Forskolin Induces Endocrine Disturbance in Human JEG-3 Placental Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Cell Line and Culture Condition
2.3. Cell Viability Evaluation
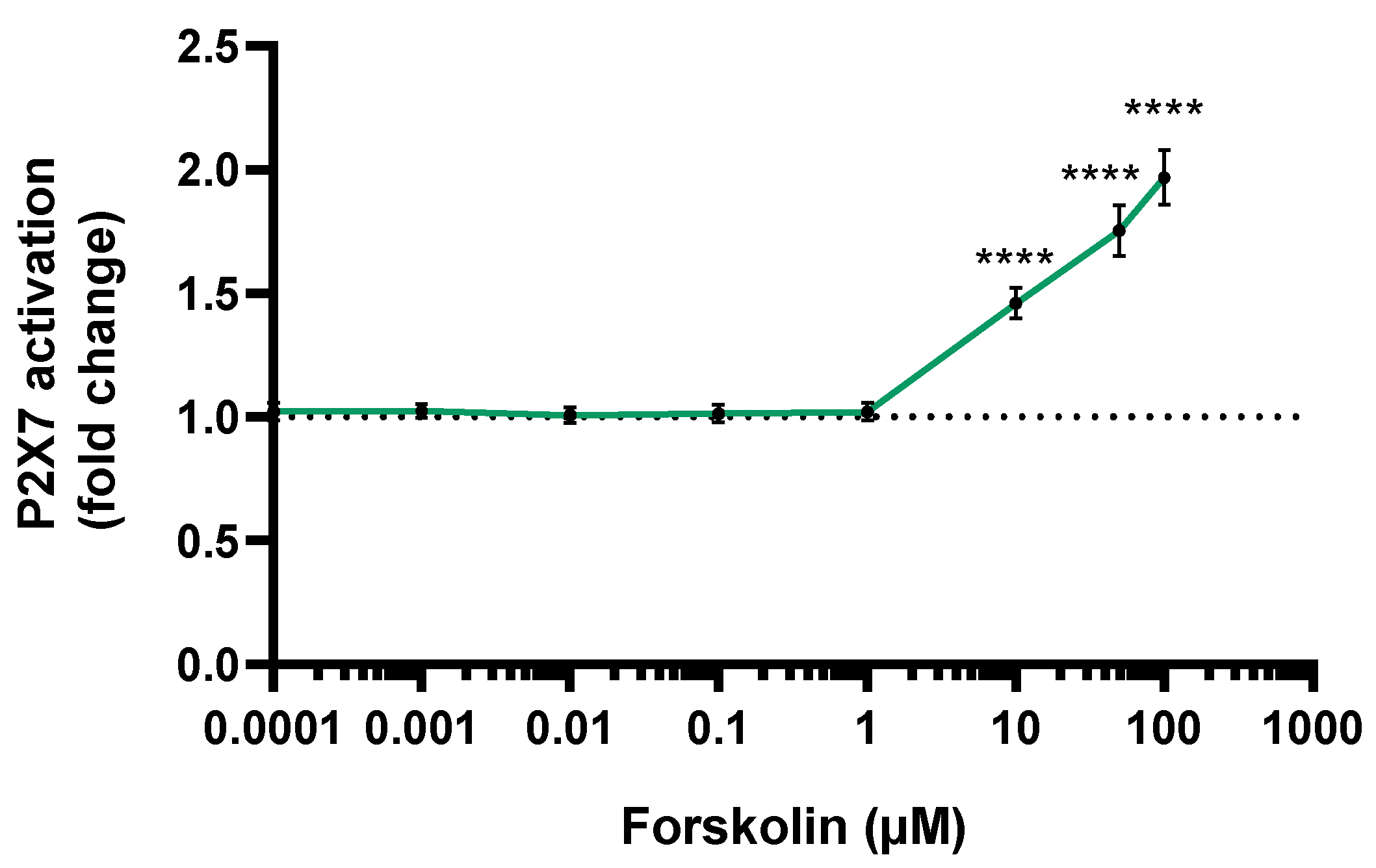
2.4. P2X7 Receptor Activation
2.5. Hormonal Dosages
2.6. Statistical Analysis
3. Results
3.1. Cell Viability
3.2. P2X7 Activation
3.3. Estradiol Secretion
3.4. Progesterone Secretion
3.5. hPL Secretion
3.6. Hyperglycosylated hCG Secretion
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chinnasamy, K.; Rajamani, K.; Vadivel, E. Coleus Forskohlii: A Comprehensive Review on Morphology, Phytochemistry and Pharmacological Aspects. J. Med. Plants Res. 2010, 4, 278–285. [Google Scholar]
- Valdés, L.J.; Mislankar, S.G.; Paul, A.G. Coleus barbatus (C.forskohlii)(Lamiaceae) and the potential new drug forskolin (Coleonol). Econ. Bot. 1987, 41, 474–483. [Google Scholar] [CrossRef]
- Tripathi, P.; Singh, A. Natural resources from plants in the treatment of cancer: An update. Asian J. Pharm. Clin. Res. 2017, 10, 13. [Google Scholar] [CrossRef]
- Sapio, L.; Gallo, M.; Illiano, M.; Chiosi, E.; Naviglio, D.; Spina, A.; Naviglio, S. The Natural cAMP Elevating Compound Forskolin in Cancer Therapy: Is It Time? J. Cell. Physiol. 2017, 232, 922–927. [Google Scholar] [CrossRef]
- Illiano, M.; Sapio, L.; Salzillo, A.; Capasso, L.; Caiafa, I.; Chiosi, E.; Spina, A.; Naviglio, S. Forskolin improves sensitivity to doxorubicin of triple negative breast cancer cells via Protein Kinase A-mediated ERK1/2 inhibition. Biochem. Pharmacol. 2018, 152, 104–113. [Google Scholar] [CrossRef]
- Wagh, V.D.; Patil, P.N.; Surana, S.J.; Wagh, K.V. Forskolin: Upcoming antiglaucoma molecule. J. Postgrad. Med. 2012, 58, 199–202. [Google Scholar] [CrossRef]
- Loskutova, E.; O’Brien, C.; Loskutov, I.; Loughman, J. Nutritional supplementation in the treatment of glaucoma: A systematic review. Surv. Ophthalmol. 2019, 64, 195–216. [Google Scholar] [CrossRef] [Green Version]
- Rolle, T.; Dallorto, L.; Rossatto, S.; Curto, D.; Nuzzi, R. Assessing the Performance of Daily Intake of a Homotaurine, Carnosine, Forskolin, Vitamin B2, Vitamin B6, and Magnesium Based Food Supplement for the Maintenance of Visual Function in Patients with Primary Open Angle Glaucoma. J. Ophthalmol. 2020, 2020, 7879436. [Google Scholar] [CrossRef] [Green Version]
- Illiano, M.; Conte, M.; Sapio, L.; Nebbioso, A.; Spina, A.; Altucci, L.; Naviglio, S. Forskolin Sensitizes Human Acute Myeloid Leukemia Cells to H3K27me2/3 Demethylases GSKJ4 Inhibitor via Protein Kinase A. Front. Pharmacol. 2018, 9, 792. [Google Scholar] [CrossRef]
- Loftus, H.L.; Astell, K.J.; Mathai, M.L.; Su, X.Q. Coleus forskohlii Extract Supplementation in Conjunction with a Hypocaloric Diet Reduces the Risk Factors of Metabolic Syndrome in Overweight and Obese Subjects: A Randomized Controlled Trial. Nutrients 2015, 7, 9508–9522. [Google Scholar] [CrossRef] [Green Version]
