo, V.P. Impact of Abandoned Mining Facility Wastes on the Aquatic Ecosystem of the Mogpog River, Marinduque, Philippines. J. Health Pollut. 2020, 10, 200611. [Google Scholar] [CrossRef]
- Agarin, C.J.M.; Mascareñas, D.R.; Nolos, R.; Chan, E.; Senoro, D.B. Transition Metals in Freshwater Crustaceans, Tilapia, and Inland Water: Hazardous to the Population of the Small Island Province. Toxics 2021, 9, 71. [Google Scholar] [CrossRef]
- Mahmood, A.; Malik, R.N. Human health risk assessment of heavy metals via consumption of contaminated vegetables collected from different irrigation sources in Lahore, Pakistan. Arab. J. Chem. 2014, 7, 91–99. [Google Scholar] [CrossRef] [Green Version]
- Venkatramanan, S.; Chung, S.Y.; Kim, T.H.; Prasanna, M.V.; Hamm, S.Y. Assessment and distribution of metals contamination in groundwater: A case study of Busan City, Korea. Water Qual. Expo. Health 2015, 7, 219–225. [Google Scholar] [CrossRef]
- Tiwari, A.K.; Singh, P.K.; Singh, A.K.; De Maio, M. Estimation of heavy metal contamination in groundwater and development of a heavy metal pollution index by using GIS technique. Bull. Environ. Contam. Toxicol. 2016, 96, 508–515. [Google Scholar] [CrossRef]
- Kong, M.; Zhong, H.; Wu, Y.; Liu, G.; Xu, Y.; Wang, G. Developing and validating intrinsic groundwater vulnerability maps in regions with limited data: A case study from Datong City in China using DRASTIC and Nemerow pollution indices. Environ. Earth Sci. 2019, 78, 262. [Google Scholar] [CrossRef]
- Jiang, C.; Zhao, Q.; Zheng, L.; Chen, X.; Li, C.; Ren, M. Distribution, source and health risk assessment based on the Monte Carlo method of heavy metals in shallow groundwater in an area affected by mining activities, China. Ecotoxicol. Environ. Saf. 2021, 224, 112679. [Google Scholar] [CrossRef]
- Jafarzadeh, N.; Heidari, K.; Meshkinian, A.; Kamani, H.; Mohammadi, A.A.; Conti, G.O. Non-carcinogenic risk assessment of exposure to heavy metals in underground water resources in Saraven, Iran: Spatial distribution, monte-carlo simulation, sensitive analysis. Environ. Res. 2022, 204, 112002. [Google Scholar] [CrossRef]
- Senoro, D.B.; de Jesus, K.L.M.; Mendoza, L.C.; Apostol, E.M.D.; Escalona, K.S.; Chan, E.B. Groundwater Quality Monitoring Using In-Situ Measurements and Hybrid Machine Learning with Empirical Bayesian Kriging Interpolation Method. Appl. Sci. 2021, 12, 132. [Google Scholar] [CrossRef]
- Salvacion, A.R.; Magcale-Macandog, D.B. Spatial analysis of human population distribution and growth in Marinduque Island, Philippines. J. Mar. Isl. Cult. 2015, 4, 27–33. [Google Scholar] [CrossRef] [Green Version]
- Salvacion, A.R. Mapping land limitations for agricultural land use planning using fuzzy logic approach: A case study for Marinduque Island, Philippines. GeoJournal 2021, 86, 915–925. [Google Scholar] [CrossRef]
- David, C.P.C.; Plumlee, G.S. Comparison of dissolved copper concentration trends in two rivers receiving ARD from an inactive copper mine (Marinduque Island, Philippines). In Proceedings of the 7th International Conference on Acid Rock Drainage (ICARD), St. Louis, MO, USA, 26 March 2006; pp. 426–438. [Google Scholar]
- Environmental Protection Agency. Groundwater Sampling. Available online: https://www.epa.gov/sites/default/files/2017-07/documents/groundwater_sampling301_af.r4.pdf (accessed on 22 March 2022).
- Environmental Protection Agency. Quick Guide to Drinking Water Sample Collection. Available online: https://www.epa.gov/sites/default/files/2015-11/documents/drinking_water_sample_collection.pdf (accessed on 22 March 2022).
- Environmental Protection Agency. In Situ Water Quality Monitoring Operating Procedure. Available online: https://www.epa.gov/sites/default/files/2015-06/documents/Insitu-Water-Quality-Mon.pdf (accessed on 22 March 2022).
