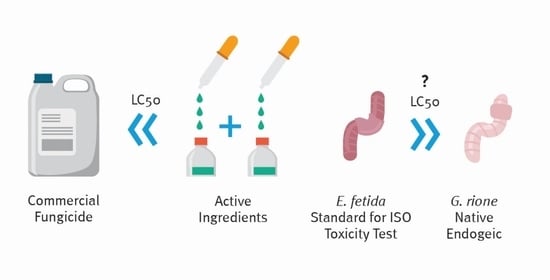
Commercial Fungicide Toxic Effects on Terrestrial Non-Target Species Might Be Underestimated When Based Solely on Active Ingredient Toxicity and Standard Earthworm Tests
Abstract
:1. Introduction
2. Materials and Methods
2.1. Review of Hazard Identification
2.2. Test Facilities
2.3. Test Organisms
2.4. Test Substances
2.5. Test Substrate
2.6. Experimental Setup
2.7. Toxicity Test 1: Sublethal Effects-Growth and Reproduction
2.8. Toxicity Test 2: Acute Effects-Lethality
2.9. Statistical Analysis
3. Results
3.1. Review of Hazard Identification
| Formulation Trade Brand | Active Ingredient | CAS Number | Earthworm Acute Toxicity, 14 Day-LC50 a (mg kg−1) | Acute Toxicity Category b [40,41,42,43] | Sub-lethal Toxicity, 56 Day-Reproduction NOEC c (mg kg−1) | Earthworm Sublethal Toxicity Category d [40,41,42,43] | Hazard Category e [30,31,44,45] |
|---|---|---|---|---|---|---|---|
| SWING PLUS® | Metconazole | 125116-23-6 | >500 | Moderate | >20 | Moderate |  |
| Epoxiconazole | 133855-98-8 | >500 | Moderate | >3.24 | Moderate |  | |
| PROSARO® | Tebuconazole | 107534-96-3 | 1381 | Low | 10 | Moderate |  |
| Prothioconazole | 178928-70-6 | >1000 | Low | 1.33 | Moderate |  |
 : May be fatal if swallowed and enters airways. Causes damage to organs. May cause damage to organs. May damage fertility or the unborn child. Suspected of damaging fertility or the unborn child. May cause cancer. Suspected of causing cancer. May cause genetic defects. Suspected of causing genetic defects. May cause allergy or asthma symptoms or breathing difficulties if inhaled.
: May be fatal if swallowed and enters airways. Causes damage to organs. May cause damage to organs. May damage fertility or the unborn child. Suspected of damaging fertility or the unborn child. May cause cancer. Suspected of causing cancer. May cause genetic defects. Suspected of causing genetic defects. May cause allergy or asthma symptoms or breathing difficulties if inhaled.  : May cause respiratory irritation. May cause drowsiness or dizziness. May cause an allergic skin reaction. Causes serious eye irritation. Causes skin irritation. Harmful if swallowed. Harmful in contact with skin. Harmful if inhaled. Harms public health and the environment by destroying ozone in the upper atmosphere.
: May cause respiratory irritation. May cause drowsiness or dizziness. May cause an allergic skin reaction. Causes serious eye irritation. Causes skin irritation. Harmful if swallowed. Harmful in contact with skin. Harmful if inhaled. Harms public health and the environment by destroying ozone in the upper atmosphere.  : Toxic or very toxic to aquatic life with long-lasting effects.
