Determination of Prostaglandins (Carboprost, Cloprostenol, Dinoprost, Dinoprostone, Misoprostol, Sulprostone) by UHPLC-MS/MS in Toxicological Investigations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Instrumentation
2.3. Working Solutions, Calibration Curve and Sample Preparation
2.4. Validation
3. Results
3.1. Optimization of Mass Spectrometry Parameters
3.2. Validation Results
3.3. Method Application and Toxicological Findings
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Bygdeman, M. Prostaglandin analogues and their uses. Baillière’s Clin. Obstet. Gynaecol. 1992, 6, 893–903. [Google Scholar] [CrossRef]
- Bearak, J.; Popinchalk, A.; Ganatra, B.; Moller, A.B.; Tunçalp, Ö.; Beavin, C.; Kwok, L.; Alkema, L. Unintended pregnancy and abortion by income, region, and the legal status of abortion: Estimates from a comprehensive model for 1990–2019. Lancet Glob. Health 2020, 8, e1152–e1161. [Google Scholar] [CrossRef]
- Ganatra, B.; Gerdts, C.; Rossier, C.; Johnson, B.R., Jr.; Tunçalp, Ö.; Assifi, A.; Sedgh, G.; Singh, S.; Bankole, A.; Popinchalk, A.; et al. Global, regional, and subregional classification of abortions by safety, 2010–2014: Estimates from a Bayesian hierarchical model. Lancet 2017, 90, 2372–2381. [Google Scholar] [CrossRef]
- Nivedita, K.; Shanthini, F. Is it safe to provide abortion pills over the counter? A study on outcome following self-medication with abortion pills. J. Clin. Diagn. Res. 2015, 9, QC01. [Google Scholar] [CrossRef] [PubMed]
- Murtagh, C.; Wells, E.; Raymond, E.G.; Coeytaux, F.; Winikoff, B. Exploring the feasibility of obtaining mifepristone and misoprostol from the internet. Contraception 2018, 97, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Mazurek, A.; Baran, P.; Blazewicz, A.; Januszewicz, P.; Jop, K.; Maurin, J. Medicines and preparations of unknown origin used illegally for pharmacological abortive purposes. Wydawnictwo UR 2012 ISSN 2082-369X. Med. J. Rzesz. Univ. Natl. Med. Inst. 2012, 10, 238–244. [Google Scholar]
