Effects of Summer and Autumn Drought on Eutrophication and the Phytoplankton Community in Dongting Lake in 2022
Abstract
1. Introduction
2. Materials and Methods
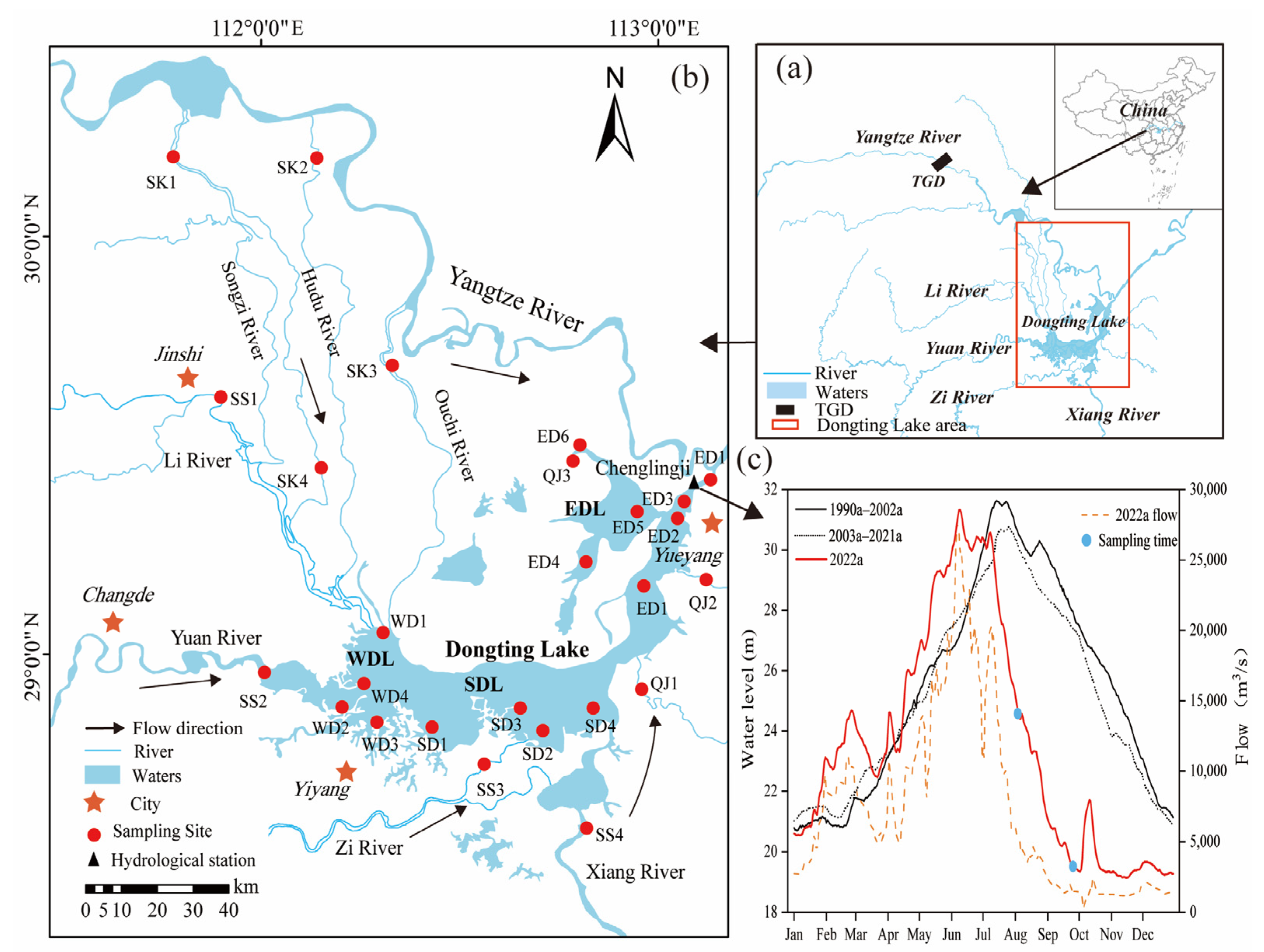
2.1. Study Area
2.2. Sampling
2.3. Sample Analysis
2.4. Data Analysis
3. Results
3.1. Environmental Parameter Variation and Eutrophication State
3.1.1. Environment Parameter Variation
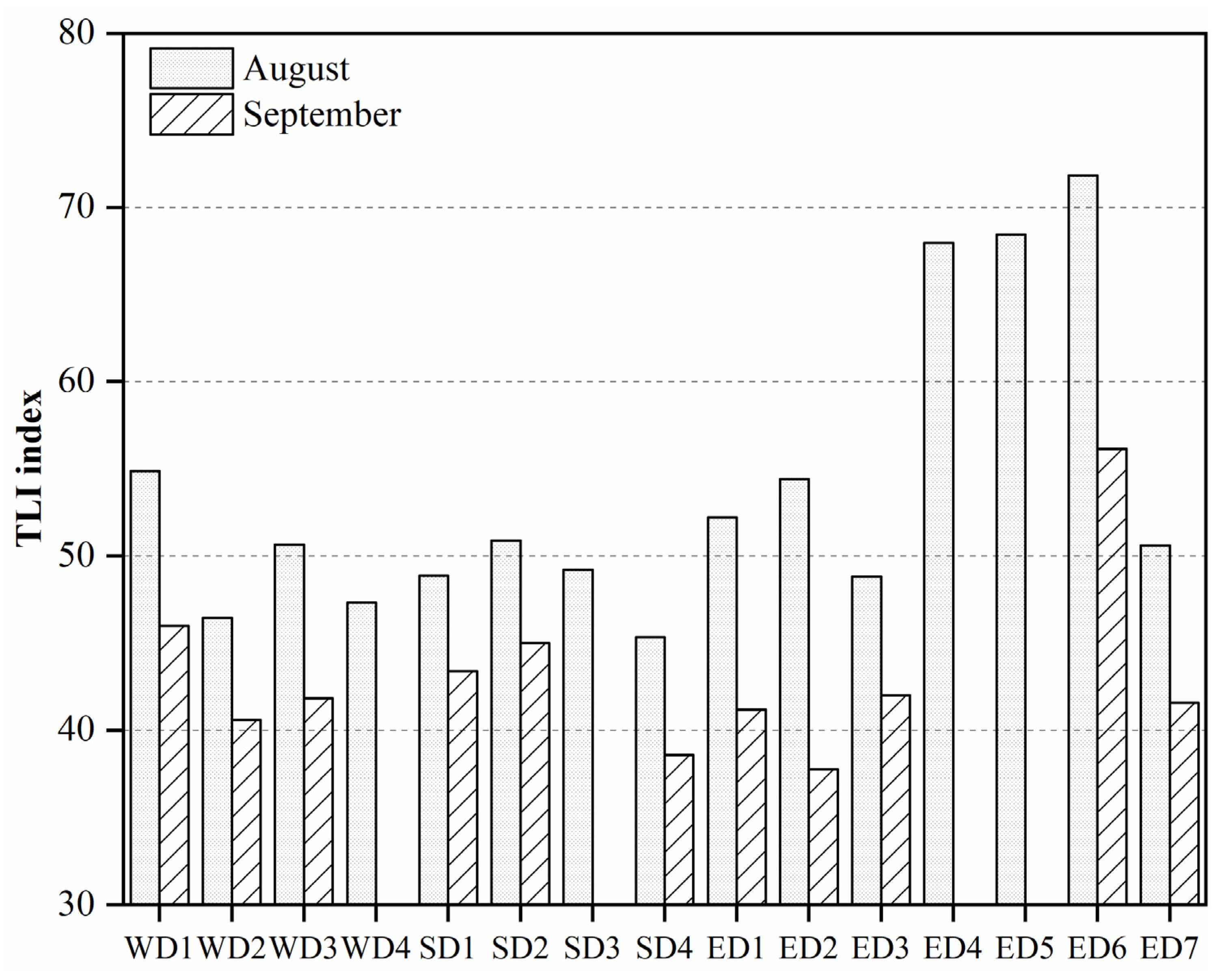
3.1.2. Eutrophication State
3.2. Distribution Characteristics of the Phytoplankton Community in Dongting Lake
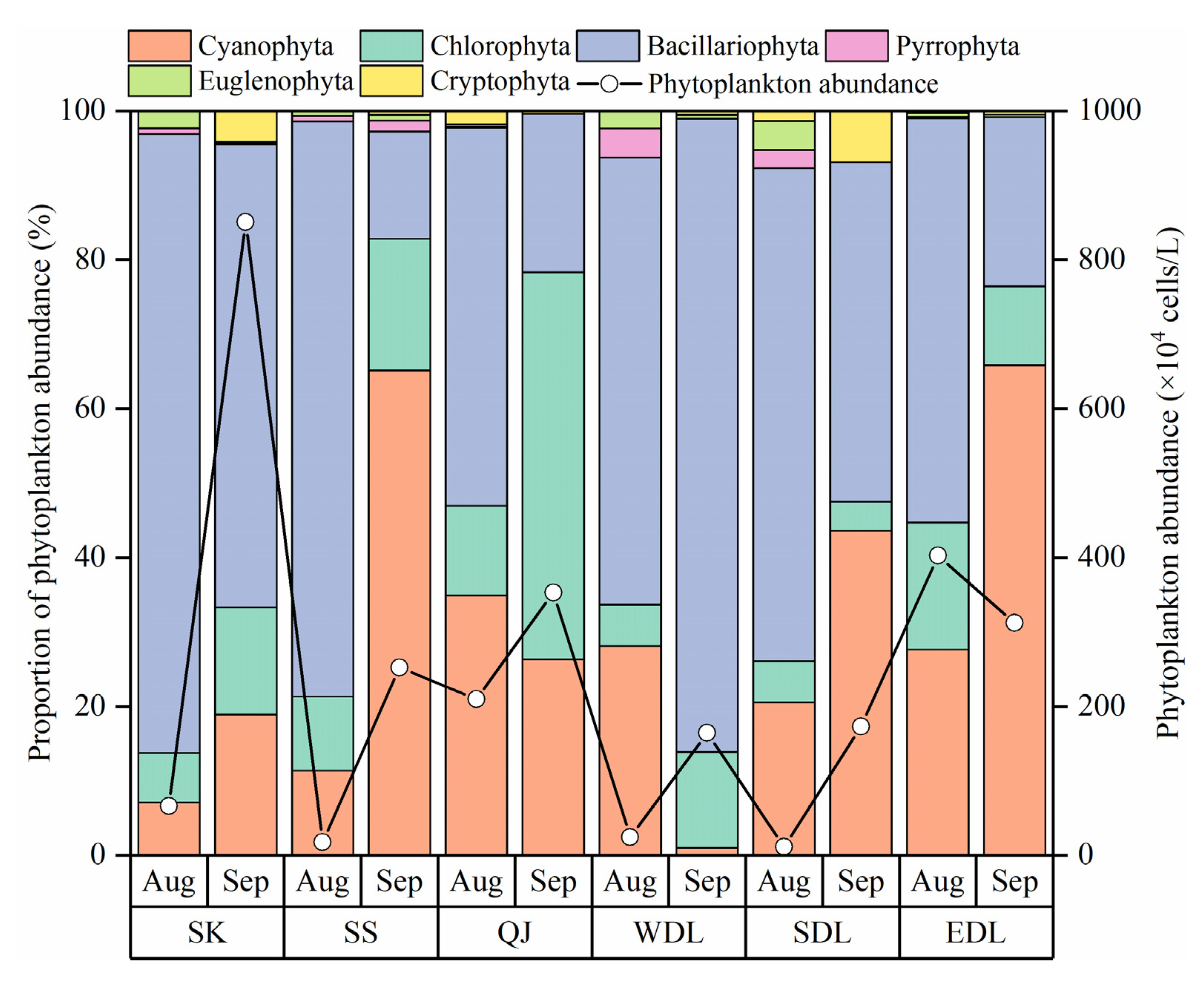
3.2.1. Phytoplankton Community Composition
3.2.2. Dominant Species of Phytoplankton
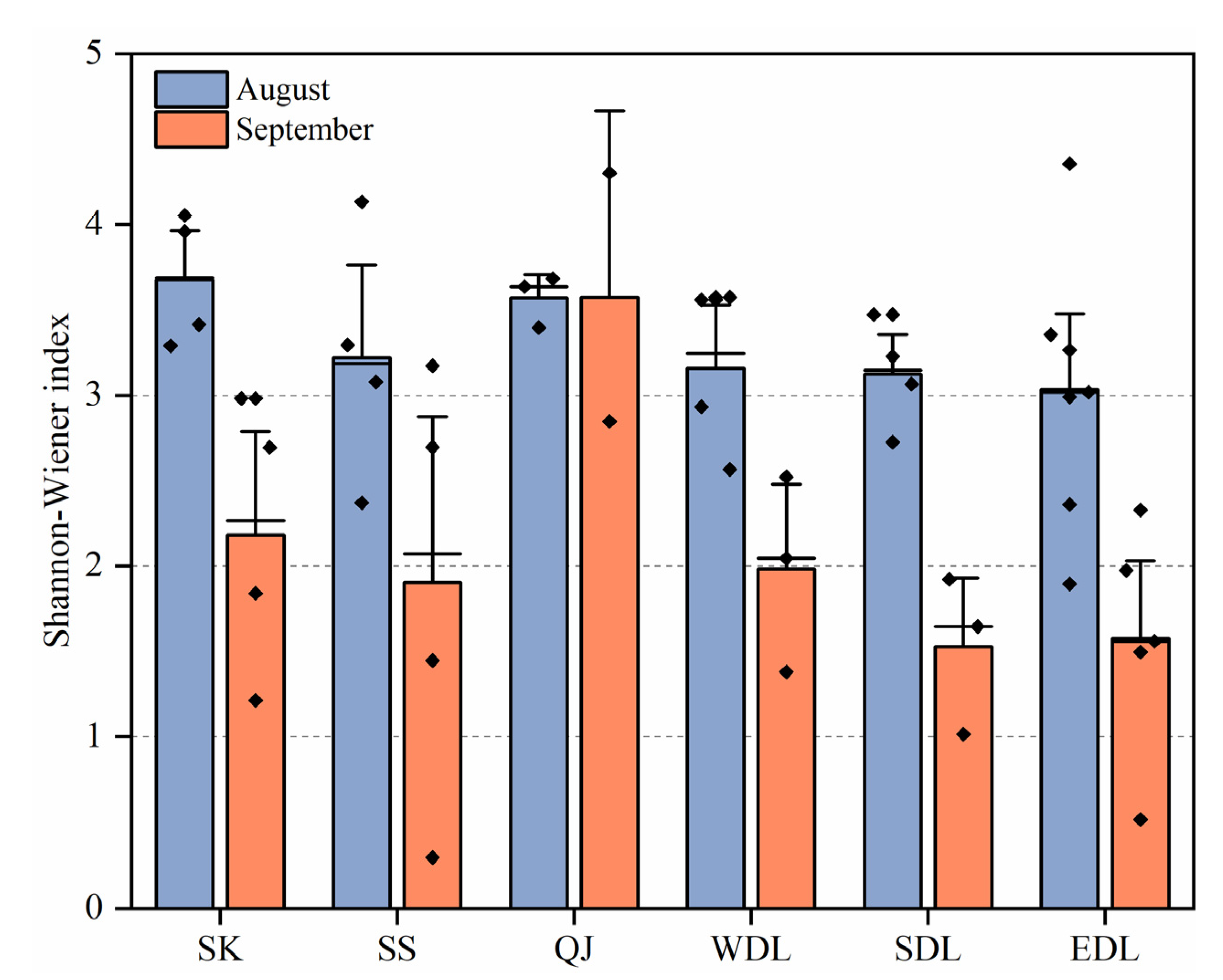
3.2.3. Phytoplankton Diversity (H) and the Community Similarity Index (J)
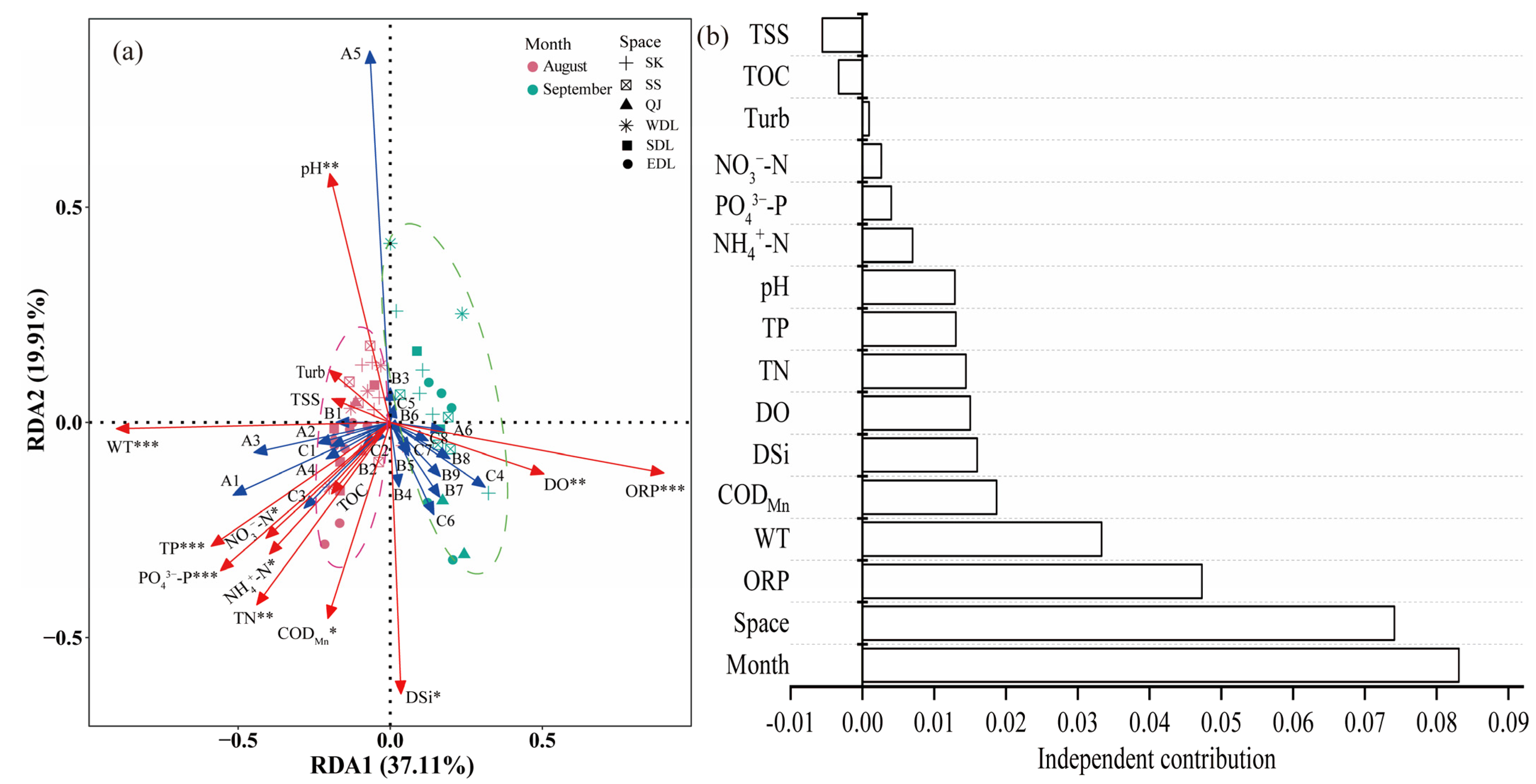
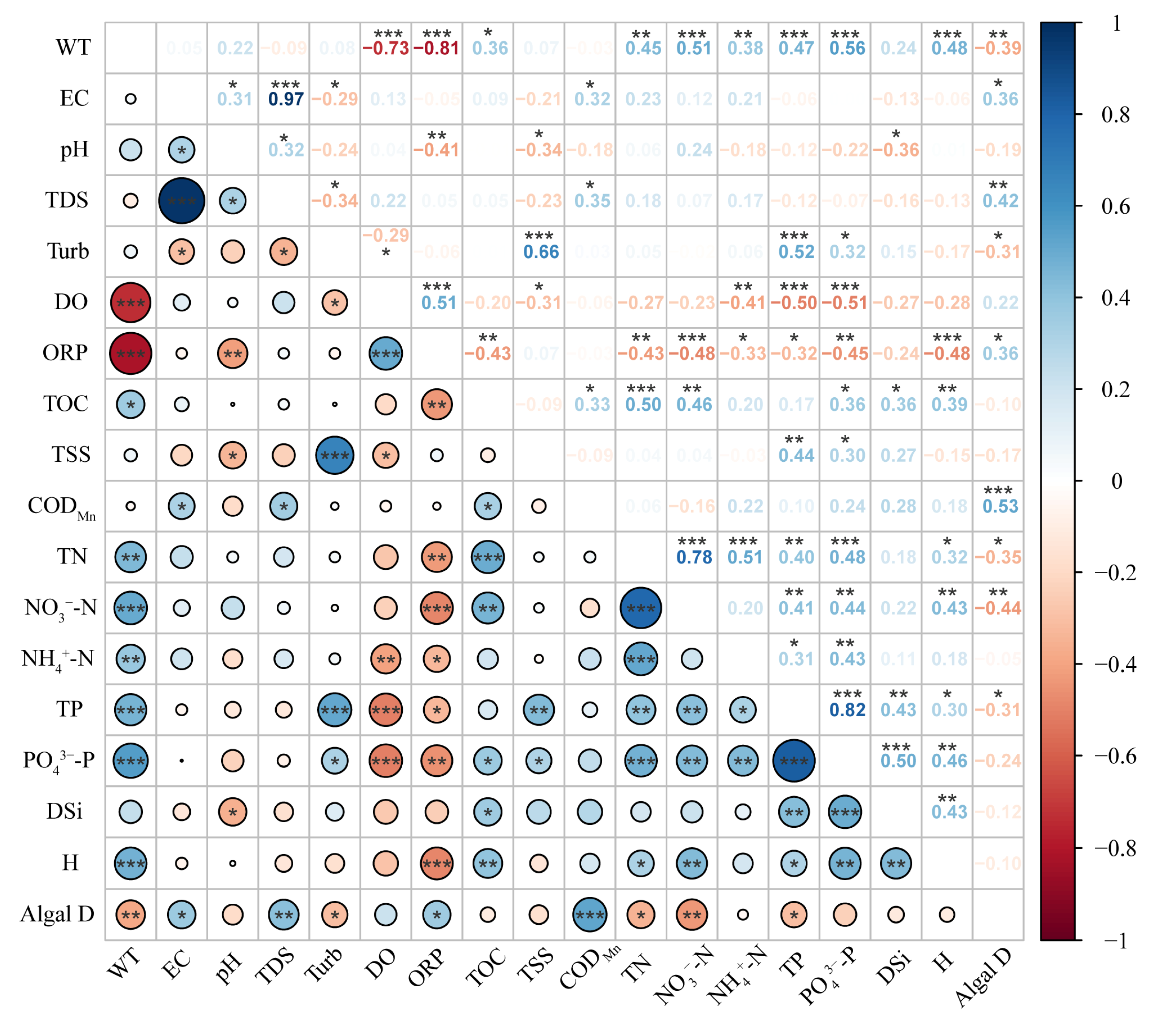
3.3. Driving Factors of the Phytoplankton Community Structure
3.4. Comparison with Other Freshwater Lakes
4. Discussion
4.1. Integrated Characterization of Eutrophication in Dongting Lake under Drought Events
4.2. Response of Phytoplankton Communities to Environmental Factors during Drought Events
4.3. Effects of Lake Hydrologic Connectivity on Phytoplankton Community Heterogeneity during Drought Events
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jeppesen, E.; Kronvang, B.; Olesen, J.E.; Audet, J.; Sondergaard, M.; Hoffmann, C.C.; Andersen, H.E.; Lauridsen, T.L.; Liboriussen, L.; Larsen, S.E.; et al. Climate change effects on nitrogen loading from cultivated catchments in Europe: Implications for nitrogen retention, ecological state of lakes and adaptation. Hydrobiologia 2011, 663, 1–21. [Google Scholar] [CrossRef]
- Strayer, D.L.; Dudgeon, D. Freshwater biodiversity conservation: Recent progress and future challenges. J. N. Am. Benthol. Soc. 2010, 29, 344–358. [Google Scholar] [CrossRef]
- Iqbal, M.M.; Li, L.; Hussain, S.; Lee, J.L.; Mumtaz, F.; Elbeltagi, A.; Waqas, M.S.; Dilawar, A. Analysis of seasonal variations in surface water quality over wet and dry regions. Water 2022, 14, 1058. [Google Scholar] [CrossRef]
- Ayele, H.S.; Atlabachew, M. Review of characterization, factors, impacts, and solutions of Lake eutrophication: Lesson for lake Tana, Ethiopia. Environ. Sci. Pollut. Res. 2021, 28, 14233–14252. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Yu, G.B.; Liu, Y.; Li, G.L.; Feng, S.; Wu, S.C.; Wong, M.H. Urbanization impairs surface water quality: Eutrophication and metal stress in the grand canal of China. River Res. Appl. 2012, 28, 1135–1148. [Google Scholar] [CrossRef]
- Iqbal, M.M.; Hussain, S.; Cheema, M.; Jehanzeb, M.; Lee, J.L.; Waqas, M.S.; Aslam, M.A. Seasonal effect of agricultural pollutants on coastline environment: A case study of the southern estuarine water ecosystem of the boseong county Korea. Pak. J. Agri. Sci. 2022, 59, 117–124. [Google Scholar]