- Henderson, S.; Magu, B.; Rasmussen, C.; Lancaster, S.; Kerksick, C.; Smith, P.; Melton, C.; Cowan, P.; Greenwood, M.; Earnest, C.; et al. Effects of Coleus Forskohlii Supplementation on Body Composition and Hematological Profiles in Mildly Overweight Women. J. Int. Soc. Sports Nutr. 2005, 2, 54–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hersey, S.; Owirodu, A.; Miller, M. Forskolin stimulation of acid and pepsinogen secretion by gastric glands. Biochim. Biophys. Acta 1983, 755, 293–299. [Google Scholar] [CrossRef]
- Shu, Y.-M.; Zeng, H.-T.; Ren, Z.; Zhuang, G.-L.; Liang, X.-Y.; Shen, H.-W.; Yao, S.-Z.; Ke, P.-Q.; Wang, N.-N. Effects of cilostamide and forskolin on the meiotic resumption and embryonic development of immature human oocytes. Hum. Reprod. 2008, 23, 504–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almeida, F.C.; Lemonica, I.P. The toxic effects of Coleus barbatus B. on the different periods of pregnancy in rats. J. Ethnopharmacol. 2000, 73, 53–60. [Google Scholar] [CrossRef]
- Griffiths, B.K.; Campbell, J.P. Placental structure, function and drug transfer. Contin. Educ. Anaesth. Crit. Care Pain 2015, 15, 84–89. [Google Scholar] [CrossRef] [Green Version]
- Murphy, V.E.; Smith, R.; Giles, W.B.; Clifton, V. Endocrine Regulation of Human Fetal Growth: The Role of the Mother, Placenta, and Fetus. Endocr. Rev. 2006, 27, 141–169. [Google Scholar] [CrossRef]
- Chatuphonprasert, W.; Jarukamjorn, K.; Ellinger, I. Physiology and Pathophysiology of Steroid Biosynthesis, Transport and Metabolism in the Human Placenta. Front. Pharmacol. 2018, 9, 1027. [Google Scholar] [CrossRef] [Green Version]
- Reis, F.M.; D’Antona, D.; Petraglia, F. Predictive Value of Hormone Measurements in Maternal and Fetal Complications of Pregnancy. Endocr. Rev. 2002, 23, 230–257. [Google Scholar] [CrossRef]
- Chaddha, V.; Viero, S.; Huppertz, B.; Kingdom, J. Developmental biology of the placenta and the origins of placental insufficiency. Semin. Fetal Neonatal Med. 2004, 9, 357–369. [Google Scholar] [CrossRef]
- Fisher, S.J. The placental problem: Linking abnormal cytotrophoblast differentiation to the maternal symptoms of preeclampsia. Reprod. Biol. Endocrinol. 2004, 2, 53. [Google Scholar] [CrossRef] [Green Version]
- Ilekis, J.V.; Tsilou, E.; Fisher, S.; Abrahams, V.M.; Soares, M.J.; Cross, J.C.; Zamudio, S.; Illsley, N.; Myatt, L.; Colvis, C.; et al. Placental origins of adverse pregnancy outcomes: Potential molecular targets: An Executive Workshop Summary of the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Am. J. Obstet. Gynecol. 2016, 215, S1–S46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgan, T.K. Role of the Placenta in Preterm Birth: A Review. Am. J. Perinatol. 