- Lomboy, M.; Riego de Dios, J.; Magtibay, B.; Quizon, R.; Molina, V.; Fadrilan-Camacho, V.; See, J.; Enoveso, A.; Barbosa, L.; Agravante, A. Updating national standards for drinking-water: A Philippine experience. J. Water Health 2017, 15, 288–295. [Google Scholar] [CrossRef]
- Abdel-Satar, A.M.; Ali, M.H.; Goher, M.E. Indices of water quality and metal pollution of Nile River, Egypt. Egypt. J. Aquat. Res. 2017, 43, 21–29. [Google Scholar] [CrossRef]
- Chidambaram, S.; Srinivasamoorthy, K.; Anandhan, P.; Selvam, S. A study on assessment of credible sources of heavy metal pollution vulnerability in groundwater of Thoothukudi districts, Tamilnadu, India. Water Qual. Expo. Health 2015, 7, 459–467. [Google Scholar]
- Zhong, S.; Geng, H.; Zhang, F.; Liu, Z.; Wang, T.; Song, B. Risk assessment and prediction of heavy metal pollution in groundwater and river sediment: A case study of a typical agricultural irrigation area in Northeast China. Int. J. Anal. Chem. 2015, 2015, 921539. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Feng, M.; Hao, X. Application of Nemerow index method and integrated water quality index method in water quality assessment of Zhangze Reservoir. IOP Conf. Ser. Earth Environ. Sci. 2018, 128, 012160. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, H.; Sun, Y.; Chen, J. Novel assessment method of heavy metal pollution in surface water: A case study of Yangping River in Lingbao City, China. Environ. Eng. Res. 2017, 22, 31–39. [Google Scholar] [CrossRef] [Green Version]
- Vu, C.T.; Lin, C.; Shern, C.C.; Yeh, G.; Tran, H.T. Contamination, ecological risk and source apportionment of heavy metals in sediments and water of a contaminated river in Taiwan. Ecol. Indic. 2017, 82, 32–42. [Google Scholar] [CrossRef]
- Pervez, S.; Dugga, P.; Siddiqui, M.N.; Bano, S.; Verma, M.; Candeias, C.; Mishra, A.; Verma, S.R.; Tamrakar, A.; Karbhal, I.; et al. Sources and health risk assessment of potentially toxic elements in groundwater in the mineral-rich tribal belt of Bastar, Central India. Groundw. Sustain. Dev. 2021, 14, 100628. [Google Scholar] [CrossRef]
- Qu, L.; Huang, H.; Xia, F.; Liu, Y.; Dahlgren, R.A.; Zhang, M.; Mei, K. Risk analysis of heavy metal concentration in surface waters across the rural-urban interface of the Wen-Rui Tang River, China. Environ. Pollut. 2018, 237, 639–649. [Google Scholar] [CrossRef] [Green Version]
- Karim, Z. Risk assessment of dissolved trace metals in drinking water of Karachi, Pakistan. Bull. Environ. Contam. Toxicol. 2011, 86, 676–678. [Google Scholar] [CrossRef] [PubMed]
- Giri, S.; Singh, A.K. Human health risk assessment via drinking water pathway due to metal contamination in the groundwater of Subarnarekha River Basin, India. Environ. Monit. Assess. 2015, 187, 63. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Zhao, D.Y.; Jia, H.Y.; Zhang, Y.; Zhang, X.X.; Cheng, S.P. Preliminary risk assessment of trace metal pollution in surface water from Yangtze River in Nanjing Section, China. Bull. Environ. Contam. Toxicol. 2009, 82, 405–409. [Google Scholar] [CrossRef]
- Bortey-Sam, N.; Nakayama, S.M.; Ikenaka, Y.; Akoto, O.; Baidoo, E.; Mizukawa, H.; Ishizuka, M. Health risk assessment of heavy metals and metalloid in drinking water from communities near gold mines in Tarkwa, Ghana. Environ. Monit. Assess. 2015, 187, 1–12. [Google Scholar] [CrossRef]
- Wongsasuluk, P.; Chotpantarat, S.; Siriwong, W.; Robson, M. Heavy metal contamination and human health risk assessment in drinking water from shallow groundwater wells in an agricultural area in Ubon Ratchathani province, Thailand. Environ. Geochem. Health 2014, 36, 169–182. [Google Scholar] [CrossRef]
- Bodrud-Doza, M.D.; Islam, A.T.; Ahmed, F.; Das, S.; Saha, N.; Rahman, M.S. Characterization of groundwater quality using water evaluation indices, multivariate statistics and geostatistics in central Bangladesh. Water Sci. 2016, 30, 19–40. [Google Scholar] [CrossRef] [Green Version]
- Lim, H.S.; Lee, J.S.; Chon, H.T.; Sager, M. Heavy metal contamination and health risk assessment in the vicinity of the abandoned Songcheon Au–Ag mine in Korea. J. Geochem. Explor. 2008, 96, 223–230. [Google Scholar] [CrossRef]
- Antoine, J.M.; Fung, L.A.H.; Grant, C.N. Assessment of the potential health risks associated with the aluminium, arsenic, cadmium and lead content in selected fruits and vegetables grown in Jamaica. Toxicol. Rep. 2017, 4, 181–187. [Google Scholar] [CrossRef]
- Oskarsson, A. Barium. In Handbook on the Toxicology of Metals, 5th ed.; Nordberg, G.F., Fowler, B.A., Nordberg, M., Friberg, L.T., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 91–100. [Google Scholar]
- Mohammadi, A.A.; Zarei, A.; Majidi, S.; Ghaderpoury, A.; Hashempour, Y.; Saghi, M.H.; Alinejad, A.; Yousefi, M.; Hosseingholizadeh, N.; Ghaderpoori, M. Carcinogenic and non-carcinogenic health risk assessment of heavy metals in drinking water of Khorramabad, Iran. MethodsX 2019, 6, 1642–1651. [Google Scholar] [CrossRef]
- Ghosh, G.C.; Khan, M.; Hassan, J.; Chakraborty, T.K.; Zaman, S.; Kabir, A.H.M.; Tanaka, H. Human health risk assessment of elevated and variable iron and manganese intake with arsenic-safe groundwater in Jashore, Bangladesh. Sci. Rep. 2020, 10, 5206. [Google Scholar] [CrossRef]
- Yang, X.; Duan, J.; Wang, L.; Li, W.; Guan, J.; Beecham, S.; Mulcahy, D. Heavy metal pollution and health risk assessment in the Wei River in China. Environ. Monit. Assess. 2015, 187, 111. [Google Scholar] [CrossRef]
- Bodrud-Doza, M.; Islam, S.D.U.; Hasan, M.T.; Alam, F.; Haque, M.M.; Rakib, M.A.; Ashadudzaman Asad, M.; Rahman, M.A. Groundwater pollution by trace metals and human health risk assessment in central west part of Bangladesh. Groundw. Sustain. Dev. 2019, 9, 100219. [Google Scholar] [CrossRef]
- Fallahzadeh, R.A.; Ghaneian, M.T.; Miri, M.; Dashti, M.M. Spatial analysis and health risk assessment of heavy metals concentration in drinking water resources. Environ. Sci. Pollut. Res. 2017, 24, 24790–24802. [Google Scholar] [CrossRef]
- De Jesus, K.L.M.; Senoro, D.B.; Dela Cruz, J.C.; Chan, E.B. A Hybrid Neural Network–Particle Swarm Optimization Informed Spatial Interpolation Technique for Groundwater Quality Mapping in a Small Island Province of the Philippines. Toxics 2021, 9, 273. [Google Scholar] [CrossRef]
- Gawad, S.S.A. Concentrations of heavy metals in water, sediment and mollusk gastropod, Lanistes carinatus from Lake Manzala, Egypt. Egypt. J. Aquat. Res. 2018, 44, 77–82. [Google Scholar] [CrossRef]
- Lim, W.Y.; Aris, A.Z.; Zakaria, M.P. Spatial variability of metals in surface water and sediment in the Langat River and geochemical factors that influence their water-sediment interactions. Sci. World J. 2012, 2012, 652150. [Google Scholar] [CrossRef] [Green Version]
- Ogunlaja, A.; Ogunlaja, O.O.; Okewole, D.M.; Morenikeji, O.A. Risk assessment and source identification of heavy metal contamination by multivariate and hazard index analyses of a pipeline vandalised area in Lagos State, Nigeria. Sci. Total Environ. 2019, 651, 2943–2952. [Google Scholar] [CrossRef]
- Sevilla, J.B.; Lee, C.H.; Lee, B.Y. Assessment of spatial variations in surface water quality of Kyeongan Stream, South Korea using multi-variate statistical techniques. In Sustainability in Food and Water, 1st ed.; Sumi, A., Fukushi, K., Honda, R., Hassan, K.M., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 39–48. [Google Scholar]
- Food and Drug Administration. Philippine National Standards for Drinking Water of 2017. Available online: https://www.fda.gov.ph/wp-content/uploads/2021/08/Administrative-Order-No.-2017-0010.pdf (accessed on 22 March 2022).