- Qiu, J. China drought highlights future climate threats. Nature 2010, 465, 142–143. [Google Scholar] [CrossRef]
- Min, S.K.; Zhang, X.B.; Zwiers, F.W.; Hegerl, G.C. Human contribution to more-intense precipitation extremes. Nature 2011, 470, 378–381. [Google Scholar] [CrossRef]
- Xia, J.; Chen, J.; She, D. Impacts and countermeasures of extreme drought in the Yangtze River Basin in 2022. J. Hydraul. Eng. 2022, 53, 1143–1153. [Google Scholar]
- Zhang, L.X.; Zhou, T.J. Drought over East Asia: A Review. J. Clim. 2015, 28, 3375–3399. [Google Scholar] [CrossRef]
- Zhang, W.J.; Jin, F.F.; Zhao, J.X.; Qi, L.; Ren, H.L. The Possible Influence of a Nonconventional El Nino on the Severe Autumn Drought of 2009 in Southwest China. J. Clim. 2013, 26, 8392–8405. [Google Scholar] [CrossRef]
- Li, S.Y.; Bush, R.T.; Mao, R.; Xiong, L.H.; Ye, C. Extreme drought causes distinct water acidification and eutrophication in the Lower Lakes (Lakes Alexandrina and Albert), Australia. J. Hydrol. 2017, 544, 133–146. [Google Scholar] [CrossRef]
- Djabri, L.; Bouhsina, S.; Hani, A.; Bosch, A.; Mudry, J.; Djouamaa, M.C. Impacts of drought on water quality: The case of aquifers in eastern Algeria. In Evolving Water Resources Systems: Understanding, Predicting and Managing Water-Society Interactions; IAHS Press: Wallingford, UK, 2014; pp. 357–362. [Google Scholar]
- Barroso, H.D.; Tavares, T.C.L.; Soares, M.D.; Garcia, T.M.; Rozendo, B.; Vieira, A.S.C.; Viana, P.B.; Pontes, T.M.; Ferreira, T.J.T.; Pereira, J.; et al. Intra-annual variability of phytoplankton biomass and nutrients in a tropical estuary during a severe drought. Estuar. Coast. Shelf Sci. 2018, 213, 283–293. [Google Scholar] [CrossRef]
- Whitton, B.A. Changing approaches to monitoring during the period of the ‘Use of Algae for Monitoring Rivers’ symposia. Hydrobiologia 2012, 695, 7–16. [Google Scholar] [CrossRef]
- Cai, Y.; Qi, L.; Shan, T.; Liu, Y.; Zhang, N.N.; Lu, X.X.; Fan, Y.W. Application of Phytoplankton Taxonomic alpha-Diversity Indices to Assess Trophic States in Barrier Lake: A Case of Jingpo Lake. Diversity 2022, 14, 1003. [Google Scholar] [CrossRef]
- McCarthy, J.J.; Goldman, J.C. Nitrogenous nutrition of marine phytoplankton in nutrient-depleted waters. Science 1979, 203, 670–672. [Google Scholar] [CrossRef] [PubMed]
- Sunda, W.G.; Huntsman, S.A. Interrelated influence of iron, light and cell size on marine phytoplankton growth. Nature 1997, 390, 389–392. [Google Scholar] [CrossRef]
- Yuan, Y.X.; Jiang, M.; Liu, X.T.; Yu, H.X.; Otte, M.L.; Ma, C.X.; Her, Y.G. Environmental variables influencing phytoplankton communities in hydrologically connected aquatic habitats in the Lake Xingkai basin. Ecol. Indic. 2018, 91, 1–12. [Google Scholar] [CrossRef]
- Anderson, S.R.; Harvey, E.L. Seasonal variability and drivers of microzooplankton grazing and phytoplankton growth in a subtropical estuary. Front. Mar. Sci. 2019, 6, 174. [Google Scholar] [CrossRef]
