The Influence of Photodynamic Antimicrobial Chemotherapy on the Microbiome, Neuroendocrine and Immune System of Crustacean Post Larvae
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Curcumin and the Rearing of Artemia nauplii and P. monodon
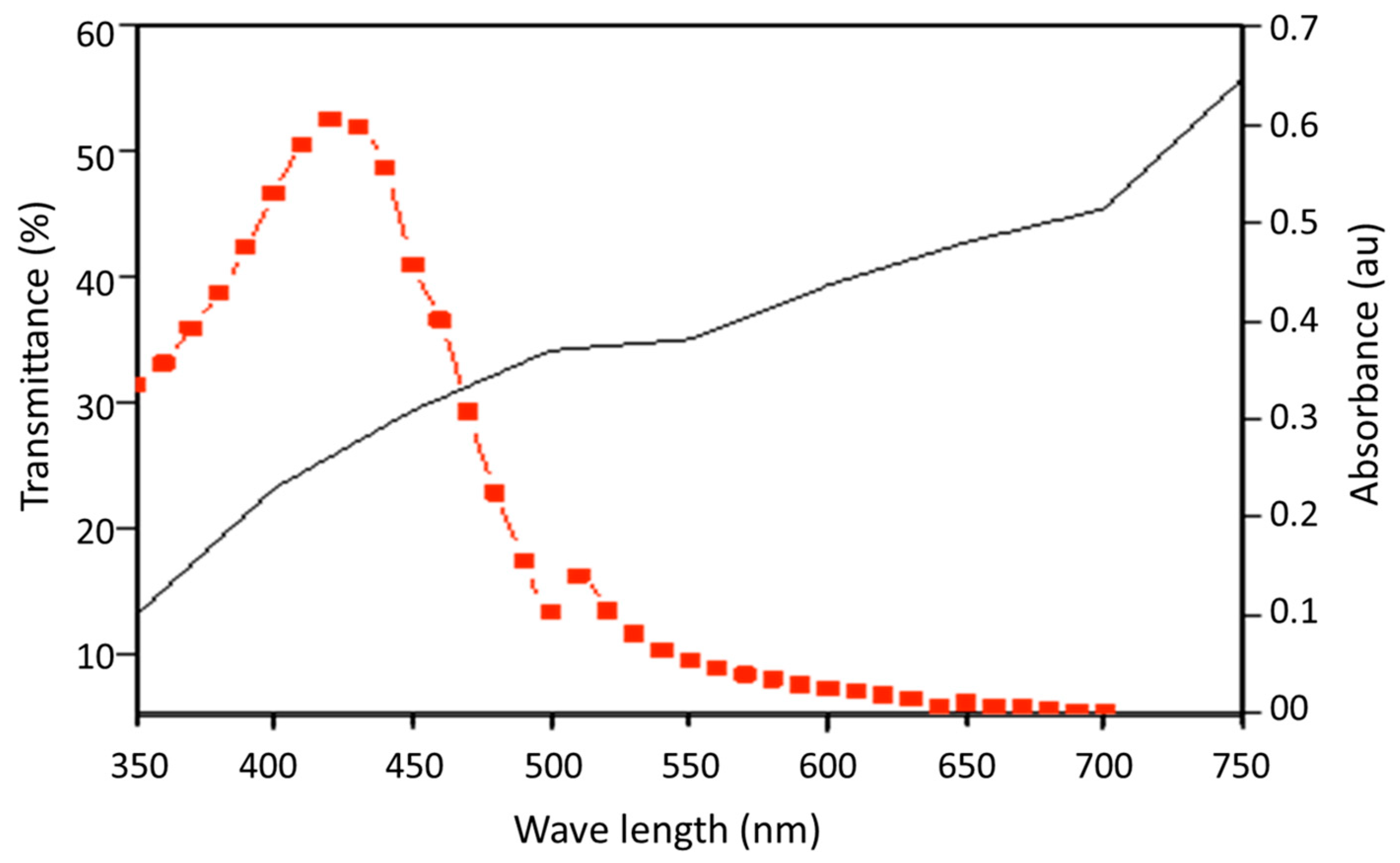
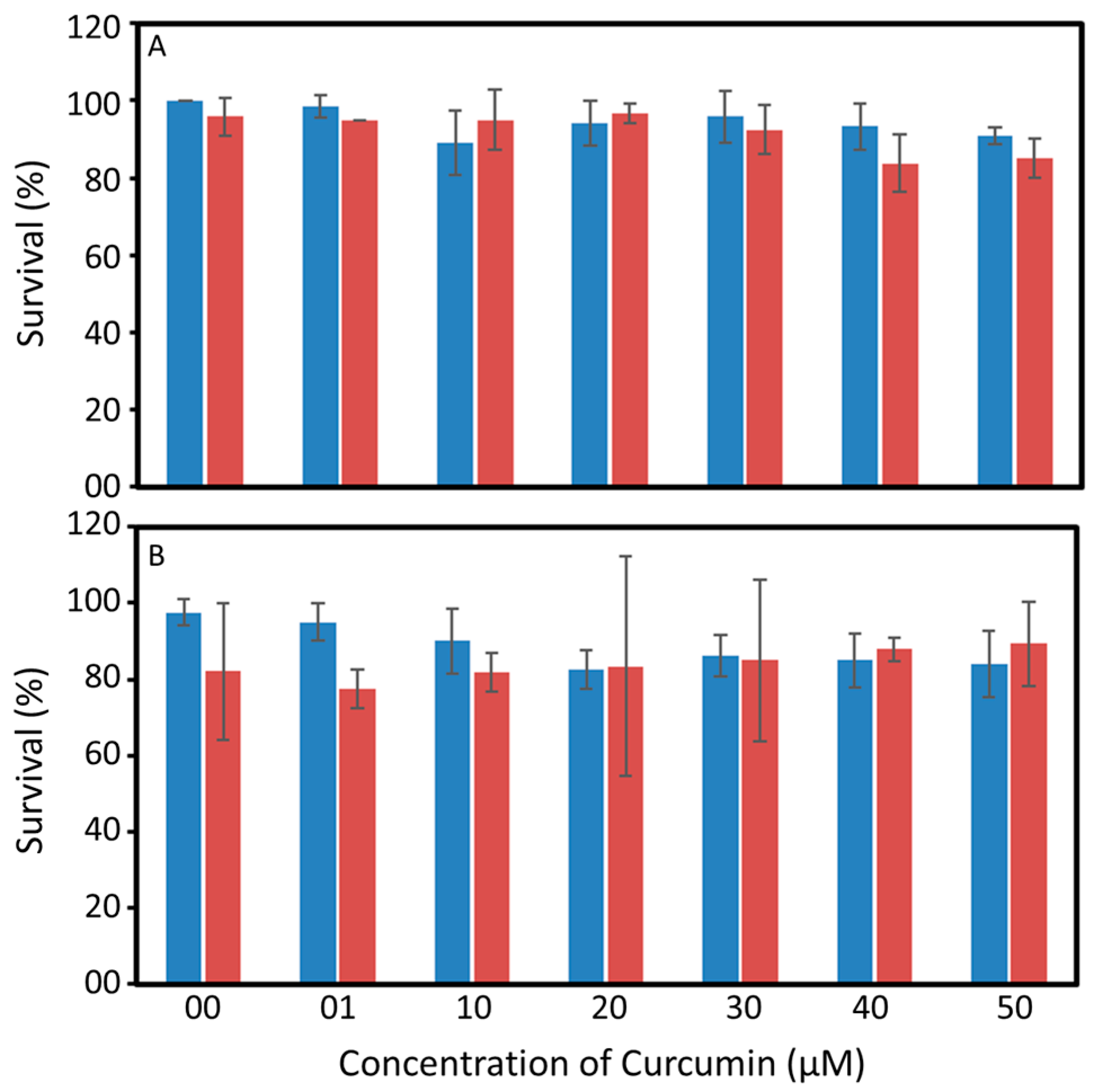
2.2. Spectral Properties and Toxicity of Curcumin
2.3. Abundance of Vibrio spp. in P. monodon Exposed to Photoexcited Curcumin
2.4. Microbiome of P. monodon Exposed to Photoexcited Curcumin
2.5. Neuroendocrine (MIH, CHH) and Immune (Crustin, PoPO) Gene Expression
2.6. Statistical Analysis
3. Results
3.1. Spectral Properties and Toxicity of Curcumin
3.2. Abundance of Vibrio spp. and Microbiome of Shrimp Larvae Exposed to Photoexcited Curcumin
3.3. Neuroendocrine and Immune Gene Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ghosh, C.; Sarkar, P.; Issa, R.; Haldar, J. Alternatives to conventional antibiotics in the era of antimicrobial resistance. Trends Microbiol. 2019, 27, 323–338. [Google Scholar] [CrossRef] [PubMed]
- Sakari, M.; Laisi, A.; Pulliainen, A.T. Exotoxin-targeted drug modalities as antibiotic alternatives. ACS Infect. Dis. 2022, 8, 433–456. [Google Scholar] [CrossRef] [PubMed]
- Anas, A.; Sukumaran, V.; Nampullipurackal Devarajan, D.; Maniyath, S.; Chekidhenkuzhiyil, J.; Mary, A.; Parakkaparambil Kuttan, S.; Tharakan, B. Probiotics inspired from natural ecosystem to inhibit the growth of Vibrio spp. causing white gut syndrome in Litopenaeus vannamei. 3 Biotech 2021, 11, 66. [Google Scholar] [CrossRef] [PubMed]