2016, 33, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Tsimis, M.E.; Lei, J.; Rosenzweig, J.M.; Arif, H.; Shabi, Y.; AlShehri, W.; Talbot, C.C.; Baig-Ward, K.M.; Segars, J.; Graham, E.M.; et al. P2X7 receptor blockade prevents preterm birth and perinatal brain injury in a mouse model of intrauterine inflammation. Biol. Reprod. 2017, 97, 230–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fodor, P.; White, B.; Khan, R. Inflammation—The role of ATP in pre-eclampsia. Microcirculation 2020, 27, e12585. [Google Scholar] [CrossRef]
- Wakx, A.; Regazzetti, A.; Dargère, D.; Auzeil, N.; Gil, S.; Evain-Brion, D.; Laprévote, O.; Rat, P. New in vitro biomarkers to detect toxicity in human placental cells: The example of benzo[A]pyrene. Toxicol. Vitro Int. J. Publ. Assoc. BIBRA 2016, 32, 76–85. [Google Scholar] [CrossRef]
- Fouyet, S.; Olivier, E.; Leproux, P.; Dutot, M.; Rat, P. Bisphenol A, Bisphenol F, and Bisphenol S: The Bad and the Ugly. Where Is the Good? Life 2021, 11, 314. [Google Scholar] [CrossRef]
- Fouyet, S.; Olivier, E.; Leproux, P.; Dutot, M.; Rat, P. Pregnant Women and Endocrine Disruptors: Role of P2X7 Receptor and Mitochondrial Alterations in Placental Cell Disorders. Cells 2022, 11, 495. [Google Scholar] [CrossRef]
- Olivier, E.; Wakx, A.; Fouyet, S.; Dutot, M.; Rat, P. JEG-3 placental cells in toxicology studies: A promising tool to reveal pregnancy disorders. Anat. Cell Biol. 2021, 54, 83–92. [Google Scholar] [CrossRef]
- Rat, P.; Olivier, E.; Tanter, C.; Wakx, A.; Dutot, M. A fast and reproducible cell- and 96-well plate-based method for the evaluation of P2X7 receptor activation using YO-PRO-1 fluorescent dye. J. Biol. Methods 2017, 4, e64. [Google Scholar] [CrossRef] [Green Version]
- Innis, S.M. Fatty acids and early human development. Early Hum. Dev. 2007, 83, 761–766. [Google Scholar] [CrossRef]
- Zhu, Y.; Mordaunt, C.E.; Yasui, D.H.; Marathe, R.; Coulson, R.L.; Dunaway, K.W.; Jianu, J.M.; Walker, C.K.; Ozonoff, S.; Hertz-Picciotto, I.; et al. Placental DNA methylation levels at CYP2E1 and IRS2 are associated with child outcome in a prospective autism study. Hum. Mol. Genet. 2019, 28, 2659–2674. [Google Scholar] [CrossRef]
- Szabó, D.; Tod, P.; Gölöncsér, F.; Román, V.; Lendvai, B.; Otrokocsi, L.; Sperlágh, B. Maternal P2X7 receptor inhibition prevents autism-like phenotype in male mouse offspring through the NLRP3-IL-1β pathway. Brain Behav. Immun. 2022, 101, 318–332. [Google Scholar] [CrossRef]
- Brănişteanu, D.D.; Mathieu, C. Progesterone in gestational diabetes mellitus: Guilty or not guilty? Trends Endocrinol. Metab. 2003, 14, 54–56. [Google Scholar] [CrossRef]
- Qi, X.; Gong, B.; Yu, J.; Shen, L.; Jin, W.; Wu, Z.; Wang, J.; Wang, J.; Li, Z. Decreased cord blood estradiol levels in related to mothers with gestational diabetes. Medicine 2017, 96, e6962. [Google Scholar] [CrossRef]