- Xu, Y.Y.; Cai, Q.H.; Ye, L.; Shao, M.L. Asynchrony of spring phytoplankton response to temperature driver within a spatial heterogeneity bay of Three-Gorges Reservoir, China. Limnologica 2011, 41, 174–180. [Google Scholar] [CrossRef]
- Ward, B.B.; Van Oostende, N. Phytoplankton assemblage during the North Atlantic spring bloom assessed from functional gene analysis. J. Plankton Res. 2016, 38, 1135–1150. [Google Scholar] [CrossRef][Green Version]
- Hrdinka, T.; Novicky, O.; Hanslik, E.; Rieder, M. Possible impacts of floods and droughts on water quality. J. Hydro-Environ. Res. 2012, 6, 145–150. [Google Scholar] [CrossRef]
- Worrall, F.; Burt, T.P. The effect of severe drought on the dissolved organic carbon (DOC) concentration and flux from British rivers. J. Hydrol. 2008, 361, 262–274. [Google Scholar] [CrossRef]
- Mosley, L.M. Drought impacts on the water quality of freshwater systems; review and integration. Earth Sci. Rev. 2015, 140, 203–214. [Google Scholar] [CrossRef]
- Wang, W.J.; Shi, K.; Wang, X.W.; Wang, S.Q.; Zhang, D.; Peng, Y.Y.; Li, N.; Zhang, Y.L.; Zhang, Y.B.; Qin, B.Q.; et al. A record-breaking extreme heat event caused unprecedented warming of lakes in China. Sci. Bull. 2023, 68, 578–582. [Google Scholar] [CrossRef]
- Yu, Y.W.; Mei, X.F.; Dai, Z.J.; Gao, J.J.; Li, J.B.; Wang, J.; Lou, Y.Y. Hydromorphological processes of Dongting Lake in China between 1951 and 2014. J. Hydrol. 2018, 562, 254–266. [Google Scholar] [CrossRef]
- Yan, G.H.; Yin, X.Y.; Huang, M.S.; Wang, X.; Huang, D.Z.; Li, D. Dynamics of phytoplankton functional groups in river-connected lakes and the major influencing factors: A case study of Dongting Lake, China. Ecol. Indic. 2023, 149, 110177. [Google Scholar] [CrossRef]
- Han, Q.Q.; Zhang, S.H.; Huang, G.X.; Zhang, R. Analysis of long-term water level variation in Dongting Lake, China. Water 2016, 8, 306. [Google Scholar] [CrossRef]
- Liang, C.; Li, H.Q.; Lei, M.J.; Du, Q.Y. Dongting Lake Water Level Forecast and Its Relationship with the Three Gorges Dam Based on a Long Short-Term Memory Network. Water 2018, 10, 1389. [Google Scholar] [CrossRef]
- Liu, Y.Z.; Jiang, C.B.; Long, Y.N.; Deng, B.; Jiang, J.Y.; Yang, Y.; Wu, Z.Y. Study on the Water Level-Discharge Relationship Changes in Dongting Lake Outlet Section over 70 Years and the Impact of Yangtze River Backwater Effect. Water 2023, 15, 2057. [Google Scholar] [CrossRef]
- Zhou, H.; Luo, Z.C.; Tangdamrongsub, N.; Wang, L.C.; He, L.J.; Xu, C.; Li, Q. Characterizing Drought and Flood Events over the Yangtze River Basin Using the HUST-Grace2016 Solution and Ancillary Data. Remote Sens. 2017, 9, 1100. [Google Scholar] [CrossRef]
- Huang, Q.; Sun, Z.D.; Opp, C.; Lotz, T.; Jiang, J.H.; Lai, X.J. Hydrological Drought at Dongting Lake: Its Detection, Characterization, and Challenges Associated With Three Gorges Dam in Central Yangtze, China. Water Resour. Manag. 2014, 28, 5377–5388. [Google Scholar] [CrossRef]