- World Health Organization. Total Dissolved Solids in Drinking-Water. Available online: https://www.who.int/water_sanitation_health/dwq/chemicals/tds.pdf (accessed on 22 March 2022).
- De Jesus, K.L.M.; Senoro, D.B.; Dela Cruz, J.C.; Chan, E.B. Neuro-Particle Swarm Optimization Based In-Situ Prediction Model for Heavy Metals Concentration in Groundwater and Surface Water. Toxics 2022, 10, 95. [Google Scholar] [CrossRef]
- Pérez Castresana, G.; Castañeda Roldán, E.; García Suastegui, W.A.; Morán Perales, J.L.; Cruz Montalvo, A.; Handal Silva, A. Evaluation of health risks due to heavy metals in a rural population exposed to Atoyac River pollution in Puebla, Mexico. Water 2019, 11, 277. [Google Scholar] [CrossRef] [Green Version]
- Environmental Protection Agency. Guidelines for Carcinogenic Risk Assessment. Available online: https://www.epa.gov/sites/default/files/2013-09/documents/cancer_guidelines_final_3-25-05.pdf (accessed on 22 March 2022).
- World Health Organization. IARC monographs on the identification of carcinogenic hazards to humans. In Agents Classified by the IARC Monographs; World Health Organisation: Geneva, Switzerland, 2020; Volume 1–125. [Google Scholar]
- Environmental Protection Agency. Risk Assessment Guidance for Superfund (RAGS) Volume III: Part A. Available online: https://www.epa.gov/risk/risk-assessment-guidance-superfund-rags-volume-iii-part (accessed on 22 March 2022).
- Nyambura, C.; Hashim, N.O.; Chege, M.W.; Tokonami, S.; Omonya, F.W. Cancer and non-cancer health risks from carcinogenic heavy metal exposures in underground water from Kilimambogo, Kenya. Groundw. Sustain. Dev. 2020, 10, 100315. [Google Scholar] [CrossRef]
- Alidadi, H.; Tavakoly Sany, S.B.; Zarif Garaati Oftadeh, B.; Mohamad, T.; Shamszade, H.; Fakhari, M. Health risk assessments of arsenic and toxic heavy metal exposure in drinking water in northeast Iran. Environ. Health Prev. Med. 2019, 24, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rezaei, A.; Hassani, H.; Hassani, S.; Jabbari, N.; Mousavi, S.B.F.; Rezaei, S. Evaluation of groundwater quality and heavy metal pollution indices in Bazman basin, southeastern Iran. Groundw. Sustain. Dev. 2019, 9, 100245. [Google Scholar] [CrossRef]
- Qureshi, S.S.; Channa, A.; Memon, S.A.; Khan, Q.; Jamali, G.A.; Panhwar, A.; Saleh, T.A. Assessment of physicochemical characteristics in groundwater quality parameters. Environ. Technol. Innov. 2021, 24, 101877. [Google Scholar] [CrossRef]
- Varghese, J.; Jaya, D.S. Metal pollution of groundwater in the vicinity of Valiathura sewage farm in Kerala, South India. Bull. Environ. Contam. Toxicol. 2014, 93, 694–698. [Google Scholar] [CrossRef]
- Kuisi, M.A.; Abdel-Fattah, A. Groundwater vulnerability to selenium in semi-arid environments: Amman Zarqa Basin, Jordan. Environ. Geochem. Health 2010, 32, 107–128. [Google Scholar] [CrossRef]
- Zamani, A.A.; Yaftian, M.R.; Parizanganeh, A. Multivariate statistical assessment of heavy metal pollution sources of groundwater around a lead and zinc plant. Iran. J. Environ. Health Sci. Eng. 2012, 9, 29. [Google Scholar] [CrossRef] [Green Version]