- Kerestes, C.; Freese, M.; Stockdale, C.K.; Hardy-Fairbanks, A.J. Googling abortion pills: The ease of buying misoprostol and mifepristone online for home use [1N]. Obset. Gynecol. 2019, 133, 150S. [Google Scholar] [CrossRef]
- Canzi, E.F.; Lopes, B.R.; Robeldo, T.; Borra, R.; Da Silva, M.F.G.F.; Oliveira, R.V.; Maia, B.H.N.S.; Cass, Q.B. Prostaglandins E2 and F2α levels in human menstrual fluid by online solid phase extraction coupled to liquid chromatography tandem mass spectrometry (SPE-LC-MS/MS). J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2019, 1109, 60–66. [Google Scholar] [CrossRef]
- Oliveira, G.M.; Dionísio, T.J.; Weckwerth, G.M.; Siqueira-Sandrin, V.S.; Polanco, N.L.D.H.; Faria, F.A.C.; Santos, C.F.; Calvo, A.M. Detection and quantification of prostaglandin E2 in saliva by liquid chromatography-tandem mass spectrometry using microextraction by packed sorbent. Prostaglandins Other Lipid Mediat. 2022, 163, 106672. [Google Scholar] [CrossRef]
- Shi, Y.; Rankin, M.M.; Norquay, L.D.; Weng, N.; Patel, S. Bioanalysis of sulprostone, a prostaglandin E2 analogue and selective EP 3 agonist, in monkey plasma by liquid chromatography-tandem mass spectrometry. J. Chrom. B 2018, 1092, 51–57. [Google Scholar] [CrossRef]
- Yin, L.; Meng, X.; Zhou, X.; Zhang, T.; Sun, H.; Yang, Z.; Yang, B.; Xiao, N.; Fawcett, J.P.; Yang, Y.; et al. Simultaneous determination of carboprost methylate and its active metabolite carboprost in dog plasma by liquid chromatography-tandem mass spectrometry with positive/negative ion-switching electrospray ionization and its application to a pharmacokinetic study. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2015, 998–999, 8–14. [Google Scholar] [CrossRef]