- Ji, H.; Wu, G.; Liu, Y. Sharp change of lake levels during the two extreme droughts and its hydroclimatic processes in Lake Dongting, China. J. Lake Sci. 2016, 28, 207–216. [Google Scholar]
- Zhang, H.; Wang, Y.Y.; Xu, J. Influence of Seasonal Water Level Fluctuations on Food Web Structure of a Large Floodplain Lake in China. Sustainability 2023, 15, 10724. [Google Scholar] [CrossRef]
- Xie, Y.H.; Chen, X.S. Effects of Three-Gorge Project on Succession of Wetland Vegetation in Dongting Lake. Res. Agric. Mod. 2008, 29, 684–687. [Google Scholar]
- Yuan, Y.; Zeng, G.M.; Liang, J.; Huang, L.; Hua, S.S.; Li, F.; Zhu, Y.; Wu, H.P.; Liu, J.Y.; He, X.X.; et al. Variation of water level in Dongting Lake over a 50-year period: Implications for the impacts of anthropogenic and climatic factors. J. Hydrol. 2015, 525, 450–456. [Google Scholar] [CrossRef]
- Tian, Z.B.; Zheng, B.H.; Wang, L.J.; Li, L.Q.; Wang, X.; Li, H.; Norra, S. Long term (1997–2014) spatial and temporal variations in nitrogen in Dongting Lake, China. PLoS ONE 2017, 12, e0170993. [Google Scholar] [CrossRef]
- Li, F.; Qin, X.Y.; Xie, Y.H.; Chen, X.S.; Hu, J.Y.; Liu, Y.Y.; Hou, Z.Y. Physiological mechanisms for plant distribution pattern: Responses to flooding and drought in three wetland plants from Dongting Lake, China. Limnology 2013, 14, 71–76. [Google Scholar] [CrossRef]
- Yan, G.H.; Yin, X.Y.; Wang, X.; Huang, M.S.; Huang, D.Z.; Wang, E.R.; Zhang, Y.Y. Driving factors of phytoplankton population and function group change in Dongting Lake and evaluation of water quality applicability. Chin. J. Environ. Sci. 2023, 44, 246–257. [Google Scholar]
- Ministry of Ecological Environment of the People’s Republic of China. Methods for the Monitoring and Analysis of Water and Wastewater, 4th ed.; Chinese Environmental Science Press: Beijing, China, 2002. [Google Scholar]
- Hu, H.J.; Wei, Y.X. The Freshwater Algae of China: Systematics, Taxonomy and Ecology; Science Press: Beijing, China, 2006. (In Chinese) [Google Scholar]
- Aksnes, D.L.; Wassmann, P. Modeling the significance of zooplankton grazing for export production. Limnol. Oceanogr. 1993, 38, 978–985. [Google Scholar] [CrossRef]
- Shannon, C.E. The Mathematical Theory of Communication; University of Illinois Press: Urbana, IL, USA, 1963; p. 117. [Google Scholar]
- Jaccard, P. Nouvelles recherches sur la distribution florale. Bull. Soc. Vaud. Sci. Nat. 1908, 44, 223–270. [Google Scholar]
- Tan, X.; Xia, X.L.; Cheng, X.L.; Zhang, Q. FTemporal and spatial pattern of phytoplankton community and its biodiversity indices in the Danjiangkou Reservoir. Chin. J. Environ. Sci. 2011, 32, 2875–2882. [Google Scholar]
- Hu, J.; Yang, Y.; Chi, S.; Shen, Q.; Hu, J. Spatial variation of phytoplankton community structure and its relationship with environmental factors at the Mangshan pumping station. Acta Ecol. Sin. 2017, 37, 1054–1062. [Google Scholar]