- Sajil Kumar, P.J.; Davis Delson, P.; Thomas Babu, P. Appraisal of heavy metals in groundwater in Chennai city using a MPI model. Bull. Environ. Contam. Toxicol. 2012, 89, 793–798. [Google Scholar] [CrossRef]
- Tong, S.; Li, H.; Tudi, M.; Yuan, X.; Yang, L. Comparison of characteristics, water quality and health risk assessment of trace elements in surface water and groundwater in China. Ecotoxicol. Environ. Saf. 2021, 219, 112283. [Google Scholar] [CrossRef]
- Nurchi, V.M.; Buha Djordjevic, A.; Crisponi, G.; Alexander, J.; Bjørklund, G.; Aaseth, J. Arsenic toxicity: Molecular targets and therapeutic agents. Biomolecules 2020, 10, 235. [Google Scholar] [CrossRef] [Green Version]
- Naujokas, M.F.; Anderson, B.; Ahsan, H.; Aposhian, H.V.; Graziano, J.H.; Thompson, C.; Suk, W.A. The broad scope of health effects from chronic arsenic exposure: Update on a worldwide public health problem. Environ. Health Perspect. 2013, 121, 295–302. [Google Scholar] [CrossRef]
- Srivastava, S.; Flora, S.J. Arsenicals: Toxicity, their use as chemical warfare agents, and possible remedial measures. In Handbook of Toxicology of Chemical Warfare Agents, 3rd ed.; Gupta, R.C., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 303–319. [Google Scholar]
- Dani, S.U.; Walter, G.F. Chronic arsenic intoxication diagnostic score (CAsIDS). J. Appl. Toxicol. 2018, 38, 122–144. [Google Scholar] [CrossRef]
- Rahman, M.A.; Rahman, A.; Khan, M.Z.K.; Renzaho, A.M. Human health risks and socio-economic perspectives of arsenic exposure in Bangladesh: A scoping review. Ecotoxicol. Environ. Saf. 2018, 150, 335–343. [Google Scholar] [CrossRef]
- Palma-Lara, I.; Martínez-Castillo, M.; Quintana-Pérez, J.C.; Arellano-Mendoza, M.G.; Tamay-Cach, F.; Valenzuela-Limón, O.L.; García-Montalvo, E.A.; Hernández-Zavala, A. Arsenic exposure: A public health problem leading to several cancers. Regul. Toxicol. Pharmacol. 2020, 110, 104539. [Google Scholar] [CrossRef]
- Wei, S.; Zhang, H.; Tao, S. A review of arsenic exposure and lung cancer. Toxicol. Res. 2019, 8, 319. [Google Scholar] [CrossRef]
- Rao, C.V.; Pal, S.; Mohammed, A.; Farooqui, M.; Doescher, M.P.; Asch, A.S.; Yamada, H.Y. Biological effects and epidemiological consequences of arsenic exposure, and reagents that can ameliorate arsenic damage in vivo. Oncotarget 2017, 8, 57605. [Google Scholar] [CrossRef] [Green Version]
- Saint-Jacques, N.; Brown, P.; Nauta, L.; Boxall, J.; Parker, L.; Dummer, T.J. Estimating the risk of bladder and kidney cancer from exposure to low-levels of arsenic in drinking water, Nova Scotia, Canada. Environ. Int. 2018, 110, 95–104. [Google Scholar] [CrossRef]
- Mendez, W.M.; Eftim, S.; Cohen, J.; Warren, I.; Cowden, J.; Lee, J.S.; Sams, R. Relationships between arsenic concentrations in drinking water and lung and bladder cancer incidence in US counties. J. Expo. Sci. Environ. Epidemiol. 2017, 27, 235–243. [Google Scholar] [CrossRef]
- Ahn, J.; Boroje, I.J.; Ferdosi, H.; Kramer, Z.J.; Lamm, S.H. Prostate cancer incidence in US counties and low levels of arsenic in drinking water. Int. J. Environ. Res. Public Health 2020, 17, 960. [Google Scholar] [CrossRef] [Green Version]
- De Souza, I.D.; de Andrade, A.S.; Dalmolin, R.J.S. Lead-interacting proteins and their implication in lead poisoning. Crit. Rev. Toxicol. 2018, 48, 375–386. [Google Scholar] [CrossRef]