- Anas, A.; Sobhanan, J.; Sulfiya, K.M.; Jasmin, C.; Sreelakshmi, P.K.; Biju, V. Advances in photodynamic antimicrobial chemotherapy. J. Photochem. Photobiol. C Photochem. Rev. 2021, 49, 100452. [Google Scholar] [CrossRef]
- Cui, J.; Zhou, Q.; Yu, M.; Liu, Y.; Teng, X.; Gu, X. 4-tert-butylphenol triggers common carp hepatocytes ferroptosis via oxidative stress, iron overload, SLC7A11/GSH/GPX4 axis, and ATF4/HSPA5/GPX4 axis. Ecotoxicol. Environ. Saf. 2022, 242, 113944. [Google Scholar] [CrossRef]
- Xu, T.; Liu, Q.; Chen, D.; Liu, Y. Atrazine exposure induces necroptosis through the P450/ROS pathway and causes inflammation in the gill of common carp (Cyprinus carpio L.). Fish Shellfish Immunol. 2022, 131, 809–816. [Google Scholar] [CrossRef]
- Kirszberg, C.; Rumjanek, V.M.; Capella, M.A.M. Methylene blue is more toxic to erythroleukemic cells than to normal peripheral blood mononuclear cells: A possible use in chemotherapy. Cancer Chemother. Pharmacol. 2005, 56, 659–665. [Google Scholar] [CrossRef]
- Tian, J.; Huang, B.; Nawaz, M.H.; Zhang, W. Recent advances of multi-dimensional porphyrin-based functional materials in photodynamic therapy. Coord. Chem. Rev. 2020, 420, 213410. [Google Scholar] [CrossRef]
- Dou, F.; Huang, K.; Nitin, N. Targeted Photodynamic Treatment of Bacterial Biofilms Using Curcumin Encapsulated in Cells and Cell Wall Particles. ACS Appl. Bio Mater. 2021, 4, 514–522. [Google Scholar] [CrossRef]