- Lai, J.S.; Zou, Y.; Zhang, J.L.; Peres-Neto, P.R. Generalizing hierarchical and variation partitioning in multiple regression and canonical analyses using the rdacca.hp R package. Methods Ecol. Evol. 2022, 13, 782–788. [Google Scholar] [CrossRef]
- Wang, M.Q.; Wang, J.C.; Wang, Q.; Yang, C.Y.; Zou, Z.H.; Qian, B. Characteristics of plankton community structure and eutrophication status in Dongting Lake in the season with normal water level. Chin. J. Ecol. 2018, 37, 2418–2429. [Google Scholar]
- Fu, Z.; Guo, J.; Huang, D.; Wang, C. The evolution and influencing factors of eutrophication in Dongting Lake. Environ. Chem. Beijing China 2022, 41, 2636–2645. [Google Scholar]
- Yang, X.; Ma, J.S.; Zhang, H.; Zhou, Q. Community structure and the water quality during different hydrological periods in Poyang Lake. Acta Hydrobiol. Sin. 2021, 45, 1093–1103. [Google Scholar]
- Wu, Z.; Zhu, C.; Tang, P.; Yang, X.; Wang, H.; Zhang, F. Correlation analysis of phytoplankton community and water quality factors in Chaohu Lake. J. Biol. 2023, 40, 79–84. [Google Scholar]
- Wu, T.; Liu, J.; Deng, J.; Dai, X.; Tang, R.; Peng, K.; Zou, W.; Cai, Y.; Gong, Z. Community structure of phytoplankton and bioassessment of water quality in a large water-carrying lake, Lake Hongze. J. Lake Sci. 2019, 31, 440–448. [Google Scholar]
- Geng, M.M.; Wang, K.L.; Yang, N.; Li, F.; Zou, Y.A.; Chen, X.S.; Deng, Z.M.; Xie, Y.H. Spatiotemporal water quality variations and their relationship with hydrological conditions in Dongting Lake after the operation of the Three Gorges Dam, China. J. Clean Prod. 2021, 283, 124644. [Google Scholar] [CrossRef]
- Guan, X.W.; Zeng, M. Characteristics and enlightenment of low water in Changjiang River Basin in 2022. Yangtze River 2022, 53, 1–5. [Google Scholar]
- Feng, Y.; Zheng, B.H.; Jia, H.F.; Peng, J.Y.; Zhou, X.Y. Influence of social and economic development on water quality in Dongting Lake. Ecol. Indic. 2021, 131, 108220. [Google Scholar] [CrossRef]
- Rodrigues, L.C.; Simoes, N.R.; Bovo-Scomparin, V.M.; Jati, S.; Santana, N.F.; Roberto, M.C.; Train, S. Phytoplankton alpha diversity as an indicator of environmental changes in a neotropical floodplain. Ecol. Indic. 2015, 48, 334–341. [Google Scholar] [CrossRef]
- Connell, J. Diversity in tropical rain forests and coral reefs. High diversity of trees and corals is maintained only in a nonequilibrium state. Science 1978, 199, 1302–1310. [Google Scholar] [CrossRef] [PubMed]
- Marengo, J.A.; Ambrizzi, T.; da Rocha, R.P.; Alves, L.M.; Cuadra, S.V.; Valverde, M.C.; Torres, R.R.; Santos, D.C.; Ferraz, S.E.T. Future change of climate in South America in the late twenty-first century: Intercomparison of scenarios from three regional climate models. Clim. Dyn. 2010, 35, 1089–1113. [Google Scholar] [CrossRef]
- Moss, B.; Kosten, S.; Meerhoff, M.; Battarbee, R.W.; Jeppesen, E.; Mazzeo, N.; Havens, K.; Lacerot, G.; Liu, Z.W.; De Meester, L.; et al. Allied attack: Climate change and eutrophication. Inland Waters 2011, 1, 101–105. [Google Scholar] [CrossRef]