- Berkane, N.; Liere, P.; Oudinet, J.-P.; Hertig, A.; Lefèvre, G.; Pluchino, N.; Schumacher, M.; Chabbert-Buffet, N. From Pregnancy to Preeclampsia: A Key Role for Estrogens. Endocr. Rev. 2017, 38, 123–144. [Google Scholar] [CrossRef]
- Harada, N.; Yoshimura, N.; Honda, S.-I. Unique regulation of expression of human aromatase in the placenta. J. Steroid Biochem. Mol. Biol. 2003, 86, 327–334. [Google Scholar] [CrossRef]
- Means, G.D.; Kilgore, M.W.; Mahendroo, M.S.; Mendelson, C.R.; Simpson, E.R. Tissue-Specific Promoters Regulate Aromatase Cytochrome P450 Gene Expression in Human Ovary and Fetal Tissues. Mol. Endocrinol. 1991, 5, 2005–2013. [Google Scholar] [CrossRef] [Green Version]
- Honkisz, E.; Wójtowicz, A.K. Modulation of estradiol synthesis and aromatase activity in human choriocarcinoma JEG-3 cells exposed to tetrabromobisphenol A. Toxicol. Vitro Int. J. Publ. Assoc. BIBRA 2015, 29, 44–50. [Google Scholar] [CrossRef]
- Meng, Y.; Lv, P.-P.; Ding, G.-L.; Yu, T.-T.; Liu, Y.; Shen, Y.; Hu, X.-L.; Lin, X.-H.; Tian, S.; Lv, M.; et al. High Maternal Serum Estradiol Levels Induce Dyslipidemia in Human Newborns via a Hepatic HMGCR Estrogen Response Element. Sci. Rep. 2015, 5, 10086. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.-L.; Feng, C.; Lin, X.-H.; Zhong, Z.-X.; Zhu, Y.-M.; Lv, P.-P.; Lv, M.; Meng, Y.; Zhang, D.; Lu, X.-E.; et al. High Maternal Serum Estradiol Environment in the First Trimester Is Associated With the Increased Risk of Small-for-Gestational-Age Birth. J. Clin. Endocrinol. Metab. 2014, 99, 2217–2224. [Google Scholar] [CrossRef] [Green Version]
- Wunsch, D.M.; Anderson, L.D.; Pepe, G.J.; Albrecht, E.D. Regulation of Progesterone Formation by Human Placental Cells in Culture. Endocrinology 1986, 119, 998–1003. [Google Scholar] [CrossRef]
- Pepe, G.J.; Albrecht, E.D. Steroid Endocrinology of Pregnancy|GLOWM. Glob. Libr. Women’s Med. 2008. [Google Scholar] [CrossRef]
- Shanker, Y.G.; Rao, A.J. Regulation of progresterone biosynthesis in the human placenta by estradiol 17β and progesterone. IUBMB Life 1997, 43, 591–599. [Google Scholar] [CrossRef]
- Caritis, S.N.; Hirsch, R.P.; Zeleznik, A.J. Adrenergic Stimulation of Placental Progesterone Production. J. Clin. Endocrinol. Metab. 1983, 56, 969–972. [Google Scholar] [CrossRef]
- Feinman, M.A.; Kliman, H.J.; Caltabiano, S.; Strauss, J.F. 8-Bromo-3′5′ -Adenosine Monophosphate Stimulates the Endocrine Activity of Human Cytotrophoblasts in Culture. J. Clin. Endocrinol. Metab. 1986, 63, 1211–1217. [Google Scholar] [CrossRef]
- Park, S.; Kim, M.-Y.; Baik, S.H.; Woo, J.-T.; Kwon, Y.J.; Daily, J.W.; Park, Y.-M.; Yang, J.-H.; Kim, S.-H. Gestational diabetes is associated with high energy and saturated fat intakes and with low plasma visfatin and adiponectin levels independent of prepregnancy BMI. Eur. J. Clin. Nutr. 2013, 67, 196–201. [Google Scholar] [CrossRef] [Green Version]