- Szpot, P.; Wachełko, O.; Zawadzki, M. Forensic toxicological aspects of misoprostol use in pharmacological abortions. Molecules 2022, 27, 6534. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.M.; Fraser, K. Misoprostol and attempted self-induction of abortion. J. R. Soc. Med. 1998, 91, 204–205. [Google Scholar] [CrossRef] [PubMed]
- Baskar, M.; Gupta, S.; Singh, A. Identification of drugs using FTIR in a case of illegal abortion: A forensic study. GSC Biol. Pharm. Sci. 2022, 18, 83–91. [Google Scholar] [CrossRef]
- Devadasu, C.H.; Harika, S.; Mallikarjuna, T.; Adilakshmi, G.; Sreenath, A.; Swetha Ratna, G. A spectrophotometric assay for the simultaneous analysis of mifepristone and misoprostol in tablets using Vierodt’s and absorbance ratio methods. Res. J. Pharm. Technol. 2012, 5, 46–49. [Google Scholar]
- Vijayasree, P.; Rubesh Kumar, S.; Manasa, K.; Gowri devi, K.C.; Charumathi, S. A simple RP-HPLC method for quantitation of carboprost tromethamine in injection dosage form. JGTPS 2014, 5, 2012–2016. [Google Scholar]
- Lee, J.H.; Park, H.N.; Kim, N.S.; Park, H.J.; Park, S.; Shin, D.; Kang, H. Detection of illegal abortion-induced drugs using rapid and simultaneous method for the determination of abortion-induced compounds by LC–MS/MS. Chromatographia 2019, 82, 1365–1371. [Google Scholar] [CrossRef]
- Chu, K.; Wang, C.C.; Pang, C.; Rogers, M. Method to determine stability and recovery of carboprost and misoprostol in infusion preparations. J. Chrom. B 2007, 857, 83–91. [Google Scholar] [CrossRef]
- De Campos, E.G.; da Costa, B.R.B.; Dos Santos, F.S.; Monedeiro, F.; Alves, M.N.R.; Santos Junior, W.J.R.; De Martinis, B.S. Alternative matrices in forensic toxicology: A critical review. Forensic Toxicol. 2022, 40, 1–18. [Google Scholar] [CrossRef]