- Jeppesen, E.; Brucet, S.; Naselli-Flores, L.; Papastergiadou, E.; Stefanidis, K.; Noges, T.; Noges, P.; Attayde, J.L.; Zohary, T.; Coppens, J.; et al. Ecological impacts of global warming and water abstraction on lakes and reservoirs due to changes in water level and related changes in salinity. Hydrobiologia 2015, 750, 201–227. [Google Scholar] [CrossRef]
- Bradbury, J.P. Diatom Stratigraphy and Human Settlement in Minnesota; Geological Society of America: Boulder, CO, USA, 1975; Volume 171, pp. 1–74. [Google Scholar]
- Liu, J.F.; Chen, Y.W.; Li, M.J.; Liu, B.G.; Liu, X.; Wu, Z.S.; Cai, Y.J.; Xu, J.Y.; Wang, J.J. Water-level fluctuations are key for phytoplankton taxonomic communities and functional groups in Poyang Lake. Ecol. Indic. 2019, 104, 470–478. [Google Scholar] [CrossRef]
- Yan, X.; Zhang, Y.; Li, Y.; Jiang, Y.; Cui, Z.; Gao, X.; Wu, N.; Nicola, F.; Han, X. Hydrologic and physicochemical factors co-drive seasonal changes of phytoplankton during dynamic water diversion processes in the Danjiangkou Reservoir. J. Lake Sci. 2021, 33, 1350–1363. [Google Scholar]
- Jiang, Z.B.; Liu, J.J.; Chen, J.F.; Chen, Q.Z.; Yan, X.J.; Xuan, J.L.; Zeng, J.N. Responses of summer phytoplankton community to drastic environmental changes in the Changjiang (Yangtze River) estuary during the past 50 years. Water Res. 2014, 54, 1–11. [Google Scholar] [CrossRef]
- Till, A.; Rypel, A.L.; Bray, A.; Fey, S.B. Fish die-offs are concurrent with thermal extremes in north temperate lakes. Nat. Clim. Chang. 2019, 9, 637–641. [Google Scholar] [CrossRef]
- Walter, K.M.; Zimov, S.A.; Chanton, J.P.; Verbyla, D.; Chapin, F.S. Methane bubbling from Siberian thaw lakes as a positive feedback to climate warming. Nature 2006, 443, 71–75. [Google Scholar] [CrossRef] [PubMed]
- de Emiliani, M.O.G. Effects of water level fluctuations on phytoplankton in a river-floodplain lake system (Parana River, Argentina). Hydrobiologia 1997, 357, 1–15. [Google Scholar] [CrossRef]
- Liu, X.; Qian, K.M.; Tan, G.L.; Xing, J.S.; Li, M.; Chen, Y.W. Phytoplankton Community Structure and Its Succession in Isolated Lakes of Poyang-Junshan Lake. Chin. J. Environ. Sci. 2014, 35, 2557–2564. [Google Scholar]
- Wang, S.Y.; Gao, Y.; Jia, J.J.; Kun, S.D.; Lyu, S.X.; Li, Z.X.; Lu, Y.; Wen, X.F. Water level as the key controlling regulator associated with nutrient and gross primary productivity changes in a large floodplain-lake system (Lake Poyang), China. J. Hydrol. 2021, 599, 126414. [Google Scholar]
- Istvanovics, V.; Honti, M.; Voros, L.; Kozma, Z. Phytoplankton dynamics in relation to connectivity, flow dynamics and resource availability-the case of a large, lowland river, the Hungarian Tisza. Hydrobiologia 2010, 637, 121–141. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, X.; Tian, S.; Cao, Y.; Chen, R. Study on phytoplankton community structure characteristics and its influencing factors of lakes in arid regions of the Yellow River basin during ice-sealing period. J. Hydraul. Eng. 2020, 51, 1070–1079. [Google Scholar]