: Toxic or very toxic to aquatic life with long-lasting effects.| Formulation Trade Brand | Other Ingredient a | CAS Number | Toxicity to Earthworms b | Hazard Category According to ECHA c | Information Source |
|---|---|---|---|---|---|
| SWING PLUS® | propanoic acid (2S)-2-hydroxy-2-ethylhexyl ester | 186817-80-1 | Not available |  | ECHA, NIH |
| oxirane, methyl-, polymer with oxirane, monoisotridecyl ether, block | 196823-11-7 | Not available |  | ECHA | |
| poly(oxy-1,2-ethanediyl), α-[2,4,6-tris(1-phenylethyl)phenyl]-ω-hydroxy-, also known as tristyrylphenol ethoxylated | 99734-09-5 | 21-d LC50, earthworm Apporectodea calignosa, >40 mg kg−1. EC50 for growth reduction, 23.9 mg kg−1. EC10 and EC50 for reproduction 3.44 mg·kg−1 and 13.7 mg kg−1, respectively |  | ECHA Krogh et al., 1996, cited by [46] | |
| naphtha solvent (oil) heavy aromatic fraction | 64742-94-5 | LC50, E. fetida 14 d: 129 mg kg−1 |  | ECHA | |
| Kerosene unspecified | 64742-81-0 | LC50, E. fetida,14 d:1079 mg kg−1 |  | ECHA, [39] | |
| Benzyl alcohol | 100-51-6 | Not available |  | ECHA | |
| PROSARO® | N,N-Dimethyldecanamide | 14433-76-2 | E. fetida, LC50 = 1032.1 mg kg−1 soil (NOEC = 562 mg kg−1 soil) |  | ECHA, NIH |
 : May be fatal if swallowed and enters airways. Causes damage to organs. May cause damage to organs. May damage fertility or the unborn child. Suspected of damaging fertility or the unborn child. May cause cancer. Suspected of causing cancer. May cause genetic defects. Suspected of causing genetic defects. May cause allergy or asthma symptoms or breathing difficulties if inhaled.
: May be fatal if swallowed and enters airways. Causes damage to organs. May cause damage to organs. May damage fertility or the unborn child. Suspected of damaging fertility or the unborn child. May cause cancer. Suspected of causing cancer. May cause genetic defects. Suspected of causing genetic defects. May cause allergy or asthma symptoms or breathing difficulties if inhaled.  : May cause respiratory irritation. May cause drowsiness or dizziness. May cause an allergic skin reaction. Causes serious eye irritation. Causes skin irritation. Harmful if swallowed. Harmful in contact with skin. Harmful if inhaled. Harms public health and the environment by destroying ozone in the upper atmosphere.
: May cause respiratory irritation. May cause drowsiness or dizziness. May cause an allergic skin reaction. Causes serious eye irritation. Causes skin irritation. Harmful if swallowed. Harmful in contact with skin. Harmful if inhaled. Harms public health and the environment by destroying ozone in the upper atmosphere.  : Toxic or very toxic to aquatic life with long-lasting effects.
: Toxic or very toxic to aquatic life with long-lasting effects.3.2. Toxicity Test 1: Sublethal Effects–Growth and Reproduction
3.3. Toxicity Test 2: Acute Effects-Lethality
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blouin, M.; Hodson, M.E.; Delgado, E.A.; Baker, G.; Brussaard, L.; Butt, K.R.; Dai, J.; Dendooven, L.; Pérès, G.; Tondoh, J.E. A Review of Earthworm Impact on Soil Function and Ecosystem Services. Eur. J. Soil Sci. 2013, 64, 161–182. [Google Scholar] [CrossRef]
- Brown, G.G.; Pashanasi, B.; Villenave, C.; Patron, J.C.; Senapati, B.K.; Giri, S.; Barois, I.; Lavelle, P.; Blanchart, E.; Blakemore, R.J. Effects of Earthworms on Plant Production in the Tropics. Earthworm Manag. Trop. Agroecosyst. 1999, 5, 87–147. [Google Scholar]
- Scheu, S. Effects of Earthworms on Plant Growth: Patterns and Perspectives: The 7th International Symposium on Earthworm Ecology·Cardiff·Wales·2002. Pedobiologia 2003, 47, 846–856. [Google Scholar] [CrossRef]
- Domínguez, A.; Brown, G.G.; Sautter, K.D.; de Oliveira, C.M.R.; de Vasconcelos, E.C.; Niva, C.C.; Bartz, M.L.C.; Bedano, J.C. Toxicity of AMPA to the Earthworm Eisenia Andrei Bouché, 1972 in Tropical Artificial Soil. Sci. Rep. 2016, 6, 19731. [Google Scholar] [CrossRef] [PubMed]
- Santadino, M.; Coviella, C.; Momo, F. Glyphosate Sublethal Effects on the Population Dynamics of the Earthworm Eisenia Fetida (Savigny, 1826). Water. Air. Soil Pollut. 2014, 225, 2207. [Google Scholar] [CrossRef]
- NPIC. Pesticide Ingredients, National Pesticide Information Center. 2019. Available online: http://npic.orst.edu/ingred/index.html (accessed on 13 May 2021).