- Molavi, N.; Meamar, R.; Hasanzadeh, A.; Eizadi-Mood, N. The Effects of Succimer and Penicillamine on Acute Lead Poisoning Patients. Int. J. Med. Toxicol. Forensic Med. 2021, 11, 33474. [Google Scholar]
- Soltaninejad, K.; Shadnia, S. Lead poisoning in opium abuser in Iran: A systematic review. Int. J. Prev. Med. 2018, 9, 3. [Google Scholar]
- Yang, F.; Massey, I.Y. Exposure routes and health effects of heavy metals on children. Biometals 2019, 32, 563–573. [Google Scholar]
- Shukla, V.; Shukla, P.; Tiwari, A. Lead poisoning. Indian J. Med. Spec. 2018, 9, 146–149. [Google Scholar] [CrossRef]
- Njati, S.Y.; Maguta, M.M. Lead-based paints and children’s PVC toys are potential sources of domestic lead poisoning—A review. Environ. Pollut. 2019, 249, 1091–1105. [Google Scholar] [CrossRef]
- Boskabady, M.; Marefati, N.; Farkhondeh, T.; Shakeri, F.; Farshbaf, A.; Boskabady, M.H. The effect of environmental lead exposure on human health and the contribution of inflammatory mechanisms, a review. Environ. Int. 2018, 120, 404–420. [Google Scholar] [CrossRef]
- Lentini, P.; Zanoli, L.; de Cal, M.; Granata, A.; Dell’Aquila, R. Lead and heavy metals and the kidney. In Critical Care Nephrology, 3rd ed.; Ronco, C., Bellomo, R., Kellum, J.A., Ricci, Z., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1324–1330. [Google Scholar]
- Alisha, V.P.; Gupta, P. A comprehensive review of environmental exposure of toxicity of lead. J. Pharmacol. Phytochem. 2018, 7, 1991–1995. [Google Scholar]
- Rehman, K.; Fatima, F.; Waheed, I.; Akash, M.S.H. Prevalence of exposure of heavy metals and their impact on health consequences. J. Cell. Biochem. 2018, 119, 157–184. [Google Scholar] [CrossRef]
- Moorthy, S.; Samuel, A.E.; Moideen, F.; Peringat, J. Interstitial nephritis presenting as acute kidney injury following ingestion of alternative medicine containing Lead: A case report. Adv. J. Emerg. Med. 2019, 3, e8. [Google Scholar]
- Conlon, E.; Ferguson, K.; Dack, S.; Cathcart, S.; Azizi, A.; Keating, A. Unusual cases of lead poisoning in the UK. Chem. Hazards Poisons Rep. 2016, 26, 73–78. [Google Scholar]
- Naja, G.M.; Volesky, B. Toxicity and sources of Pb, Cd, Hg, Cr, As, and radionuclides in the environment. In Handbook of Advanced Industrial and Hazardous Wastes Management, 1st ed.; Wang, L.K., Wang, M.S., Hung, Y., Shammas, N.K., Chen, J.P., Eds.; CRC Press: Boca Raton, FL, USA, 2017; pp. 855–903. [Google Scholar]
- Carocci, A.; Catalano, A.; Lauria, G.; Sinicropi, M.S.; Genchi, G. Lead toxicity, antioxidant defense and environment. In Reviews of Environmental Contamination and Toxicology; de Voogt, P., Ed.; Springer: Cham, Switzerland, 2016; pp. 45–67. [Google Scholar]
- Mohammadyan, M.; Moosazadeh, M.; Borji, A.; Khanjani, N.; Rahimi Moghadam, S. Investigation of occupational exposure to lead and its relation with blood lead levels in electrical solderers. Environ. Monit. Assess. 2019, 191, 1–9. [Google Scholar] [CrossRef]