- Liu, X.; Li, Y.A.; Liu, B.G.; Qian, K.M.; Chen, Y.W.; Gao, J.F. Cyanobacteria in the complex river-connected Poyang Lake: Horizontal distribution and transport. Hydrobiologia 2016, 768, 95–110. [Google Scholar] [CrossRef]

| Classification | Shannon–Wiener Index (H) | Jaccard Similarity Index (J) | ||
|---|---|---|---|---|
| Range | Description | Range | Description | |
| I | 0–1 | Heavy pollution | 0 | No similar |
| II | 1–2 | α-Medium pollution | 0.01–0.25 | Highly dissimilar |
| III | 2–3 | β-Medium pollution | 0.26–0.50 | Slightly similar |
| IV | >3 | Light or no pollution | 0.51–0.75 | Moderate similarity |
| V | 0.76–0.99 | Highly similar | ||
| VI | 1 | Completely similar | ||
| Phyla | Code | Species | SK | SS | QJ | WDL | SDL | EDL | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| August | September | August | September | August | September | August | September | August | September | August | September | |||
| Bacillariophyta | A1 | Synedra sp. | 0.115 | - | 0.107 | - | 0.147 | - | - | - | 0.097 | - | 0.243 | - |
| A2 | Navicula sp. | 0.049 | - | 0.022 | - | 0.047 | - | - | - | 0.060 | - | - | - | |
| A3 | Nitzschia sp. | 0.130 | 0.022 | 0.049 | - | 0.119 | - | 0.106 | - | 0.026 | - | 0.104 | - | |
| A4 | Melosira granulata | 0.060 | - | 0.241 | 0.027 | 0.057 | - | 0.344 | 0.034 | 0.199 | 0.131 | - | 0.025 | |
| A5 | Melosira granulata var. angutissima | 0.167 | 0.252 | 0.191 | - | - | 0.036 | 0.199 | 0.471 | 0.043 | - | - | 0.059 | |
| A6 | Cyclotella sp. | 0.126 | 0.046 | - | 0.027 | - | 0.051 | 0.026 | - | - | - | - | 0.027 | |
| Chlorophyta | B1 | Chlorella sp. | - | - | - | - | 0.022 | - | - | - | - | - | - | - |
| B2 | Ankistrodesmus acicularis | - | - | - | - | 0.022 | - | - | - | - | - | - | - | |
| B3 | Ulothrix sp. | - | - | - | - | - | - | - | 0.024 | - | - | - | - | |
| B4 | Scenedesmus quadricauda | - | - | - | - | 0.035 | 0.116 | - | - | - | - | - | - | |
| B5 | Scenedesmus bicaudatus | - | - | - | - | - | 0.029 | - | - | - | - | - | - | |
| B6 | Crucigenia tetrapedia | - | - | - | - | - | 0.029 | - | - | - | - | - | - | |
| B7 | Crucigenia rectangularis | - | - | - | - | - | 0.029 | - | - | - | - | - | - | |
| B8 | Eudorina sp. | - | - | - | 0.053 | - | 0.029 | - | - | - | - | - | - | |
| B9 | Pediastrum simplex | - | - | - | 0.023 | - | 0.029 | - | - | - | - | - | - | |
| Cyanophyta | C1 | Pseudanabaena sp. | 0.037 | - | 0.024 | - | 0.041 | - | 0.087 | - | 0.035 | 0.034 | - | - |
| C2 | Raphidiopsis sp. | - | - | - | - | 0.029 | - | - | - | - | - | - | - | |
| C3 | Planktothricoides | - | - | - | - | 0.035 | - | - | - | 0.053 | - | 0.049 | - | |
| C4 | Phormidium tenue | - | 0.052 | - | - | - | - | - | - | - | - | - | 0.064 | |
| C5 | Microcystis sp. | - | - | - | 0.146 | - | 0.027 | - | - | - | - | - | - | |