- Nagy, K.; Duca, R.C.; Lovas, S.; Creta, M.; Scheepers, P.T.; Godderis, L.; Ádám, B. Systematic Review of Comparative Studies Assessing the Toxicity of Pesticide Active Ingredients and Their Product Formulations. Environ. Res. 2020, 181, 108926. [Google Scholar] [CrossRef]
- Gomes, S.I.L.; Ammendola, A.; Casini, S.; Amorim, M.J.B. Toxicity of Fungicides to Terrestrial Non-Target Fauna—Formulated Products versus Active Ingredients (Azoxystrobin, Cyproconazole, Prothioconazole, Tebuconazole)—A Case Study with Enchytraeus Crypticus (Oligochaeta). Sci. Total Environ. 2021, 754, 142098. [Google Scholar] [CrossRef]
- De Silva, P.M.C.S.; Pathiratne, A.; van Gestel, C.A.M. Toxicity of Chlorpyrifos, Carbofuran, Mancozeb and Their Formulations to the Tropical Earthworm Perionyx Excavatus. Appl. Soil Ecol. 2010, 44, 56–60. [Google Scholar] [CrossRef]
- Robinson, C.; Portier, C.J.; Čavoški, A.; Mesnage, R.; Roger, A.; Clausing, P.; Whaley, P.; Muilerman, H.; Lyssimachou, A. Achieving a High Level of Protection from Pesticides in Europe: Problems with the Current Risk Assessment Procedure and Solutions. Eur. J. Risk Regul. 2020, 11, 450–480. [Google Scholar] [CrossRef]
- Chen, C.; Wang, Y.; Qian, Y.; Zhao, X.; Wang, Q. The Synergistic Toxicity of the Multiple Chemical Mixtures: Implications for Risk Assessment in the Terrestrial Environment. Environ. Int. 2015, 77, 95–105. [Google Scholar] [CrossRef]
- Defarge, N.; De Vendômois, J.S.; Séralini, G.E. Toxicity of Formulants and Heavy Metals in Glyphosate-Based Herbicides and Other Pesticides. Toxicol. Rep. 2018, 5, 156–163. [Google Scholar] [CrossRef] [PubMed]
- USEPA. Pesticide Registration Manual: Chapter 2—Registering a Pesticide Product. 2022. Available online: https://www.epa.gov/pesticide-registration/pesticide-registration-manual-chapter-2-registering-pesticide-product (accessed on 15 February 2022).
- Commission Regulation (EU). No 284/2013. Official Journal of the European Union. 2013. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2013:093:0085:0152:EN:PDF (accessed on 13 May 2021).
- MGAP. Form. 233 Solicitud de Registro de Producto Fitosanitario, Trámites y Servicios. Ministerio de Agricultura y Pesca. 2008. Available online: https://www.gub.uy/ministerio-ganaderia-agricultura-pesca/tramites-y-servicios/servicios/autorizacion-venta-productos-fitosanitarios (accessed on 10 February 2021).
- Method Development and Applications Section. Guidance Document on Statistical Methods for Environmental Toxicity Tests, Ottawa: Environment Canada. 2007. Available online: https://publications.gc.ca/collections/collection_2012/ec/En49-7-1-46-eng.pdf (accessed on 1 March 2021).
- Method Development and Applications Section. Biological Test Method: Tests for Toxicity of Contaminated Soil to Earthworms (Eisenia Andrei, Eisenia Fetida, or Lumbricus Terrestris), Guidance Document on Statistical Methods for Environmental Toxicity Tests. Ottawa: Environment Canada. 2004. Available online: https://publications.gc.ca/collections/collection_2013/ec/En49-7-1-43-eng.pdf (accessed on 5 March 2018).