- Ma, W.; Liu, C.; Li, J.; Hao, M.; Ji, Y.; Zeng, X. The effects of aloe emodin-mediated antimicrobial photodynamic therapy on drug-sensitive and resistant Candida albicans. Photochem. Photobiol. Sci. 2020, 19, 485–494. [Google Scholar] [CrossRef]
- Zaki Ahmad, M.; Akhter, S.; Mohsin, N.; A Abdel-Wahab, B.; Ahmad, J.; Husain Warsi, M.; Rahman, M.; Mallick, N.; Jalees Ahmad, F. Transformation of curcumin from food additive to multifunctional medicine: Nanotechnology bridging the gap. Curr. Drug Discov. Technol. 2014, 11, 197–213. [Google Scholar] [CrossRef] [PubMed]
- Dias, L.D.; Blanco, K.C.; Mfouo-Tynga, I.S.; Inada, N.M.; Bagnato, V.S. Curcumin as a photosensitizer: From molecular structure to recent advances in antimicrobial photodynamic therapy. J. Photochem. Photobiol. C Photochem. Rev. 2020, 45, 100384. [Google Scholar] [CrossRef]
- Moideen, S.K.; Anas, A.; Sobhanan, J.; Zhao, H.; Biju, V. Photoeradication of aquatic pathogens by curcumin for clean and safe drinking water. J. Photochem. Photobiol. A Chem. 2022, 432, 114104. [Google Scholar] [CrossRef]
- Unicef. Over 300,000 Choldren under Five Died from Diarrhoeal Diseases Linked to Limited access to Safe Water, Sanitation and Hygiene in 2015; Mekki, N., Tidey, C., Eds.; Unicef: New York, NY, USA, 2016; Available online: https://www.unicef.org/media/media_92918.html (accessed on 25 December 2022).