- Iskierka, M.; Zawadzki, M.; Szpot, P.; Jurek, T. Detection of drugs in postmortem specimens of blood, vitreous humor and bone marrow aspirate. J. Anal. Toxicol. 2021, 45, 348–355. [Google Scholar] [CrossRef]
- Szpot, P.; Wachełko, O.; Zawadzki, M. Diclofenac concentrations in post-mortem specimens—Distribution, case reports, and validated method (UHPLC-QqQ-MS/MS) for its determination. Toxics 2022, 10, 421. [Google Scholar] [CrossRef]
- Gagliano-Candela, R.; Aventaggiato, L. The detection of toxic substances in entomological specimens. Int. J. Leg. Med. 2001, 114, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Szpot, P.; Buszewicz, G.; Jurek, T.; Teresiński, G. Fragmentation patterns involving ammonium adduct fragment ions: A comparison of the determination of metaldehyde in human blood by HPLC-QqQ-MS/MS and UHPLC-Q-TOF-MS. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2018, 1085, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Focardi, M.; Bosco, A.; Castiglione, F.; Bartolucci, G.; Gualco, B.; Bugelli, V. Abortion caused by intravaginal self-administration of Misoprostol: A case report. Rom. J. Leg. Med. 2019, 27, 340–342. [Google Scholar] [CrossRef]
- Watzer, B.; Lusthof, K.J.; Schweer, H. Abortion after deliberate Arthrotec® addition to food. Mass spectrometric detection of diclofenac, misoprostol acid, and their urinary metabolites. Int. J. Leg. Med. 2015, 129, 759–769. [Google Scholar] [CrossRef]
- Hopson, D.L.; Ross, J. Maternal abortifacient use for clandestine abortion. Acad. Forensic Pathol. 2016, 6, 663–672. [Google Scholar] [CrossRef]
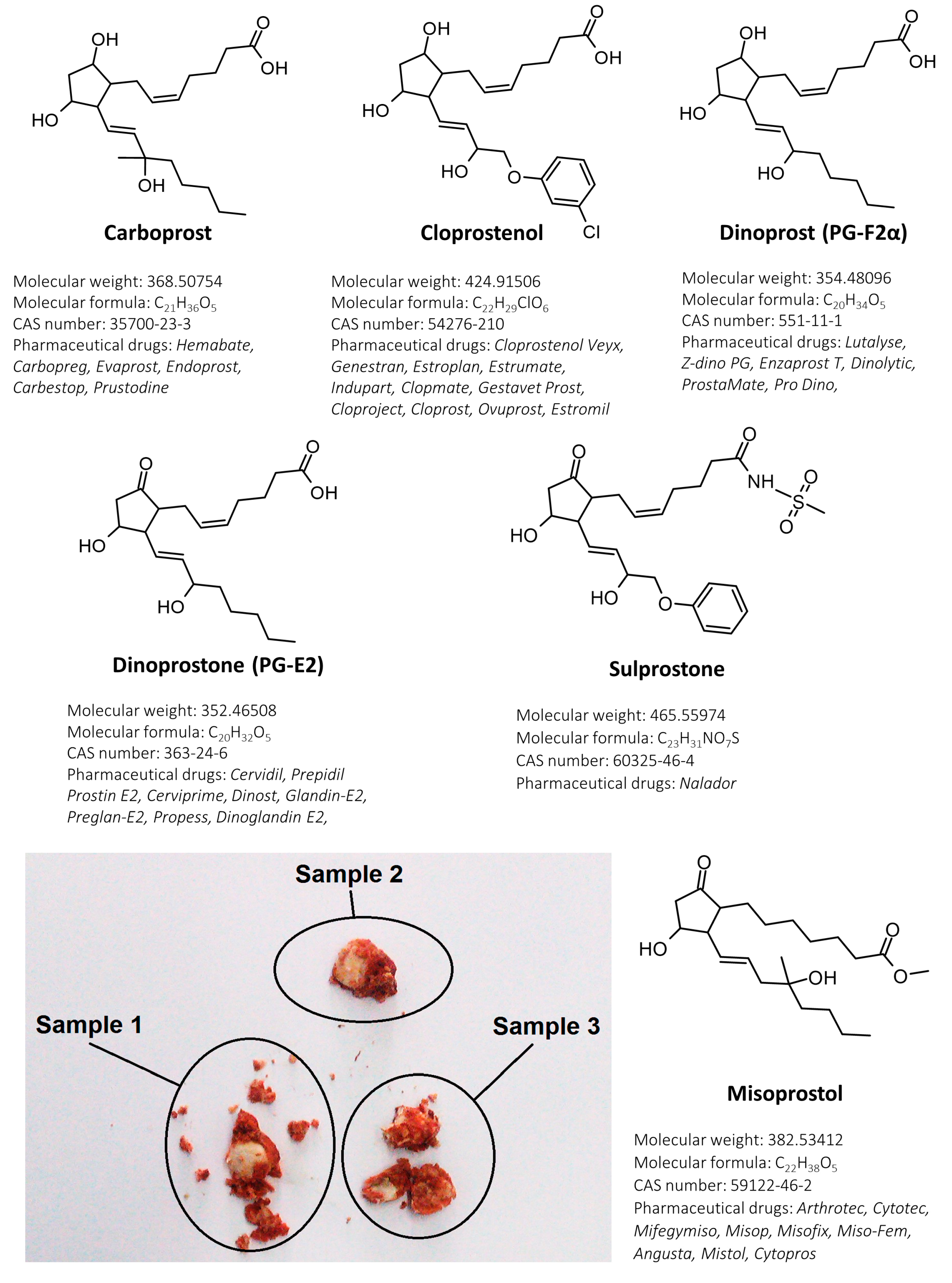
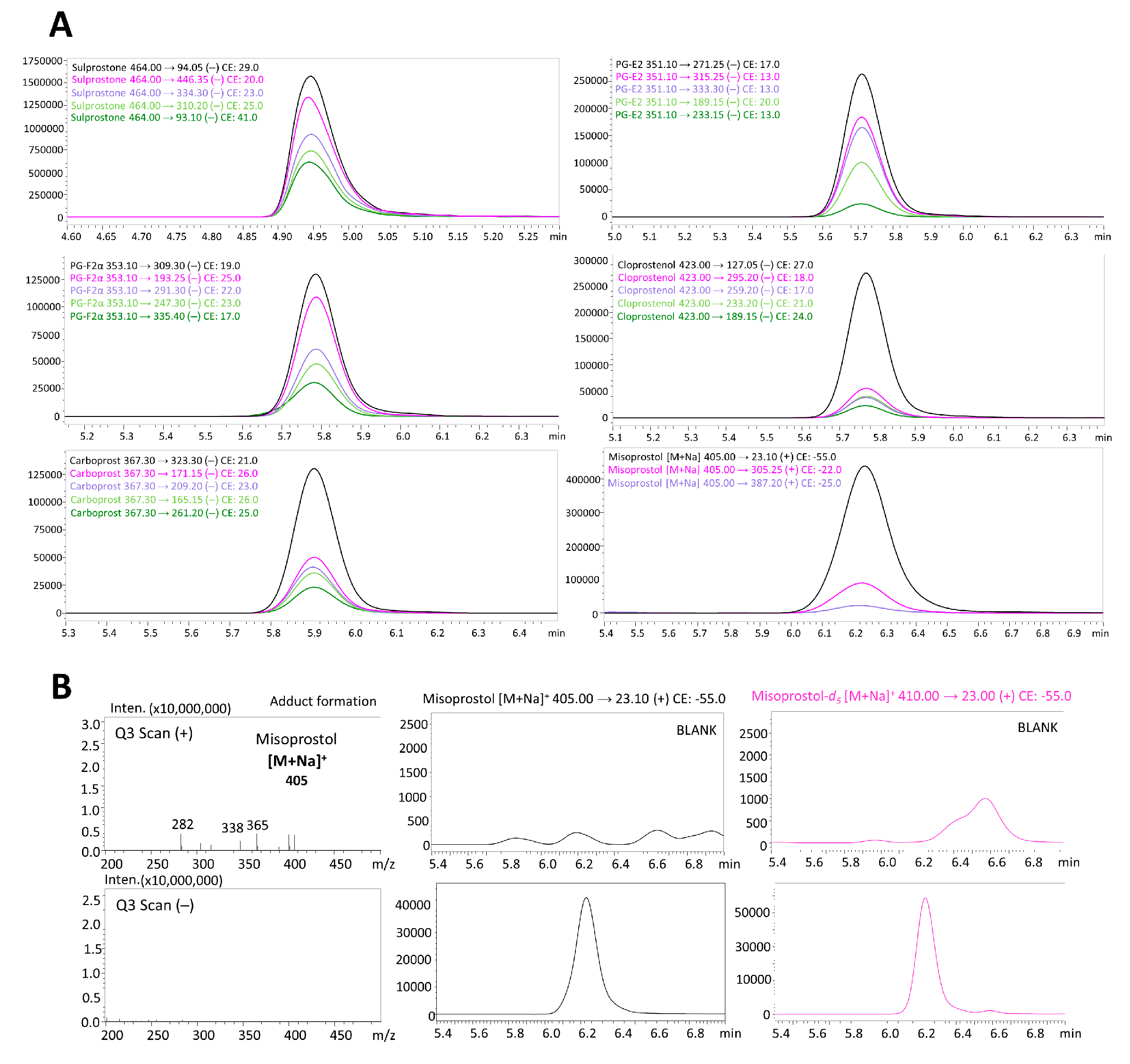
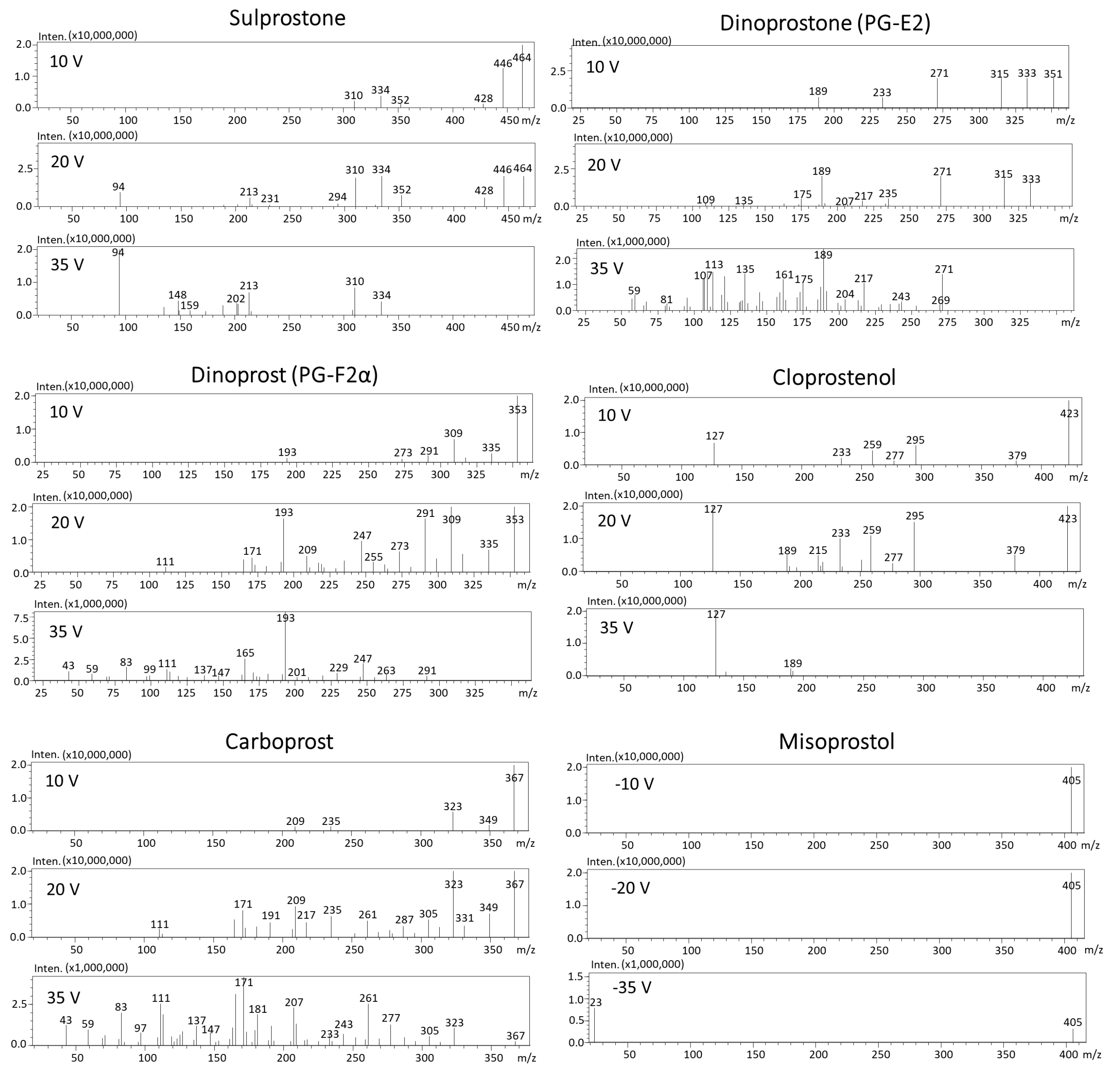
| No. | Substance | Ionization /Ion | Precursor Ion [m/z] | Product Ion [m/z] | Dwell Time (msec) | Q1 Pre-Bias [V] | Collision Energy [V] | Q3 Pre-Bias [V] | Retention Time [min] |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Carboprost | Negative [M−H]− | 367.3 | 323.3 * | 1 | 19 | 21 | 12 | 5.914 |