- ISO 17512-1; Soil Quality—Avoidance Test for Determining the Quality of Soils and Effects of Chemicals on Behaviour—Part 1: Test with Earthworms (Eisenia Fetida and Eisenia Andrei). ISO: Geneve, Switzerland, 2008.
- ISO 11268-1 & ISO 11268-2; Soil Quality—Effects of Pollutants on Earthworms—Part 1: Determination of Acute Toxicity to Eisenia Fetida/Eisenia Andrei and Part 2: Determination of Effects on Reproduction of Eisenia fetida/Eisenia andrei. ISO: Geneve, Switzerland, 2012.
- OECD. Guideline for the Testing of Chemicals No. 207. Earthworm Acute Toxicity Tests; Organisation for Economic Co-Operation and Development: Paris, France, 1984. [Google Scholar]
- Pelosi, C.; Joimel, S.; Makowski, D. Searching for a More Sensitive Earthworm Species to Be Used in Pesticide Homologation Tests—A Meta-Analysis. Chemosphere 2013, 90, 895–900. [Google Scholar] [CrossRef] [PubMed]
- Buch, A.C.; Brown, G.G.; Niva, C.C.; Sautter, K.D.; Sousa, J.P. Toxicity of Three Pesticides Commonly Used in Brazil to Pontoscolex Corethrurus (Müller, 1857) and Eisenia Andrei (Bouché, 1972). Appl. Soil Ecol. 2013, 69, 32–38. [Google Scholar] [CrossRef]
- Garcia, M.; Römbke, J.; de Brito, M.T.; Scheffczyk, A. Effects of Three Pesticides on the Avoidance Behavior of Earthworms in Laboratory Tests Performed under Temperate and Tropical Conditions. Environ. Pollut. 2008, 153, 450–456. [Google Scholar] [CrossRef] [PubMed]
- De Silva, P.M.C.S.; van Gestel, C.A.M. Comparative Sensitivity of Eisenia Andrei and Perionyx Excavatus in Earthworm Avoidance Tests Using Two Soil Types in the Tropics. Chemosphere 2009, 77, 1609–1613. [Google Scholar] [CrossRef]
- Cordero, E.H. Oligoquetos Sudamericanos de La Familia Glossoscolecidae, I. El Género Glossoscolex En El Uruguay, Con Una Sinopsis de Las Especies Del Grupo Truncatus. Comun. Zoológicas Mus. Hist. Nat. Montev. 1943, 20, 1–9. [Google Scholar]
- Jorge-Escudero, G. Potencial Aporte de las Lombrices en el Control Biológico de Fusarium Graminearum en Agroecosistemas Uruguayos. Ph.D. Thesis, Agronomy Faculty, Universidad de la República, Montevideo, Uruguay, 2018. [Google Scholar]
- Machado, F.J.; Del Ponte, E.M. Fungicide Efficacy for Managing Fusarium Head Blight of Wheat in Brazil: Systematic Review and Meta-Analysis. In Proceedings of the P47, Fifth ISFHB: Session 4—Epidemiology and Management, 5th International Symposium on Fusarium Head Blight, Florianopolis, Brazil, 6–10 April 2016; p. 94. [Google Scholar]
- Balzarini, M.G.; González, L.A.; Tablada, E.M.; Casanoves, F.; Di Rienzo, J.A.; Robledo, C.W. Infostat: Manual Del Usuario; Editorial Brujas: Córdoba, Argentina, 2008. [Google Scholar]
- Grubbs, F.E. Procedures for Detecting Outlying Observations in Samples. Technometrics 1969, 11, 1–22. [Google Scholar] [CrossRef]
- ECHAa. Epoxiconazole, in Substance Infocard. 2022. Available online: https://echa.europa.eu/substance-information/-/substanceinfo/100.114.955 (accessed on 15 February 2022).
- ECHAb. (1RS,5RS;1RS,5SR)-5-(4-Chlorobenzyl)-2,2-Dimethyl-1-(1H-1,2,4-Triazol-1-Ylmethyl)Cyclopentanol;Metconazole (ISO), in Substance Infocard. 2022. Available online: https://echa.europa.eu/substance-information/-/substanceinfo/100.125.390 (accessed on 15 February 2022).