- Sathyendranath, S.; Anas, A.; Menon, N.; George, G.; Evers-King, H.; Kulk, G.; Colwell, R.; Jutla, A.; Platt, T. Building capacity and resilience against disease transmitted via water under climate perturbations and extreme weather stress. In Space Capacity Building in the XXI Century; Studies in Space Policy; Ferretti, S., Ed.; Springer: Cham, Switzerland, 2020; Volume 22. [Google Scholar]
- Patil, P.K.; Geetha, R.; Ravisankar, T.; Avunje, S.; Solanki, H.G.; Abraham, T.J.; Vinoth, S.P.; Jithendran, K.P.; Alavandi, S.V.; Vijayan, K.K. Economic loss due to diseases in Indian shrimp farming with special reference to Enterocytozoon hepatopenaei (EHP) and white spot syndrome virus (WSSV). Aquaculture 2021, 533, 736231. [Google Scholar] [CrossRef]
- Wu, J.; Mou, H.; Xue, C.; Leung, A.W.; Xu, C.; Tang, Q.-J. Photodynamic effect of curcumin on Vibrio parahaemolyticus. Photodiagnosis Photodyn. Ther. 2016, 15, 34–39. [Google Scholar] [CrossRef]
- Andrade, G.C.; Brancini, G.T.P.; Abe, F.R.; de Oliveira, D.P.; Nicolella, H.D.; Tavares, D.C.; Micas, A.F.D.; Savazzi, E.A.; Silva-Junior, G.J.; Wainwright, M.; et al. Phenothiazinium dyes for photodynamic treatment present lower environmental risk compared to a formulation of trifloxystrobin and tebuconazole. J. Photochem. Photobiol. B Biol. 2022, 226, 112365. [Google Scholar] [CrossRef] [PubMed]
- Fischer, B.B.; Krieger-Liszkay, A.; Eggen, R.I.L. Photosensitizers Neutral Red (Type I) and Rose Bengal (Type II) Cause Light-Dependent Toxicity in Chlamydomonas reinhardtii and Induce the Gpxh Gene via Increased Singlet Oxygen Formation. Environ. Sci. Technol. 2004, 38, 6307–6313. [Google Scholar] [CrossRef]
- Pelletier, É.; Sargian, P.; Payet, J.; Demers, S. Ecotoxicological Effects of Combined UVB and Organic Contaminants in Coastal Waters: A Review. Photochem. Photobiol. 2006, 82, 981–993. [Google Scholar] [CrossRef]
- Luo, Z.; Li, Z.; Xie, Z.; Sokolova, I.M.; Song, L.; Peijnenburg, W.J.G.M.; Hu, M.; Wang, Y. Rethinking Nano-TiO2 Safety: Overview of Toxic Effects in Humans and Aquatic Animals. Small 2020, 16, 2002019. [Google Scholar] [CrossRef]
- Blasco, J.; Corsi, I. Ecotoxicology of Nanoparticles in Aquatic Systems; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Sorgeloos, P.; Bossuyt, E.; Laviña, E.; Baeza-Mesa, M.; Persoone, G. Decapsulation of Artemia cysts: A simple technique for the improvement of the use of brine shrimp in aquaculture. Aquaculture 1977, 12, 311–315. [Google Scholar] [CrossRef]
- Asok, A.; Arshad, E.; Jasmin, C.; Somnath Pai, S.; Bright Singh, I.S.; Mohandas, A.; Anas, A. Reducing Vibrio load in Artemia nauplii using antimicrobial photodynamic therapy: A promising strategy to reduce antibiotic application in shrimp larviculture. Microb. Biotechnol. 2012, 5, 59–68. [Google Scholar] [CrossRef] [Green Version]
- Cook, H.L. A Method of Rearing Penaeid Shrimp Larvae for Experimental Studies; FAO Fisheries Report; FAO: Rome, Italy, 1969; Volume 57, pp. 709–715. [Google Scholar]
