A Novel Chemiluminescent Method for Efficient Evaluation of Heterogeneous Fenton Catalysts Using Cigarette Tar
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Preparation of Cigarette-Tar-Methanol Extracts (CTME)
2.3. Synthesis of Catalysts
2.4. CL Measurements
2.5. Degradation of Rhodamine B
3. Results
3.1. CL Property of CTME
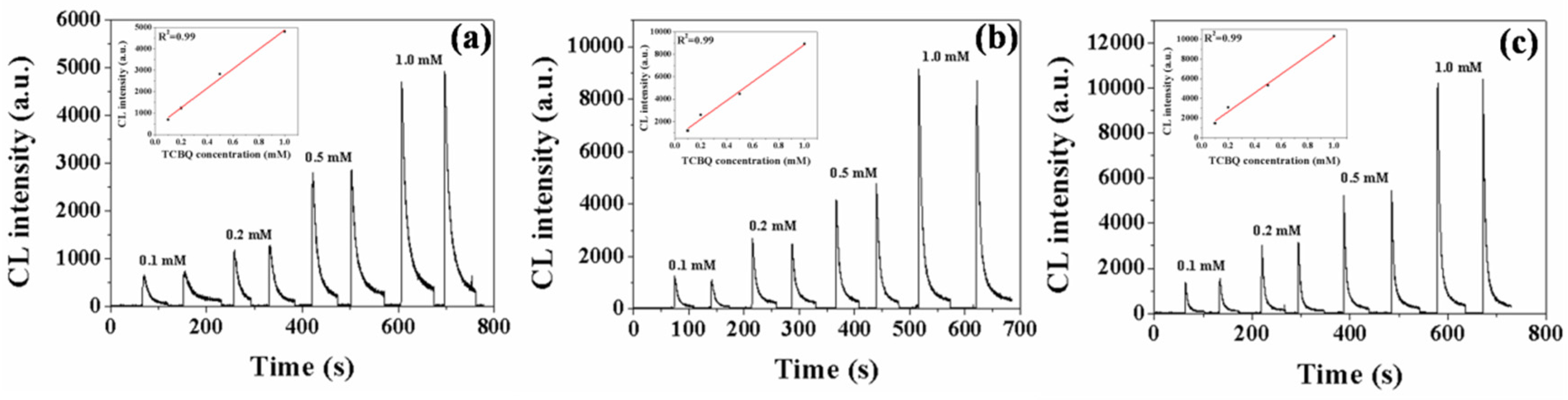
3.2. •OH Detection
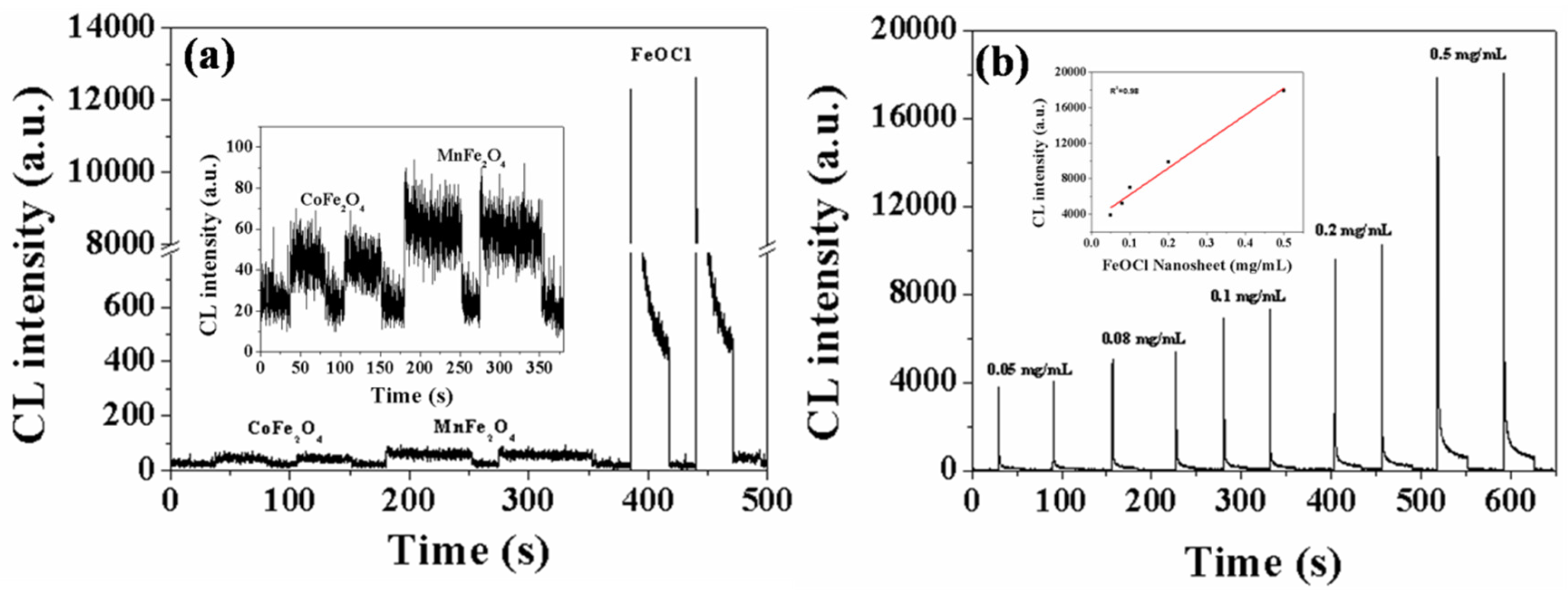
3.3. Evaluation of the Catalytic Capacity of Catalysts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, Y.; Zhu, R.; Xi, Y.; Zhu, J.; Zhu, G.; He, H. Strategies for enhancing the heterogeneous Fenton catalytic reactivity: A review. Appl. Catal. B 2019, 255, 117739. [Google Scholar] [CrossRef]
- Lu, C.; Deng, K.; Hu, C.; Lyu, L. Dual-reaction-center catalytic process continues Fenton’s story. Front. Environ. Sci. Eng. 2020, 14, 82. [Google Scholar] [CrossRef]
- Vorontsov, A.V. Advancing Fenton and photo-Fenton water treatment through the catalyst design. J. Hazard. Mater. 2019, 372, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Gao, Y.; Wu, R.; Gao, J. Research progress of degradation of organic pollutants in wastewater by heterogeneous Fenton reaction. Appl. Chem. Ind. 2019, 48, 717–720. [Google Scholar]
- Bokare, A.D.; Choi, W. Review of iron-free Fenton-like systems for activating H2O2 in advanced oxidation processes. J. Hazard. Mater. 2014, 275, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Lyu, L.; Hu, C. Heterogeneous Fenton catalytic water treatment technology and mechanism. Prog. Chem. 2017, 29, 981–999. [Google Scholar]
- Mao, J.; Quan, X.; Wang, J.; Gao, C.; Chen, S.; Yu, H.; Zhang, Y. Enhanced heterogeneous Fenton-like activity by Cu-doped BiFeO3 perovskite for degradation of organic pollutants. Front. Environ. Sci. Eng. 2018, 12, 10. [Google Scholar] [CrossRef]
- Wei, S.; Zeng, C.; Lu, Y.; Liu, G.; Luo, H.; Zhang, R. Degradation of antipyrine in the Fenton-like process with a La-doped heterogeneous catalyst. Front. Environ. Sci. Eng. 2019, 13, 66. [Google Scholar] [CrossRef]