- NIHa. PubChem Compound Summary for CID 6451142, Prothioconazole. 2022. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Prothioconazole (accessed on 15 February 2022).
- NIHb. PubChem Compound Summary for CID 86102, Tebuconazole. 2022. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Tebuconazole (accessed on 15 February 2022).
- Toni, C.; Loro, V.L.; Santi, A.; De Menezes, C.C.; Cattaneo, R.; Clasen, B.E.; Zanella, R. Exposure to Tebuconazol in Rice Field and Laboratory Conditions Induces Oxidative Stress in Carp (Cyprinus Carpio). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2011, 153, 128–132. [Google Scholar] [CrossRef]
- ECHAc. Benzyl Alcohol, in Substance Infocard. 2022. Available online: https://echa.europa.eu/substance-information/-/substanceinfo/100.002.600 (accessed on 15 February 2022).
- ECHAd. Oxirane, Methyl-, Polymer with Oxirane, Monoisotridecyl Ether, Block, in Substance Infocard. 2022. Available online: https://echa.europa.eu/substance-information/-/substanceinfo/100.202.890 (accessed on 15 February 2022).
- NIHc. PubChem Compound Summary for CID 26690, N,N-Dimethyldecanamide. 2022. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/N_N-Dimethyldecanamide (accessed on 15 February 2022).
- ECHAe. N,N-Dimethyldecanamide, in Substance Infocard. 2022. Available online: https://echa.europa.eu/substance-information/-/substanceinfo/100.034.898 (accessed on 15 February 2022).
- Moon, Y.; Yim, U.-H.; Kim, H.-S.; Kim, Y.-J.; Shin, W.S.; Hwang, I. Toxicity and Bioaccumulation of Petroleum Mixtures with Alkyl PAHs in Earthworms. Hum. Ecol. Risk Assess. Int. J. 2013, 19, 819–835. [Google Scholar] [CrossRef]
- IUPACa. Metconazole, in PPDB: Pesticide Properties DataBase. 2022. Available online: http://sitem.herts.ac.uk/aeru/ppdb/en/Reports/451.htm (accessed on 15 February 2022).
- IUPACb. Epoxiconazole (Ref: BAS 480F). In PPDB: Pesticide Properties DataBase. 2022. Available online: http://sitem.herts.ac.uk/aeru/ppdb/en/Reports/267.htm (accessed on 15 February 2022).
- IUPACc. Tebuconazole (Ref: HWG 1608). In PPDB: Pesticide Properties DataBase. 2022. Available online: http://sitem.herts.ac.uk/aeru/ppdb/en/Reports/610.htm (accessed on 15 February 2022).
- IUPACd. Prothioconazole (Ref: JAU 6476). In PPDB: Pesticide Properties DataBase. 2022. Available online: http://sitem.herts.ac.uk/aeru/ppdb/en/Reports/559.htm (accessed on 15 February 2022).
- ECHAf. (RS)-1-(4-Chloro-Phenyl)-4,4-Dimethyl-3-[1,2,4]Triazol-1-Ylmethyl-Pentan-3-Ol, in Substance Infocard. 2022. Available online: https://echa.europa.eu/substance-information/-/substanceinfo/100.127.435 (accessed on 15 February 2022).
- ECHAg. Prothioconazole (ISO); 2-[2-(1-Chlorocyclopropyl)-3-(2-Chlorophenyl)-2-Hydroxypropyl]-2,4-Dihydro-3H-1,2,4-Triazole-3-Thione, in Substance Infocard. 2022. Available online: https://echa.europa.eu/substance-information/-/substanceinfo/100.114.615 (accessed on 15 February 2022).
- Canadian Council of Ministers of the Environment. Nonylphenol and Its Ethoxylates. Canadian Soil Quality Guidelines for the Protection of Environmental and Human Health; Excerpt from Publication No. 1299; ISBN 1-896997-34-1. Available online: https://ccme.ca/en/res/nonylphenol-and-its-ethoxylates-canadian-soil-quality-guidelines-for-the-protection-of-environmental-and-human-health-en.pdf (accessed on 15 February 2022).