- Racault, M.-F.; Menon, A.A.; Jasmin, C.; Minu, P.; McConville, K.; Loveday, B.; Platt, T.; Sathyendranath, S.; Vijayan, V.; George, G. Environmental reservoirs of Vibrio cholerae: Challenges and opportunities for Ocean-color remote sensing. Remote Sens. 2019, 11, 2763. [Google Scholar] [CrossRef] [Green Version]
- De Coster, W.; D’hert, S.; Schultz, D.T.; Cruts, M.; Van Broeckhoven, C. NanoPack: Visualizing and processing long-read sequencing data. Bioinformatics 2018, 34, 2666–2669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wood, D.E.; Lu, J.; Langmead, B. Improved metagenomic analysis with Kraken 2. Genome Biol. 2019, 20, 257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H. Minimap2: Pairwise alignment for nucleotide sequences. Bioinformatics 2018, 34, 3094–3100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Vrinda, S.; Abdulaziz, A.; Abhilash, K.S.; Jasmin, C.; Kripa, V.; Bright Singh, I.S. Neuroendocrine and immunotoxicity of polyaromatic hydrocarbon, chrysene in crustacean post larvae. Ecotoxicology 2019, 28, 964–972. [Google Scholar] [CrossRef]
- Dechamma, M.M.; Rajeish, M.; Maiti, B.; Mani, M.K.; Karunasagar, I. Expression of Toll-like receptors (TLR), in lymphoid organ of black tiger shrimp (Penaeus monodon) in response to Vibrio harveyi infection. Aquac. Rep. 2015, 1, 1–4. [Google Scholar] [CrossRef]
- Arshad, E.; Anas, A.; Asok, A.; Jasmin, C.; Pai, S.S.; Singh, I.B.; Mohandas, A.; Biju, V. Fluorescence detection of the pathogenic bacteria Vibrio harveyi in solution and animal cells using semiconductor quantum dots. RSC Adv. 2016, 6, 15686–15693. [Google Scholar] [CrossRef]
- Penha, C.B.; Bonin, E.; Silva, A.F.d.; Hioka, N.; Zanqueta, E.B.; Nakamura, T.U.; Filho, B.A.d.A.; Campanerut-Sa, P.A.Z.; Mikcha, J.M.G. Photodynamic inactivation of foodborne and food spoilage bacteria by curcumin. LWT-Food Sci. Technol. 2017, 76, 198–202. [Google Scholar] [CrossRef]
- Vahedian-Azimi, A.; Abbasifard, M.; Rahimi-Bashar, F.; Guest, P.C.; Majeed, M.; Mohammadi, A.; Banach, M.; Jamialahmadi, T.; Sahebkar, A. Effectiveness of Curcumin on Outcomes of Hospitalized COVID-19 Patients: A Systematic Review of Clinical Trials. Nutrients 2022, 14, 256. [Google Scholar] [CrossRef] [PubMed]
- Chignell, C.F.; Bilskj, P.; Reszka, K.J.; Motten, A.G.; Sik, R.H.; Dahl, T.A. Spectral and photochemical properties of curcumin. Photochem. Photobiol. 1994, 59, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.; Gordon, O.N.; Edwards, R.L.; Luis, P.B. Degradation of Curcumin: From Mechanism to Biological Implications. J. Agric. Food Chem. 2015, 63, 7606–7614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santezi, C.; Reina, B.D.; Dovigo, L.N. Curcumin-mediated Photodynamic Therapy for the treatment of oral infections—A review. Photodiagnosis Photodyn. Ther. 2018, 21, 409–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Najafi, S.; Khayamzadeh, M.; Paknejad, M.; Poursepanj, G.; Kharazi Fard, M.J.; Bahador, A. An in vitro comparison of antimicrobial effects of curcumin-based photodynamic therapy and chlorhexidine, on aggregatibacter actinomycetemcomitans. J. Lasers Med. Sci. 2016, 7, 5. [Google Scholar] [CrossRef] [Green Version]