- Plakas, K.V.; Mantza, A.; Sklari, S.D.; Zaspalis, V.T.; Karabelas, A.J. Heterogeneous Fenton-like oxidation of pharmaceutical diclofenac by a catalytic iron-oxide ceramic microfiltration membrane. Chem. Eng. J. 2019, 373, 700–708. [Google Scholar] [CrossRef]
- Huang, X.; Chen, Y.; Walter, E.; Zong, M.; Wang, Y.; Zhang, X.; Qafoku, O.; Wang, Z.; Rosso, K.M. Facet-specific photocatalytic degradation of organics by heterogeneous Fenton chemistry on hematite nanoparticles. Environ. Sci. Technol. 2019, 53, 10197–10207. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, K.; Zhou, Y.; Sun, D.; Zhang, C.; Zhao, W.; Bai, J. Enhanced degradation effect of nano-PAA-CuCl2 with controllable 3D structure as heterogeneous Fenton-like catalyst over a wide pH range. J. Mater. Sci. 2019, 54, 7850–7866. [Google Scholar] [CrossRef]
- Chen, A.; Potschke, P.; Pionteck, J.; Voit, B.; Qi, H. Fe3O4 nanoparticles grown on cellulose/GO hydrogels as advanced catalytic materials for the heterogeneous Fenton-like reaction. ACS Omega 2019, 4, 5117–5125. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, C.; Qi, J.; Li, J.; Sun, X.; Shen, J.; Han, W.; Wang, L. Enhanced heterogeneous Fenton-like systems based on highly dispersed Fe0-Fe2O3 nanoparticles embedded ordered mesoporous carbon composite catalyst. Environ. Pollut. 2018, 243, 1068–1077. [Google Scholar] [CrossRef] [PubMed]
- Lyu, L.; Yan, D.; Yu, G.; Cao, W.; Hu, C. Efficient destruction of pollutants in water by a dual-reaction-center Fenton-like process over carbon nitride compounds-complexed Cu(II)-CuAlO2. Environ. Sci. Technol. 2018, 52, 4294–4304. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yan, D.; Lyu, L.; Hu, C.; Jiang, N.; Zhang, L. Notable light-free catalytic activity for pollutant destruction over flower-like BiOI microspheres by a dual-reaction-center Fenton-like Process. J. Colloid Interface Sci. 2018, 527, 251–259. [Google Scholar] [CrossRef]
- Fernandezcastro, P.; Vallejo, M.; Roman, M.F.; Ortiz, I. Insight on the fundamentals of advanced oxidation processes. Role and review of the determination methods of reactive oxygen species. J. Chem. Technol. Biotechnol. 2015, 90, 796–820. [Google Scholar] [CrossRef]