- Kim, H.C.; Jang, T.W.; Chae, H.J.; Choi, W.J.; Ha, M.N.; Ye, B.J.; Kim, B.G.; Jeon, M.J.; Kim, S.Y.; Hong, Y.S. Evaluation and management of lead exposure. Ann. Occup. Environ. Med. 2015, 27, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, K.K.; Reddy, R.C.; Bagoji, I.B.; Das, S.; Bagali, S.; Mullur, L.; Khodnapur, J.P.; Biradar, M.S. Primary concept of nickel toxicity–an overview. J. Basic Clin. Physiol. Pharmacol. 2019, 30, 141–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.C.; Ning, H.C.; Chen, S.S.; Lin, C.N.; Wang, I.K.; Weng, S.M.; Hsu, C.W.; Huang, W.H.; Lu, J.J.; Wu, T.L.; et al. Survey of urinary nickel in peritoneal dialysis patients. Oncotarget 2017, 8, 60469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Wu, M.; Xu, B.; Kang, L. Association between the urinary nickel and the diastolic blood pressure in general population. Chemosphere 2022, 286, 131900. [Google Scholar] [CrossRef]
- Karaouzas, I.; Kapetanaki, N.; Mentzafou, A.; Kanellopoulos, T.D.; Skoulikidis, N. Heavy metal contamination status in Greek surface waters: A review with application and evaluation of pollution indices. Chemosphere 2021, 263, 128192. [Google Scholar] [CrossRef]
- Nolos, R.C.; Agarin, C.J.M.; Domino, M.Y.R.; Bonifacio, P.B.; Chan, E.B.; Mascareñas, D.R.; Senoro, D.B. Health Risks Due to Metal Concentrations in Soil and Vegetables from the Six Municipalities of the Island Province in the Philippines. Int. J. Environ. Res. Public Health 2022, 19, 1587. [Google Scholar] [CrossRef]









| Method of Indexing | Range | Degree of Pollution |
|---|---|---|
| Heavy Metal Pollution Index (MPI) | <90 | Low |
| 90–180 | Medium | |
| >180 | High |
| NPI | Contamination Degree |
|---|---|
| <1.0 | Unpolluted |
| 1.0 ≤ PN < 2.5 | Slightly polluted |
| 2.5 ≤ PN < 7.0 | Moderately polluted |
| ≥7.0 | Heavily polluted |
| Parameter | Unit | Oral Values | Investigator(s) |
|---|---|---|---|
| Ingestion rate (IR) | |||
| Liters/day | 2.20 | [37] |
| Liters/day | 1.00 | [38] |
| Exposure frequency (EF) | Days/year | 365 | [39] |
| Exposure duration (ED) | |||
| Year | 70 | [40] |
| Year | 10 | [40] |
| Body weight (BW) | |||
| Kilograms | 70 | [37] |
| Kilograms | 25 | [38] |
| Average time (AT) | |||
| Days | 25,550 | [41] |
| Days | 3650 | [42] |
| Heavy Metal | Oral RfD (mg/kg/day) | SF (mg/kg/day)−1 | Reference |
|---|---|---|---|
| Arsenic | 3 × 10−4 | 1.5 | [44] |
| Barium | 2 × 10−1 | - | [45] |
| Copper | 0.04 | - | [46] |
| Iron | 7 × 10−1 | - | [47] |
| Manganese | 1.4 × 10−1 | - | [47] |
| Nickel | 0.02 | 0.84 | [46] |
| Zinc | 0.3 | - | [46] |
| Lead | 0.0014 | 8.5 × 10−3 | [46] |
| Risk Level | TCR Value | Cancer Risk |
|---|---|---|
| 1 | TCR < 10−6 | Very low |
| 2 | 10−6 < TCR < 10−5 | Low |
| 3 | 10−5 < TCR < 10−4 | Medium |
| 4 | 10−4 < TCR < 10−3 | High |
| 5 | TCR > 10−3 | Very high |
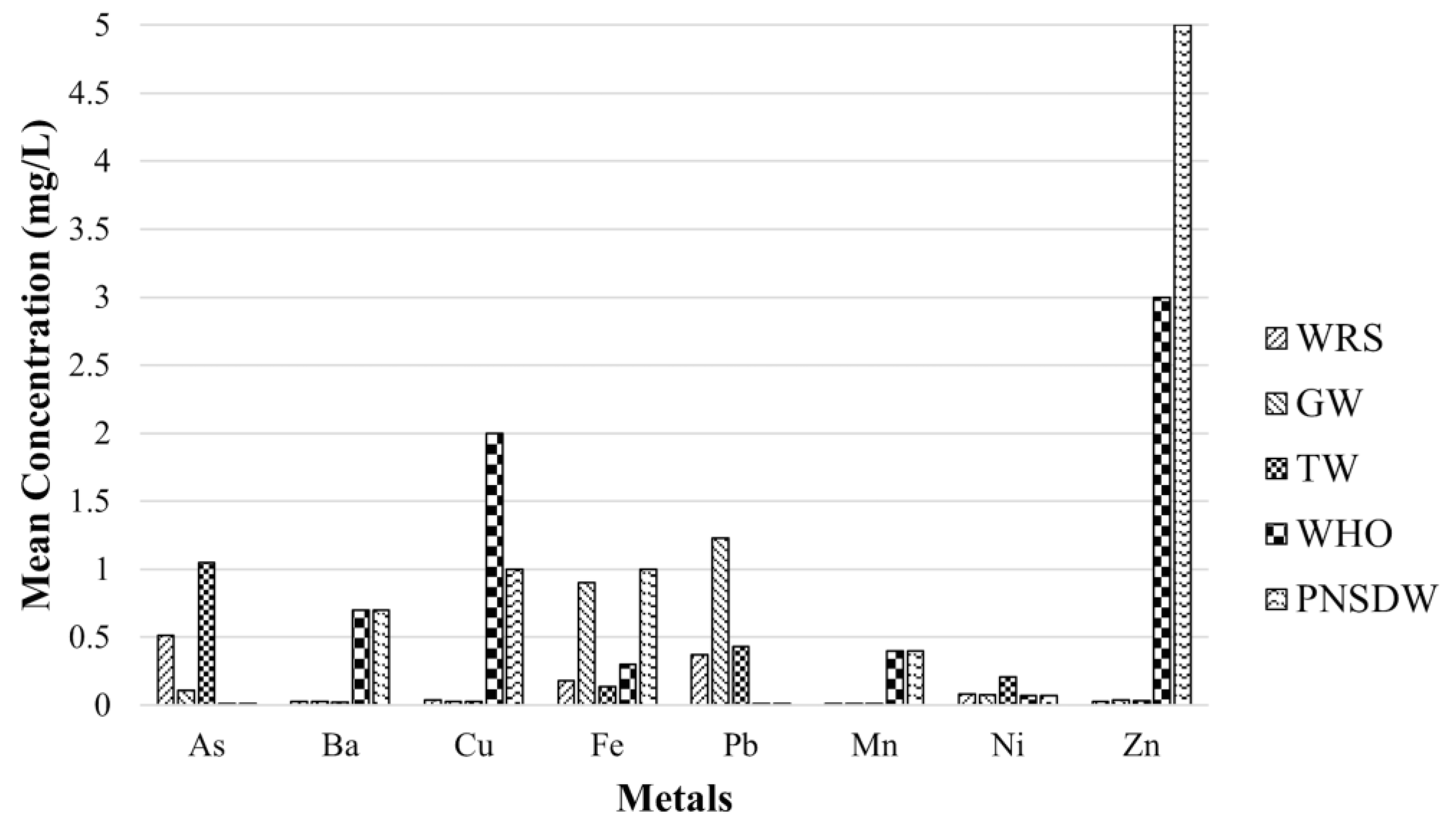
| Parameters | Unit | WRS (n = 25) | GW (n = 26) | TW (n = 49) | WHO [1] | PNSDW [48] | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | CV | Mean | SD | CV | Mean | SD | CV | ||||
| Temperature | °C | 26.6 | 3.69 | 13.9 | 29.3 | 1.99 | 6.78 | 29.4 | 1.61 | 5.48 | - | - |
| pH | - | 6.74 | 0.89 | 13.2 | 7.03 | 0.48 | 6.78 | 6.91 | 1.03 | 14.9 | 6.5–9.5 | 6.5–8.5 |
| EC | μS/cm | 51.6 | 84.6 | 164 | 680 | 735 | 108 | 378 | 286 | 75.7 | - | - |