- Chen, B.; Huang, J.; Li, H.; Zeng, Q.-H.; Wang, J.J.; Liu, H.; Pan, Y.; Zhao, Y. Eradication of planktonic Vibrio parahaemolyticus and its sessile biofilm by curcumin-mediated photodynamic inactivation. Food Control 2020, 113, 107181. [Google Scholar] [CrossRef]
- dos Santos, R.F.; Campos, B.S.; Rego Filho, F.d.A.M.G.; Moraes, J.d.O.; Albuquerque, A.L.I.; da Silva, M.C.D.; dos Santos, P.V.; de Araujo, M.T. Photodynamic inactivation of S. aureus with a water-soluble curcumin salt and an application to cheese decontamination. Photochem. Photobiol. Sci. 2019, 18, 2707–2716. [Google Scholar] [CrossRef]
- Zheng, Z.; Aweya, J.J.; Bao, S.; Yao, D.; Li, S.; Tran, N.T.; Ma, H.; Zhang, Y. The Microbial Composition of Penaeid Shrimps’ Hepatopancreas Is Modulated by Hemocyanin. J. Immunol. 2021, 207, 2733–2743. [Google Scholar] [CrossRef]
- Duan, Y.; Wang, Y.; Huang, J.; Li, H.; Dong, H.; Zhang, J. Toxic effects of cadmium and lead exposure on intestinal histology, oxidative stress response, and microbial community of Pacific white shrimp Litopenaeus vannamei. Mar. Pollut. Bull. 2021, 167, 112220. [Google Scholar] [CrossRef] [PubMed]
- Wood, S.; Metcalf, D.; Devine, D.; Robinson, C. Erythrosine is a potential photosensitizer for the photodynamic therapy of oral plaque biofilms. J. Antimicrob. Chemother. 2006, 57, 680–684. [Google Scholar] [CrossRef]
- Anas, A.; Akita, H.; Harashima, H.; Itoh, T.; Ishikawa, M.; Biju, V. Photosensitized breakage and damage of DNA by CdSe− ZnS quantum dots. J. Phys. Chem. B 2008, 112, 10005–10011. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Lei, W.; Jiang, G.; Hou, Y.; Zhang, B.; Zhou, Q.; Wang, X. Selective photodynamic inactivation of bacterial cells over mammalian cells by new triarylmethanes. Langmuir 2014, 30, 14573–14580. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; He, H.; Luo, Z.; Zhou, H.; Liang, R.; Pan, H.; Ma, Y.; Cai, L. In Situ Photocatalyzed Oxygen Generation with Photosynthetic Bacteria to Enable Robust Immunogenic Photodynamic Therapy in Triple-Negative Breast Cancer. Adv. Funct. Mater. 2020, 30, 1910176. [Google Scholar] [CrossRef]
- Xu, D.; Zhou, C.; Zhan, C.; Wang, Y.; You, Y.; Pan, X.; Jiao, J.; Zhang, R.; Dong, Z.; Wang, W. Enzymatic Micromotors as a Mobile Photosensitizer Platform for Highly Efficient On-Chip Targeted Antibacteria Photodynamic Therapy. Adv. Funct. Mater. 2019, 29, 1807727. [Google Scholar] [CrossRef]
- Lin, T.; Chen, W.; Cai, B. The use of chlorine dioxide for the inactivation of copepod zooplankton in drinking water treatment. Environ. Technol. 2014, 35, 2846–2851. [Google Scholar] [CrossRef]
- Jiang, W.; Dong, S.; Xu, F.; Chen, J.; Gong, C.; Wang, A.; Hu, Z. Mechanisms of thermal treatment on two dominant copepod species in O3/BAC processing of drinking water. Ecotoxicology 2021, 30, 945–953. [Google Scholar] [CrossRef]
- Fu, Z.; Han, F.; Huang, K.; Zhang, J.; Qin, J.G.; Chen, L.; Li, E. Impact of imidacloprid exposure on the biochemical responses, transcriptome, gut microbiota and growth performance of the Pacific white shrimp Litopenaeus vannamei. J. Hazard. Mater. 2022, 424, 127513. [Google Scholar] [CrossRef]