| 171.15 | 1 | 27 | 26 | 12 | |||||
| 209.2 | 1 | 14 | 23 | 14 | |||||
| 165.15 | 1 | 14 | 26 | 11 | |||||
| 261.2 | 1 | 27 | 25 | 17 | |||||
| 2 | Cloprostenol | Negative [M−H]− | 423.0 | 127.05 * | 1 | 13 | 27 | 23 | 5.775 |
| 295.2 | 1 | 22 | 18 | 21 | |||||
| 259.2 | 1 | 23 | 17 | 18 | |||||
| 233.2 | 1 | 16 | 21 | 16 | |||||
| 189.15 | 1 | 16 | 24 | 13 | |||||
| 3 | Dinoprost (PGF2α) | Negative [M−H]− | 353.1 | 309.3 | 1 | 26 | 19 | 11 | 5.791 |
| 193.25 * | 1 | 26 | 25 | 13 | |||||
| 291.3 | 1 | 26 | 22 | 21 | |||||
| 247.3 | 1 | 26 | 23 | 17 | |||||
| 335.4 | 1 | 26 | 17 | 12 | |||||
| 4 | Dinoprostone (PGE2) | Negative [M−H]− | 351.1 | 271.25 * | 1 | 18 | 17 | 13 | 5.719 |
| 315.25 | 1 | 26 | 13 | 11 | |||||
| 333.3 | 1 | 25 | 13 | 12 | |||||
| 189.15 | 1 | 25 | 20 | 13 | |||||
| 233.15 | 1 | 18 | 13 | 17 | |||||
| 5 | Misoprostol | Positive [M+Na]+ | 405.0 | 23.1 * | 1 | −19 | −55 | −26 | 6.237 |
| 305.25 | 1 | −23 | −22 | −14 | |||||
| 387.2 | 1 | −25 | −25 | −13 | |||||
| 6 | Sulprostone | Negative [M−H]− | 464.0 | 446.35 | 1 | 11 | 20 | 16 | 4.951 |
| 334.3 | 1 | 24 | 23 | 12 | |||||
| 310.2 | 1 | 25 | 25 | 22 | |||||
| 94.05 * | 1 | 18 | 29 | 16 | |||||
| 93.1 | 1 | 14 | 41 | 15 | |||||
| IS1 | Dinoprost-d4 (PGF2α-d4) | Negative [M−H]− | 357.3 | 313.3 * | 1 | 20 | 19 | 11 | 5.785 |
| 197.25 | 1 | 19 | 25 | 14 | |||||
| 295.4 | 1 | 19 | 22 | 21 | |||||
| 251.3 | 1 | 19 | 23 | 16 | |||||
| 213.3 | 1 | 19 | 17 | 15 | |||||
| IS2 | Misoprostol-d5 | Positive [M+Na]+ | 410.0 | 23.0 * | 1 | −22 | −55 | −26 | 6.232 |
| 305.2 | 1 | −19 | −22 | −21 | |||||
| 392.3 | 1 | −20 | −24 | −26 |
| Calibration Curve | Validation Results | ||||||
|---|---|---|---|---|---|---|---|