| TDS | ppm | 19.2 | 38.8 | 202 | 328 | 367 | 112 | 180 | 143 | 79.0 | - | 600 |
| As | mg L−1 | 0.515 | 1.86 | 361 | 0.106 | 0.19 | 184 | 1.05 | 3.84 | 365 | 0.01 | 0.01 |
| Ba | mg L−1 | 0.027 | 0.02 | 70.2 | 0.025 | 0.02 | 60.7 | 0.023 | 0.02 | 80.8 | 0.70 | 0.70 |
| Cu | mg L−1 | 0.038 | 0.08 | 222 | 0.025 | 0.06 | 250 | 0.027 | 0.09 | 319 | 2.00 | 1.00 |
| Fe | mg L−1 | 0.178 | 0.31 | 173 | 0.901 | 2.93 | 325 | 0.138 | 0.31 | 225 | 0.30 | 1.00 |
| Pb | mg L−1 | 0.371 | 0.59 | 158 | 1.23 | 3.03 | 247 | 0.432 | 0.80 | 184 | 0.01 | 0.01 |
| Mn | mg L−1 | 0.009 | 0.004 | 41.8 | 0.009 | 0.01 | 68.2 | 0.010 | 0.01 | 49.3 | 0.40 | 0.40 |
| Ni | mg L−1 | 0.082 | 0.02 | 30.5 | 0.077 | 0.04 | 55.2 | 0.208 | 0.58 | 277 | 0.07 | 0.07 |
| Zn | mg L−1 | 0.029 | 0.01 | 47.7 | 0.035 | 0.03 | 93.6 | 0.030 | 0.02 | 50.7 | 3.00 | 5.00 |
| Water Sources | Trend |
|---|---|
| WRS | As > Pb > Fe > Ni > Cu > Zn > Ba > Mn |
| GW | Pb > Fe > As > Ni > Zn > Ba > Cu > Mn |
| TW | As > Pb > Ni > Fe > Zn > Cu > Ba > Mn |
| Water Sources | HMMs | |||||||
|---|---|---|---|---|---|---|---|---|
| As | Ba | Cu | Fe | Pb | Mn | Ni | Zn | |
| TW (adult) | 0.0331 | 0.0007 | 0.0009 | 0.0043 | 0.0136 | 0.0003 | 0.0065 | 0.0010 |
| GW (adult) | 0.0033 | 0.0008 | 0.0008 | 0.0283 | 0.0386 | 0.0003 | 0.0024 | 0.0011 |
| WRS (adult) | 0.0162 | 0.0008 | 0.0011 | 0.0056 | 0.0117 | 0.0003 | 0.0026 | 0.0009 |
| TW (child) | 0.0421 | 0.0009 | 0.0011 | 0.0055 | 0.0173 | 0.0004 | 0.0083 | 0.0012 |
| GW (child) | 0.0042 | 0.0010 | 0.0010 | 0.0360 | 0.0491 | 0.0004 | 0.0031 | 0.0014 |
| WRS (child) | 0.0206 | 0.0011 | 0.0015 | 0.0071 | 0.0148 | 0.0004 | 0.0033 | 0.0011 |
| Water Sources | HQ | HI | |||||||
|---|---|---|---|---|---|---|---|---|---|
| As | Ba | Cu | Fe | Pb | Mn | Ni | Zn | ||
| Tap water (adult) | 110 * | 0.004 | 0.022 | 0.006 | 9.71 * | 0.002 | 0.327 | 0.003 | 120 * |
| GW (adult) | 3.14 * | 0.004 | 0.006 | 0.011 | 35.0 * | 0.002 | 0.123 | 0.002 | 38.2 * |
| WRS (adult) | 30.6 * | 0.005 | 0.015 | 0.005 | 7.50 * | 0.002 | 0.129 | 0.003 | 38.3 * |
| Tap water (child) | 140 * | 0.005 | 0.028 | 0.008 | 12.4 * | 0.003 | 0.416 | 0.004 | 153 * |
| GW (child) | 14.2 * | 0.005 | 0.025 | 0.052 | 35.1 * | 0.003 | 0.155 | 0.005 | 49.5 * |
| WRS (child) | 68.7 * | 0.005 | 0.037 | 0.010 | 10.6 * | 0.003 | 0.164 | 0.004 | 79.5 * |
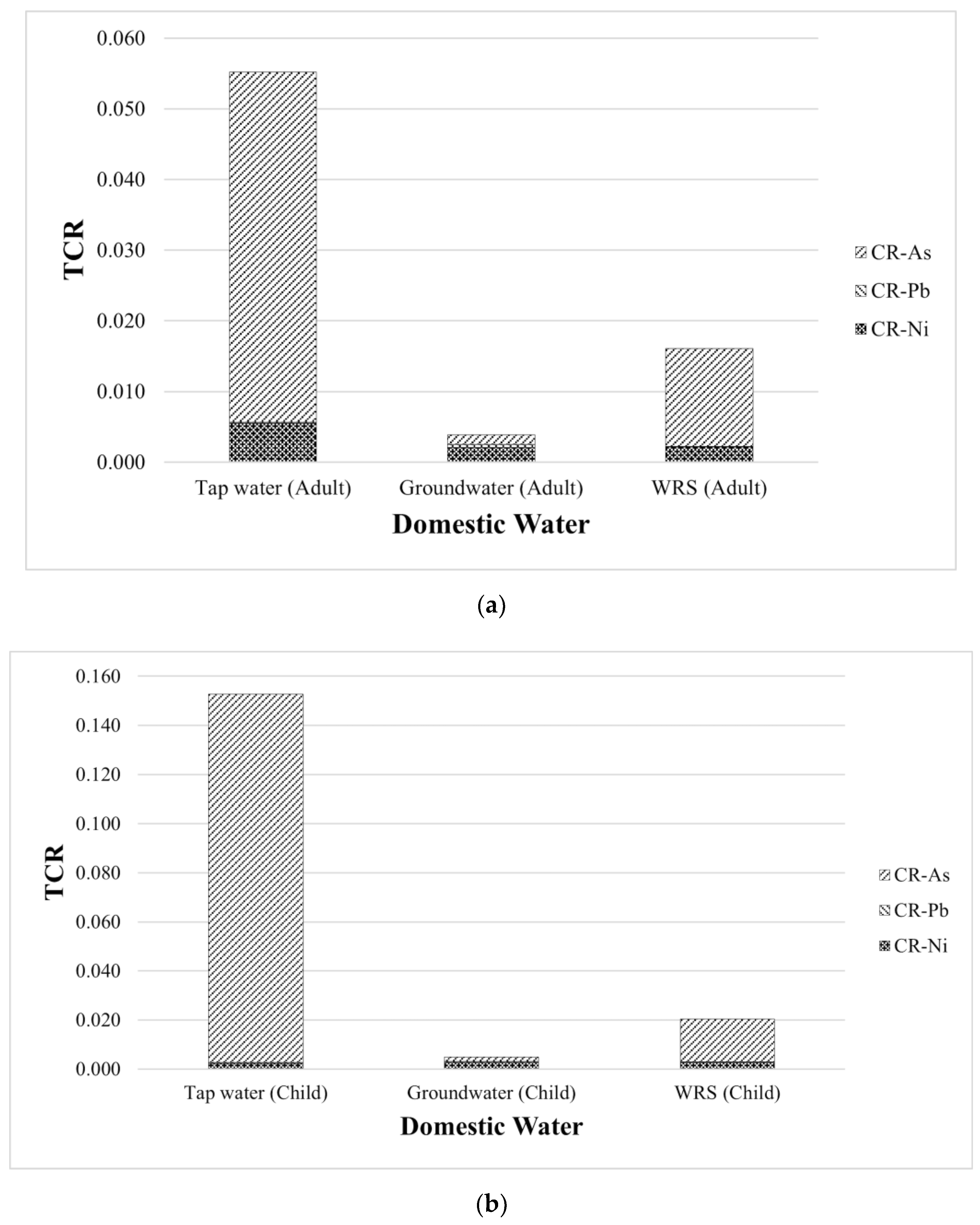
| Water Sources | CR | TCR | ||
|---|---|---|---|---|
| As | Pb | Ni | ||
| Tap water (adult) | 4.96 × 10−2 | 1.16 × 10−4 | 5.50 × 10−3 | 5.53 × 10−2 |
| Groundwater (adult) | 1.41 × 10−3 | 4.16 × 10−4 | 2.07 × 10−3 | 3.90 × 10−3 |
| WRS (adult) | 1.38 × 10−2 | 8.92 × 10−5 | 2.16 × 10−3 | 1.60 × 10−2 |
| Tap water (child) | 1.50 × 10−1 | 3.32 × 10−4 | 2.35 × 10−3 | 1.53 × 10−1 |
| Groundwater (child) | 1.80 × 10−3 | 5.29 × 10−4 | 2.63 × 10−3 | 4.96 × 10−3 |
| WRS (child) | 1.75 × 10−2 | 1.14 × 10−4 | 2.76 × 10−3 | 2.04 × 10−2 |
| Hidden Neurons | No. of Particles | No. of Iterations | Elapsed Time (sec) | MSE | R | ||
|---|---|---|---|---|---|---|---|
| Validation | Testing | ||||||
| TW | 27 | 10 | 2000 | 179.07227 | 0.00946 | 0.99943 | 0.97347 |
| GW | 29 | 6 | 2000 | 158.96334 | 0.00269 | 0.98592 | 0.95757 |