- Backa, S.; Jansbo, K.; Reitberger, T. Detection of hydroxyl radicals by a chemiluminescence method—A critical review. Holzforschung 1997, 51, 557–564. [Google Scholar] [CrossRef]
- Rahmani, H.; Ghavamipour, F.; Sajedi, R.H. Bioluminescence detection of superoxide anion using aequorin. Anal. Chem. 2019, 91, 12768–12774. [Google Scholar] [CrossRef]
- Sun, M.; Su, Y.; Yang, W.; Zhang, L.; Hu, J.; Lv, Y. Organosiloxane and polyhedral oligomeric silsesquioxanes compounds as chemiluminescent molecular probes for direct monitoring hydroxyl radicals. Anal. Chem. 2019, 91, 8926–8932. [Google Scholar] [CrossRef]
- Wardman, P. Fluorescent and luminescent probes for measurement of oxidative and nitrosative species in cells and tissues: Progress, pitfalls, and prospects. Free Radic. Biol. Med. 2007, 43, 995–1022. [Google Scholar] [CrossRef]
- Lu, C.; Song, G.; Lin, J.-M. Reactive oxygen species and their chemiluminescence-detection methods. TrAC Trends Anal. Chem. 2006, 25, 985–995. [Google Scholar] [CrossRef]
- Yu, W.; Zhao, L. Chemiluminescence detection of reactive oxygen species generation and potential environmental applications. TrAC Trends Anal. Chem. 2021, 136, 116197. [Google Scholar] [CrossRef]
- Wang, D.; Zhao, L.; Guo, L.-H.; Zhang, H. Online detection of reactive oxygen species in ultraviolet (UV)-irradiated nano-TiO2 suspensions by continuous flow chemiluminescence. Anal. Chem. 2014, 86, 10535–10539. [Google Scholar] [CrossRef] [PubMed]
- Church, D.F.; Pryor, W.A. Free-radical chemistry of cigarette smoke and its toxicological implications. Environ. Health Perspect. 1985, 64, 111–126. [Google Scholar] [CrossRef]
- Valavanidis, A.; Vlachogianni, T.; Fiotakis, K. Tobacco smoke: Involvement of reactive oxygen species and stable free radicals in mechanisms of oxidative damage, carcinogenesis and synergistic effects with other respirable particles. Int. J. Environ. Res. Public Health 2015, 6, 445–462. [Google Scholar] [CrossRef]