- Billionnet, C.; Mitanchez, D.; Weill, A.; Nizard, J.; Alla, F.; Hartemann, A.; Jacqueminet, S. Gestational diabetes and adverse perinatal outcomes from 716,152 births in France in 2012. Diabetologia 2017, 60, 636–644. [Google Scholar] [CrossRef]
- Fox, H.; Sebire, N.J. 3-Physiology of the Placenta. In Pathology of the Placenta, 3rd ed.; Fox, H., Sebire, N.J., Eds.; Saunders: Edinburgh, Scotland, 2007; pp. 57–67. ISBN 978-1-4160-2592-4. [Google Scholar]
- Sibiak, R.; Jankowski, M.; Gutaj, P.; Mozdziak, P.; Kempisty, B.; Wender-Ożegowska, E. Placental Lactogen as a Marker of Maternal Obesity, Diabetes, and Fetal Growth Abnormalities: Current Knowledge and Clinical Perspectives. J. Clin. Med. 2020, 9, 1142. [Google Scholar] [CrossRef]
- Makrigiannakis, A.; Vrekoussis, T.; Zoumakis, E.; Kalantaridou, S.N.; Jeschke, U. The Role of HCG in Implantation: A Mini-Review of Molecular and Clinical Evidence. Int. J. Mol. Sci. 2017, 18, 1305. [Google Scholar] [CrossRef]
- Cole, L. Hyperglycosylated hCG. Placenta 2007, 28, 977–986. [Google Scholar] [CrossRef]
- Cole, L.A.; Butler, S. Hyperglycosylated hCG, hCGβ and Hyperglycosylated hCGβ: Interchangeable cancer promoters. Mol. Cell. Endocrinol. 2012, 349, 232–238. [Google Scholar] [CrossRef]
- Cole, L.A. Hyperglycosylated hCG and pregnancy failures. J. Reprod. Immunol. 2012, 93, 119–122. [Google Scholar] [CrossRef]
- Keikkala, E.; Vuorela, P.; Laivuori, H.; Romppanen, J.; Heinonen, S.; Stenman, U.-H. First trimester hyperglycosylated human chorionic gonadotrophin in serum—A marker of early-onset preeclampsia. Placenta 2013, 34, 1059–1065. [Google Scholar] [CrossRef]
- Maad, M.S.; Shaymaa, K.J.; Huda, K.A. Prediction of Placenta Accreta Using Hyperglycosylated Human Chorionic Gonadotropin. Int. J. Women’s Health Reprod. Sci. 2020, 8, 142–146. [Google Scholar]
- Maglich, J.M.; Kuhn, M.; Chapin, R.E.; Pletcher, M.T. More than just hormones: H295R cells as predictors of reproductive toxicity. Reprod. Toxicol. 2014, 45, 77–86. [Google Scholar] [CrossRef] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rat, P.; Leproux, P.; Fouyet, S.; Olivier, E. Forskolin Induces Endocrine Disturbance in Human JEG-3 Placental Cells. Toxics 2022, 10, 355. https://doi.org/10.3390/toxics10070355
Rat P, Leproux P, Fouyet S, Olivier E. Forskolin Induces Endocrine Disturbance in Human JEG-3 Placental Cells. Toxics. 2022; 10(7):355. https://doi.org/10.3390/toxics10070355
Chicago/Turabian StyleRat, Patrice, Pascale Leproux, Sophie Fouyet, and Elodie Olivier. 2022. "Forskolin Induces Endocrine Disturbance in Human JEG-3 Placental Cells" Toxics 10, no. 7: 355. https://doi.org/10.3390/toxics10070355
APA StyleRat, P., Leproux, P., Fouyet, S., & Olivier, E. (2022). Forskolin Induces Endocrine Disturbance in Human JEG-3 Placental Cells. Toxics, 10(7), 355. https://doi.org/10.3390/toxics10070355