| Substance | Internal Standard | The Coefficient of Determination (R2) | Concentration Level [µg/mL] | Intraday | Interday | ||
| Precision [%] * | Accuracy [%] * | Precision [%] * | Accuracy [%] * | ||||
| Carboprost | Dinoprost-d4 (PG-F2α-d4) | >0.9988 | 0.1 1 10 | 2.9 2.4 3.3 | −4.5 −3.8 1.4 | 3.0 4.4 1.2 | 0.3 −0.2 −0.6 |
| Cloprostenol | Dinoprost-d4 (PG-F2α-d4) | >0.9988 | 0.1 1 10 | 2.4 1.5 2.4 | 4.3 −4.1 1.8 | 2.5 1.0 1.1 | 4.0 −3.8 1.0 |
| Dinoprost (PG-F2α) | Dinoprost-d4 (PG-F2α-d4) | >0.9980 | 0.1 1 10 | 5.0 4.2 1.4 | 3.0 −0.9 3.6 | 3.8 4.7 2.4 | 5.0 2.6 3.0 |
| Dinoprostone (PG-E2) | Dinoprost-d4 (PG-F2α-d4) | >0.9977 | 0.1 1 10 | 4.4 3.5 1.8 | 4.3 −3.1 4.3 | 2.4 0.8 0.7 | 4.7 −0.9 3.0 |
| Misoprostol | Misoprostol-d5 | >0.9991 | 0.1 1 10 | 1.0 4.2 2.2 | 5.0 2.1 4.8 | 4.2 2.2 1.6 | 4.0 −1.2 3.1 |
| Sulprostone | Dinoprost-d4 (PG-F2α-d4) | >0.9988 | 0.1 1 10 | 1.9 4.1 4.9 | 5.0 2.7 −3.4 | 3.5 4.5 2.9 | 4.0 2.0 −1.4 |
| Method | Prostaglandins | Details of the Method | Tested Samples | Qualitative/ Quantitative | Year | Reference |
|---|---|---|---|---|---|---|
| ATR-FTIR | Misoprostol | Characteristic wavelengths: 3304, 1737, 1364, 1016 cm−1 | Pills | Qualitative | 2022 | [14] |
| Spectrophotometry | Misoprostol | Absorbance measured at 247.6 nm | Pills | Quantitative | 2011 | [15] |
| HPLC-UV | Carboprost | Chromatographic column: Symmetry C18 (100 × 4.6 mm × 3.5 µm); Absorbance measured at 200 nm; | Injection liquids | Quantitative | 2014 | [16] |
| LC-MS | Misoprostol | Comparison of chromatograms obtained during analysis of authentic samples with chromatograms obtained for Cytotec® tablets (reference material) | Abortion pills collected from vagina during gynecological examination | Qualitative | 2019 | [24] |
| LC-QTOF-MS/MS | Carboprost Misoprostol | Chromatographic column: Suplex™PKB (250 × 2.1 mm × 5 µm); Mass spectrometry: ESI (sodium adducts in positive ionization); MRM | Infusion preparations | Quantitative | 2007 | [18] |