| C6 | Chroococcus limneticus | - | - | - | - | - | 0.051 | - | - | - | - | - | - | |
| C7 | Merismopedia punctata | - | - | - | - | - | 0.058 | - | - | - | - | - | - | |
| C8 | Merismopedia tenuissima | - | - | - | - | - | - | - | - | - | 0.105 | - | 0.092 | |
| Month (Hydrological Period) | Space | SK | SS | QJ | WDL | SDL | EDL |
|---|---|---|---|---|---|---|---|
| August (water connected) | SK | 1.00 | 0.38 | 0.42 | 0.49 | 0.37 | 0.48 |
| SS | III | 1.00 | 0.40 | 0.41 | 0.35 | 0.38 | |
| QJ | III | III | 1.00 | 0.31 | 0.42 | 0.59 | |
| WDL | IV | III | 0.31 | 1.00 | 0.40 | 0.30 | |
| SDL | III | III | III | 0.40 | 1.00 | 0.27 | |
| EDL | III | III | IV | III | III | 1.00 | |
| September (water disconnected) | SK | 1.00 | 0.11 | 0.27 | 0.18 | 0.19 | 0.24 |
| SS | II | 1.00 | 0.25 | 0.16 | 0.15 | 0.20 | |
| QJ | III | II | 1.00 | 0.17 | 0.20 | 0.34 | |
| WDL | II | II | II | 1.00 | 0.22 | 0.24 | |
| SDL | II | II | II | 0.22 | 1.00 | 0.24 | |
| EDL | II | II | III | II | II | 1.00 |
| Lake | Month | Abundance (×104 cells/L) | Shannon–Wiener Index | Eutrophication Levels (TLI Index) | Reference |
|---|---|---|---|---|---|
| DTL | August (2022a) | 122.06 | 3.30 | Light eutrophic (53.85) | This study |
| September (2022a) | 351.18 | 2.12 | Mesotrophic (43.09) | This study | |
| SK | August (2017a) | 100.9 | 1.58 | Light–middle eutrophic (50–63.66) | [49] |
| SS | 291.8 | 2.39 | |||
| DTL | 576.6 | 1.86 | |||
| DTL | September (2019a) | 31.10 | 2.81 | Mesotrophic (46.48) | [40] |
| DTL | 1991a–2020a | 10.0–95.7 | - | Mesotrophic–light eutrophic (41.09–51.68) | [50] |
| Poyang | August (2017a) | 1630–5756 | 3.95 | - | [51] |
| Chaohu | October (2020a) | 239 | 3.12 | Light eutrophic (57.3) | [52] |
| Hongze | 2015a–2016a | 5350 | - | Light eutrophic (58.07) | [53] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, G.; Yin, X.; Wang, X.; Zhang, Y.; Wang, E.; Yu, Z.; Ma, X.; Huang, M. Effects of Summer and Autumn Drought on Eutrophication and the Phytoplankton Community in Dongting Lake in 2022. Toxics 2023, 11, 822. https://doi.org/10.3390/toxics11100822
Yan G, Yin X, Wang X, Zhang Y, Wang E, Yu Z, Ma X, Huang M. Effects of Summer and Autumn Drought on Eutrophication and the Phytoplankton Community in Dongting Lake in 2022. Toxics. 2023; 11(10):822. https://doi.org/10.3390/toxics11100822
Chicago/Turabian StyleYan, Guanghan, Xueyan Yin, Xing Wang, Yunyu Zhang, Enrui Wang, Zhibing Yu, Xingliang Ma, and Minsheng Huang. 2023. "Effects of Summer and Autumn Drought on Eutrophication and the Phytoplankton Community in Dongting Lake in 2022" Toxics 11, no. 10: 822. https://doi.org/10.3390/toxics11100822
APA StyleYan, G., Yin, X., Wang, X., Zhang, Y., Wang, E., Yu, Z., Ma, X., & Huang, M. (2023). Effects of Summer and Autumn Drought on Eutrophication and the Phytoplankton Community in Dongting Lake in 2022. Toxics, 11(10), 822. https://doi.org/10.3390/toxics11100822