- Bailer, A.J.; Hughes, M.R.; Denton, D.L.; Oris, J.T. An Empirical Comparison of Effective Concentration Estimators for Evaluating Aquatic Toxicity Test Responses. Environ. Toxicol. Chem. 2000, 19, 141–150. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Baldwin, L.A. The Dose Determines the Stimulation (and Poison): Development of A Chemical Hormesis Database. Int. J. Toxicol. 1997, 16, 545–559. [Google Scholar] [CrossRef]
- ISO/TS 20281:2006; Water Quality—Guidance on Statistical Interpretation of Ecotoxicity Data. ISO: Geneve, Switzerland, 2006.
- Mesnage, R.; Antoniou, M.N. Ignoring Adjuvant Toxicity Falsifies the Safety Profile of Commercial Pesticides. Front. Public Health 2018, 5, 361. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhou, Z.; Zhu, W. Evaluating the Effects of the Tebuconazole on the Earthworm, Eisenia Fetida by H-1 NMR-Based Untargeted Metabolomics and MRNA Assay. Ecotoxicol. Environ. Saf. 2020, 194, 110370. [Google Scholar] [CrossRef]
- Suthar, S. Toxicity of Methyl Parathion on Growth and Reproduction of Three Ecologically Different Tropical Earthworms. Int. J. Environ. Sci. Technol. 2014, 11, 191–198. [Google Scholar] [CrossRef]
- Kuperman, R.G.; Checkai, R.T.; Garcia, M.V.B.; Römbke, J.; Stephenson, G.L.; Sousa, J.P. State of the Science and the Way Forward for the Ecotoxicological Assessment of Contaminated Land. Pesqui. Agropecuária Bras. 2009, 44, 811–824. [Google Scholar] [CrossRef]
- Pelosi, C.; Lebrun, M.; Beaumelle, L.; Cheviron, N.; Delarue, G.; Nélieu, S. Sublethal Effects of Epoxiconazole on the Earthworm Aporrectodea Icterica. Environ. Sci. Pollut. Res. 2016, 23, 3053–3061. [Google Scholar] [CrossRef]
- Relyea, R.A. A Cocktail of Contaminants: How Mixtures of Pesticides at Low Concentrations Affect Aquatic Communities. Oecologia 2009, 159, 363–376. [Google Scholar] [CrossRef] [PubMed]
- Tu, C.; Wang, Y.; Duan, W.; Hertl, P.; Tradway, L.; Brandenburg, R.; Lee, D.; Snell, M.; Hu, S. Effects of Fungicides and Insecticides on Feeding Behavior and Community Dynamics of Earthworms: Implications for Casting Control in Turfgrass Systems. Appl. Soil Ecol. 2011, 47, 31–36. [Google Scholar] [CrossRef]
- Van Hoesel, W.; Tiefenbacher, A.; König, N.; Dorn, V.M.; Hagenguth, J.F.; Prah, U.; Widhalm, T.; Wiklicky, V.; Koller, R.; Bonkowski, M.; et al. Single and Combined Effects of Pesticide Seed Dressings and Herbicides on Earthworms, Soil Microorganisms, and Litter Decomposition. Front. Plant Sci. 2017, 8, 215. [Google Scholar] [CrossRef]


| Treatment a | SP 10−1 | SP 100 | SP 101 | SP 102 | SP 103 | P 10−1 | P 100 | P 101 | P 102 | P 103 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Commercial fungicide concentration in soil | (mg kg−1) b | 0.25 | 2.50 | 25.00 | 250.00 | 2500.00 | 0.17 | 1.67 | 16.70 | 167.00 | 1670.00 |
| (L ha−1) c | 0.15 | 1.50 | 15.00 | 150.00 | 1500.00 | 0.10 | 1.00 | 10.00 | 100.00 | 1000.00 | |