| LC-QTRAP-MS/MS | Misoprostol | Chromatographic column: Capcell PAK C18 MGII (50 × 2.0 mm × 3 µm); Mass spectrometry: ESI (negative ionization); MRM | Counterfeit drugs distributed in the illegal marketplaces | Quantitative | 2019 | [17] |
| UHPLC-QqQ-MS/MS | Carboprost Cloprostenol Dinoprost (PG-F2α) Dinoprstone (PG-E2) Misoprostol Sulprostone | Chromatographic column: Acquity UPLC BEH C18 (50 × 2.1 mm × 1.7 µm); Mass spectrometry: ESI (sodium adducts in positive ionization & negative ionization); MRM | Abortion pills collected from vagina during gynecological examination | Quantitative | 2022 | Presented method |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szpot, P.; Wachełko, O.; Zawadzki, M. Determination of Prostaglandins (Carboprost, Cloprostenol, Dinoprost, Dinoprostone, Misoprostol, Sulprostone) by UHPLC-MS/MS in Toxicological Investigations. Toxics 2023, 11, 802. https://doi.org/10.3390/toxics11100802
Szpot P, Wachełko O, Zawadzki M. Determination of Prostaglandins (Carboprost, Cloprostenol, Dinoprost, Dinoprostone, Misoprostol, Sulprostone) by UHPLC-MS/MS in Toxicological Investigations. Toxics. 2023; 11(10):802. https://doi.org/10.3390/toxics11100802
Chicago/Turabian StyleSzpot, Paweł, Olga Wachełko, and Marcin Zawadzki. 2023. "Determination of Prostaglandins (Carboprost, Cloprostenol, Dinoprost, Dinoprostone, Misoprostol, Sulprostone) by UHPLC-MS/MS in Toxicological Investigations" Toxics 11, no. 10: 802. https://doi.org/10.3390/toxics11100802
APA StyleSzpot, P., Wachełko, O., & Zawadzki, M. (2023). Determination of Prostaglandins (Carboprost, Cloprostenol, Dinoprost, Dinoprostone, Misoprostol, Sulprostone) by UHPLC-MS/MS in Toxicological Investigations. Toxics, 11(10), 802. https://doi.org/10.3390/toxics11100802