- Chae, Y.; Kim, D.; Choi, M.-J.; Cho, Y.; An, Y.-J. Impact of nano-sized plastic on the nutritional value and gut microbiota of whiteleg shrimp Litopenaeus vannamei via dietary exposure. Environ. Int. 2019, 130, 104848. [Google Scholar] [CrossRef]
- Qian, D.; Xu, C.; Chen, C.; Qin, J.G.; Chen, L.; Li, E. Toxic effect of chronic waterborne copper exposure on growth, immunity, anti-oxidative capacity and gut microbiota of Pacific white shrimp Litopenaeus vannamei. Fish Shellfish Immunol. 2020, 100, 445–455. [Google Scholar] [CrossRef]
- Chang, E.S. Comparative endocrinology of molting and reproduction: Insects and crustaceans. Annu. Rev. Entomol. 1993, 38, 161–180. [Google Scholar] [CrossRef]
- Qiao, H.; Jiang, F.; Xiong, Y.; Jiang, S.; Fu, H.; Li, F.; Zhang, W.; Sun, S.; Jin, S.; Gong, Y.; et al. Characterization, expression patterns of molt-inhibiting hormone gene of Macrobrachium nipponense and its roles in molting and growth. PLoS ONE 2018, 13, e0198861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.; Migaud, H.; Shi, C.; Song, C.; Wang, C.; Ye, Y.; Ren, Z.; Wang, H.; Mu, C. Light intensity impacts on growth, molting and oxidative stress of juvenile mud crab Scylla paramamosain. Aquaculture 2021, 545, 737159. [Google Scholar] [CrossRef]
- Xu, L.; Pan, L.; Zhang, X.; Wei, C. Effects of crustacean hyperglycemic hormone (CHH) on regulation of hemocyte intracellular signaling pathways and phagocytosis in white shrimp Litopenaeus vannamei. Fish Shellfish Immunol. 2019, 93, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chen, H.; Xu, J.; Xu, Q.; Wang, M.; Zhao, D.; Wang, L.; Song, L. Crustacean hyperglycemic hormones directly modulate the immune response of hemocytes in shrimp Litopenaeus vannamei. Fish Shellfish Immunol. 2017, 62, 164–174. [Google Scholar] [CrossRef] [Green Version]
- Lorenzon, S.; Edomi, P.; Giulianini, P.G.; Mettulio, R.; Ferrero, E.A. Variation of crustacean hyperglycemic hormone (cHH) level in the eyestalk and haemolymph of the shrimp Palaemon elegans following stress. J. Exp. Biol. 2004, 207, 4205–4213. [Google Scholar] [CrossRef] [Green Version]
- Ren, X.; Wang, Q.; Shao, H.; Xu, Y.; Liu, P.; Li, J. Effects of Low Temperature on Shrimp and Crab Physiology, Behavior, and Growth: A Review. Front. Mar. Sci. 2021, 8, 746177. [Google Scholar] [CrossRef]
- Bhoopathy, S.; Inbakandan, D.; Rajendran, T.; Chandrasekaran, K.; Prabha, S.B.; Reddy, B.A.; Kasilingam, R.; RameshKumar, V.; Dharani, G. Dietary supplementation of curcumin-loaded chitosan nanoparticles stimulates immune response in the white leg shrimp Litopenaeus vannamei challenged with Vibrio harveyi. Fish Shellfish Immunol. 2021, 117, 188–191. [Google Scholar] [CrossRef]
- Li, M.; Kong, Y.; Wu, X.; Guo, G.; Sun, L.; Lai, Y.; Zhang, J.; Niu, X.; Wang, G. Effects of dietary curcumin on growth performance, lipopolysaccharide-induced immune responses, oxidative stress and cell apoptosis in snakehead fish (Channa argus). Aquac. Rep. 2022, 22, 100981. [Google Scholar] [CrossRef]