- Wang, D.; Yu, W.; Pang, X.; Cao, J.; Qiu, J.; Kong, F. A new method for hydroxyl radical detection by chemiluminescence of flue-cured tobacco extracts. Spectrochim. Acta Part A 2018, 204, 436–439. [Google Scholar] [CrossRef]
- Guo, S.; Li, D.; Zhang, L.; Li, J.; Wang, E. Monodisperse mesoporous superparamagnetic single-crystal magnetite nanoparticles for drug delivery. Biomaterials 2009, 30, 1881–1889. [Google Scholar] [CrossRef]
- Sun, M.; Chu, C.; Geng, F.; Lu, X.; Qu, J.; Crittenden, J.; Elimelech, M.; Kim, J.-H. Reinventing Fenton chemistry: Iron oxychloride nanosheet for pH-insensitive H2O2 activation. Environ. Sci. Technol. Lett. 2018, 5, 186–191. [Google Scholar] [CrossRef]
- Zhu, B.-Z.; Mao, L.; Huang, C.-H.; Qin, H.; Fan, R.-M.; Kalyanaraman, B.; Zhu, J.-G. Unprecedented hydroxyl radical-dependent two-step chemiluminescence production by polyhalogenated quinoid carcinogens and H2O2. Proc. Natl. Acad. Sci. USA 2012, 109, 16046–16051. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Yu, W.; Jiang, B.; Zeng, T.; Song, D.; Fang, S.; Zhang, Y.; Zhang, J. A Novel Chemiluminescent Method for Efficient Evaluation of Heterogeneous Fenton Catalysts Using Cigarette Tar. Toxics 2023, 11, 30. https://doi.org/10.3390/toxics11010030
Wang D, Yu W, Jiang B, Zeng T, Song D, Fang S, Zhang Y, Zhang J. A Novel Chemiluminescent Method for Efficient Evaluation of Heterogeneous Fenton Catalysts Using Cigarette Tar. Toxics. 2023; 11(1):30. https://doi.org/10.3390/toxics11010030
Chicago/Turabian StyleWang, Dabin, Weisong Yu, Bin Jiang, Tao Zeng, Dean Song, Song Fang, Yizhi Zhang, and Jiguang Zhang. 2023. "A Novel Chemiluminescent Method for Efficient Evaluation of Heterogeneous Fenton Catalysts Using Cigarette Tar" Toxics 11, no. 1: 30. https://doi.org/10.3390/toxics11010030
APA StyleWang, D., Yu, W., Jiang, B., Zeng, T., Song, D., Fang, S., Zhang, Y., & Zhang, J. (2023). A Novel Chemiluminescent Method for Efficient Evaluation of Heterogeneous Fenton Catalysts Using Cigarette Tar. Toxics, 11(1), 30. https://doi.org/10.3390/toxics11010030