| Metconazole | (mg kg−1) d | 0.01 | 0.07 | 0.69 | 6.88 | 68.75 | |||||
| Epoxiconazole | 0.01 | 0.09 | 0.94 | 9.38 | 93.75 | ||||||
| Tebuconazole | 0.02 | 0.21 | 2.08 | 20.83 | 208.33 | ||||||
| Protioconazole | 0.02 | 0.21 | 2.08 | 20.83 | 208.33 |
| Treatments with E. fetida | E P 1 | E P 1.8 | E P 3.2 | E P 5.6 | E P 10 | |
|---|---|---|---|---|---|---|
| Treatments with G. rione | G P 1 | G P 1.8 | G P 3.2 | G P 5.6 | G P 10 | |
| Commercial fungicide concentration in soil | (mg kg−1) a | 164 | 295 | 524 | 916 | 1637 |
| (L ha−1) b | 100 | 180 | 320 | 560 | 1000 | |
| Tebuconazole | (mg kg−1) c | 20.83 | 37.50 | 66.67 | 116.67 | 208.33 |
| Protioconazole | 20.83 | 37.50 | 66.67 | 116.67 | 208.33 | |
| AIC | PROSARO® | SWING-PLUS® |
|---|---|---|
| Hormetic model | 275.82 | 272.91 |
| Gompertz model | 281.25 | 275.15 |
| Logistic model | 283.96 | 276.30 |
| PROSARO® mg fungicide kg Soil−1 | SWING-PLUS® mg fungicide kg Soil−1 | |
|---|---|---|
| IC10 | 133.3 (20.13–1776) | 1544 (153.9–>>9000) |
| IC20 | 233.3 (35.95–3269) | 2987 (327.8–>>9000) |
| Commercial Fungicide Concentration | Mean Biomass Variation in Surviving Earthworms a | |||
|---|---|---|---|---|
| (L ha−1) | (%+SD) | |||
| E.fetida | ||||
| 0 | −0.37 * | ± | 1.48 | A |
| 100 | −0.90 | ± | 1.55 | A |
| 180 | −1.32 | ± | 2.77 | A |
| 320 | −0.59 | ± | 1.08 | A |
| 560 | −0.33 | ± | 3.19 | AB |
| 1000 | 0.98 | ± | 2.83 | B |
| G.rione b | ||||
| 0 | −1.23 | ± | 3.57 | |
| 100 | 5.35 | ± | 2.98 | |
| 180 | 6.36 | ± | 11.24 | |
| 320 | 4.78 | ± | 15.47 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jorge-Escudero, G.; Pérez Polanco, M.; Lagerlöf, J.E.; Pérez, C.A.; Míguez, D. Commercial Fungicide Toxic Effects on Terrestrial Non-Target Species Might Be Underestimated When Based Solely on Active Ingredient Toxicity and Standard Earthworm Tests. Toxics 2022, 10, 488. https://doi.org/10.3390/toxics10090488
Jorge-Escudero G, Pérez Polanco M, Lagerlöf JE, Pérez CA, Míguez D. Commercial Fungicide Toxic Effects on Terrestrial Non-Target Species Might Be Underestimated When Based Solely on Active Ingredient Toxicity and Standard Earthworm Tests. Toxics. 2022; 10(9):488. https://doi.org/10.3390/toxics10090488
Chicago/Turabian StyleJorge-Escudero, Gabriella, Mariana Pérez Polanco, Jan Erland Lagerlöf, Carlos Alberto Pérez, and Diana Míguez. 2022. "Commercial Fungicide Toxic Effects on Terrestrial Non-Target Species Might Be Underestimated When Based Solely on Active Ingredient Toxicity and Standard Earthworm Tests" Toxics 10, no. 9: 488. https://doi.org/10.3390/toxics10090488
APA StyleJorge-Escudero, G., Pérez Polanco, M., Lagerlöf, J. E., Pérez, C. A., & Míguez, D. (2022). Commercial Fungicide Toxic Effects on Terrestrial Non-Target Species Might Be Underestimated When Based Solely on Active Ingredient Toxicity and Standard Earthworm Tests. Toxics, 10(9), 488. https://doi.org/10.3390/toxics10090488