| WRS | 25 | 10 | 2000 | 118.08149 | 0.00240 | 0.99995 | 0.99063 |
| Hidden Neurons | No. of Particles | No. of Iterations | Elapsed Time (sec) | MSE | R | ||
|---|---|---|---|---|---|---|---|
| Validation | Testing | ||||||
| TW | 28 | 2 | 2000 | 156.89022 | 0.004781 | 0.99111 | 0.98528 |
| GW | 26 | 8 | 2000 | 160.20618 | 0.001309 | 0.97545 | 0.98381 |
| WRS | 29 | 10 | 2000 | 162.05585 | 0.001384 | 0.99959 | 0.96495 |
| Hidden Neurons | No. of Particles | No. of Iterations | Elapsed Time (sec) | MSE | R | ||
|---|---|---|---|---|---|---|---|
| Validation | Testing | ||||||
| TW | 30 | 5 | 2000 | 175.55656 | 0.00275 | 0.99856 | 0.99881 |
| GW | 25 | 5 | 2000 | 168.88374 | 0.00386 | 0.97732 | 0.99998 |
| WRS | 28 | 9 | 2000 | 124.37414 | 0.00142 | 0.99935 | 0.99994 |
| Hidden Neurons | No. of Particles | No. of Iterations | Elapsed Time (sec) | MSE | R | ||
|---|---|---|---|---|---|---|---|
| Validation | Testing | ||||||
| TW | 28 | 10 | 2000 | 159.46539 | 0.00585 | 0.99913 | 0.99628 |
| GW | 30 | 6 | 2000 | 178.66654 | 0.00237 | 0.99985 | 0.99978 |
| WRS | 26 | 3 | 2000 | 186.69597 | 0.00173 | 0.99637 | 0.99758 |
| Hidden Neurons | No. of Particles | No. of Iterations | Elapsed Time (sec) | MSE | R | ||
|---|---|---|---|---|---|---|---|
| Validation | Testing | ||||||
| TW | 30 | 7 | 2000 | 132.47061 | 0.00082 | 0.99628 | 0.99924 |
| GW | 26 | 7 | 2000 | 169.82680 | 0.00002 | 0.99950 | 0.99589 |
| WRS | 29 | 6 | 2000 | 169.27427 | 0.00001 | 0.99957 | 0.99629 |
| Hidden Neurons | No. of Particles | No. of Iterations | Elapsed Time (sec) | MSE | R | ||
|---|---|---|---|---|---|---|---|
| Validation | Testing | ||||||
| Tap Water | 29 | 7 | 2000 | 166.27182 | 0.00028 | 0.99871 | 0.94267 |
| GW | 26 | 7 | 2000 | 169.06680 | 0.00002 | 0.99873 | 0.99979 |
| WRS | 29 | 6 | 2000 | 130.73092 | 0.00001 | 0.97401 | 0.98393 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Senoro, D.B.; de Jesus, K.L.M.; Nolos, R.C.; Lamac, M.R.L.; Deseo, K.M.; Tabelin, C.B. In Situ Measurements of Domestic Water Quality and Health Risks by Elevated Concentration of Heavy Metals and Metalloids Using Monte Carlo and MLGI Methods. Toxics 2022, 10, 342. https://doi.org/10.3390/toxics10070342
Senoro DB, de Jesus KLM, Nolos RC, Lamac MRL, Deseo KM, Tabelin CB. In Situ Measurements of Domestic Water Quality and Health Risks by Elevated Concentration of Heavy Metals and Metalloids Using Monte Carlo and MLGI Methods. Toxics. 2022; 10(7):342. https://doi.org/10.3390/toxics10070342
Chicago/Turabian StyleSenoro, Delia B., Kevin Lawrence M. de Jesus, Ronnel C. Nolos, Ma. Rowela L. Lamac, Khainah M. Deseo, and Carlito B. Tabelin. 2022. "In Situ Measurements of Domestic Water Quality and Health Risks by Elevated Concentration of Heavy Metals and Metalloids Using Monte Carlo and MLGI Methods" Toxics 10, no. 7: 342. https://doi.org/10.3390/toxics10070342
APA StyleSenoro, D. B., de Jesus, K. L. M., Nolos, R. C., Lamac, M. R. L., Deseo, K. M., & Tabelin, C. B. (2022). In Situ Measurements of Domestic Water Quality and Health Risks by Elevated Concentration of Heavy Metals and Metalloids Using Monte Carlo and MLGI Methods. Toxics, 10(7), 342. https://doi.org/10.3390/toxics10070342