- Ming, J.; Ye, J.; Zhang, Y.; Xu, Q.; Yang, X.; Shao, X.; Qiang, J.; Xu, P. Optimal dietary curcumin improved growth performance, and modulated innate immunity, antioxidant capacity and related genes expression of NF-κB and Nrf2 signaling pathways in grass carp (Ctenopharyngodon idella) after infection with Aeromonas hydrophila. Fish Shellfish Immunol. 2020, 97, 540–553. [Google Scholar] [CrossRef]



| Sl No. | Gene | Primer Sequence (5′-3′) | Tm (°C) | Amplicon Size (bp) | Reference |
|---|---|---|---|---|---|
| 1. | MIH | F- TAGTGCGTGTGTGTGAGGAT R- CCTGTTGGCAGCCTTTAGAC | 56 | 119 | [31] |
| 2. | CHH | F- GCCGAATGCAGGAGTAACTG R- TTGCCGAGCCTCTGTAGG | 56 | 113 | [31] |
| 3. | Crustin | F- AGTTCCTGGAGTTGGAGGTGGATT R- ACCTCGTTCTGCAGTAATTGCACTC | 56 | 119 | [32] |
| 4 | ProPO | F- CGGTGACAAAGTTCCTCTTC R- GCAGGTCGCCGTAGTAAG | 56 | 122 | [32] |
| Without Photoexcitation | With Photoexcitation | |||||||
|---|---|---|---|---|---|---|---|---|
| Curcumin (µM) | 0 | 10 | 30 | 50 | 0 | 10 | 30 | 50 |
| Total read of nanopore sequencing | 671,518 | 119,439 | 145,340 | 158,835 | 366,319 | 330,755 | 178,973 | 169,091 |
| Reads filtered (>300 bp) | 439,981 | 84,764 | 98,935 | 95,529 | 249,984 | 222,984 | 139,451 | 131,852 |
| Diversity indices | ||||||||
| Chao 1 | 174,075.9 | 50,176.9 | 74,701.9 | 74,791.1 | 120,947.1 | 136,122.7 | 115,726.3 | 116,070.5 |
| Shannon | 13.78 | 10.26 | 12.97 | 12.15 | 12.98 | 12.17 | 9.65 | 10.42 |
| Top 5 abundant bacterial phylum (%) | ||||||||
| Proteobacteria | 15.39 | 8.26 | 9.87 | 13.43 | 14.42 | 9.13 | 2.5 | 3.7 |
| Firmicutes | 1.75 | 5.26 | 2.42 | 1.51 | 2.48 | 1.82 | 0.71 | 0.17 |
| Actinobacteria | 0.64 | 0.47 | 0.38 | 0.25 | 0.89 | 0.26 | 0.11 | 0.03 |
| Bacteroidetes | 0.64 | 0.11 | 0.44 | 0.41 | 0.57 | 0.39 | 0.14 | 0.19 |
| Thermotogae | 0.10 | 0.20 | 0.30 | 0.27 | 0.49 | 0.35 | 00 | 00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdulaziz, A.; Pramodh, A.V.; Sukumaran, V.; Raj, D.; John, A.M.V.B. The Influence of Photodynamic Antimicrobial Chemotherapy on the Microbiome, Neuroendocrine and Immune System of Crustacean Post Larvae. Toxics 2023, 11, 36. https://doi.org/10.3390/toxics11010036
Abdulaziz A, Pramodh AV, Sukumaran V, Raj D, John AMVB. The Influence of Photodynamic Antimicrobial Chemotherapy on the Microbiome, Neuroendocrine and Immune System of Crustacean Post Larvae. Toxics. 2023; 11(1):36. https://doi.org/10.3390/toxics11010036
Chicago/Turabian StyleAbdulaziz, Anas, Athira Vengalil Pramodh, Vrinda Sukumaran, Devika Raj, and Ann Mary Valathuparambil Baby John. 2023. "The Influence of Photodynamic Antimicrobial Chemotherapy on the Microbiome, Neuroendocrine and Immune System of Crustacean Post Larvae" Toxics 11, no. 1: 36. https://doi.org/10.3390/toxics11010036
APA StyleAbdulaziz, A., Pramodh, A. V., Sukumaran, V., Raj, D., & John, A. M. V. B. (2023). The Influence of Photodynamic Antimicrobial Chemotherapy on the Microbiome, Neuroendocrine and Immune System of Crustacean Post Larvae. Toxics, 11(1), 36. https://doi.org/10.3390/toxics11010036









