Preparation and Density Functional Theory Studies of Aluminosilicate-Based Ceramic Solidified Products for Sr Immobilization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Solidified Products
2.3. Characterization
2.4. DFT Calculation
3. Results and Discussion
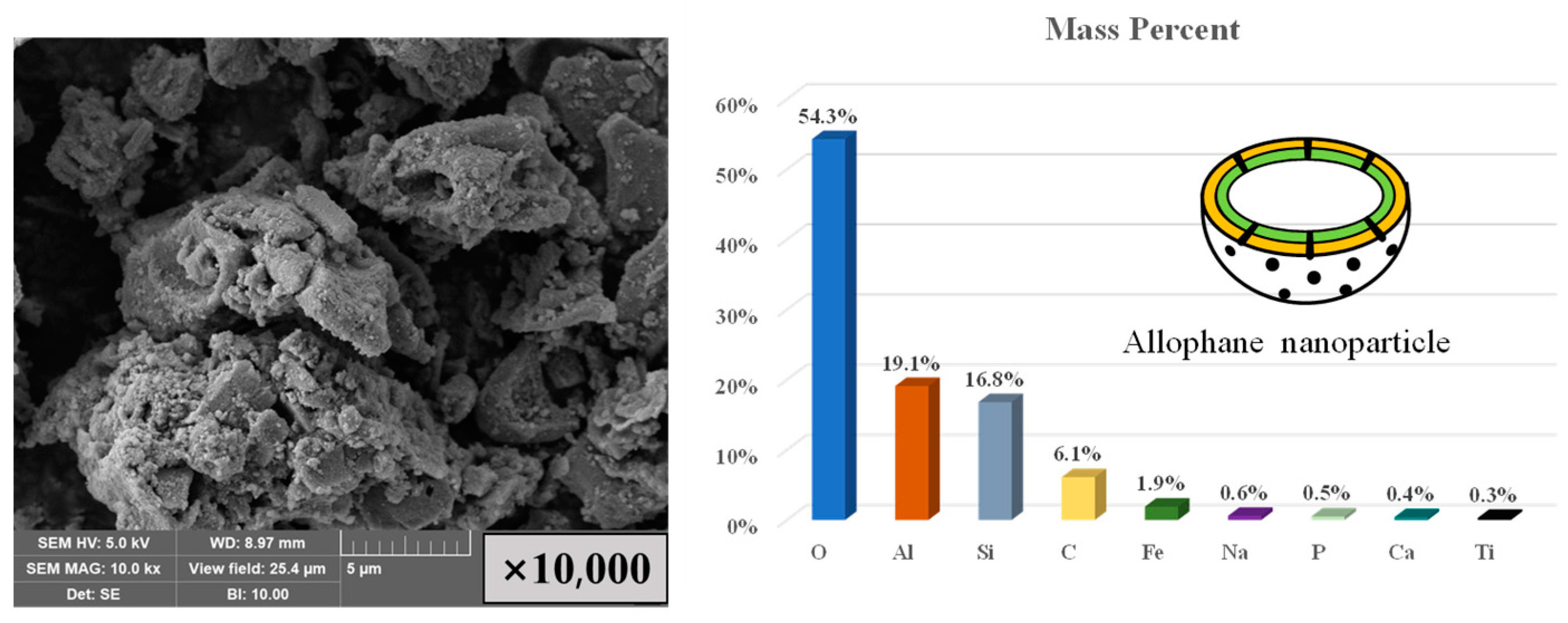
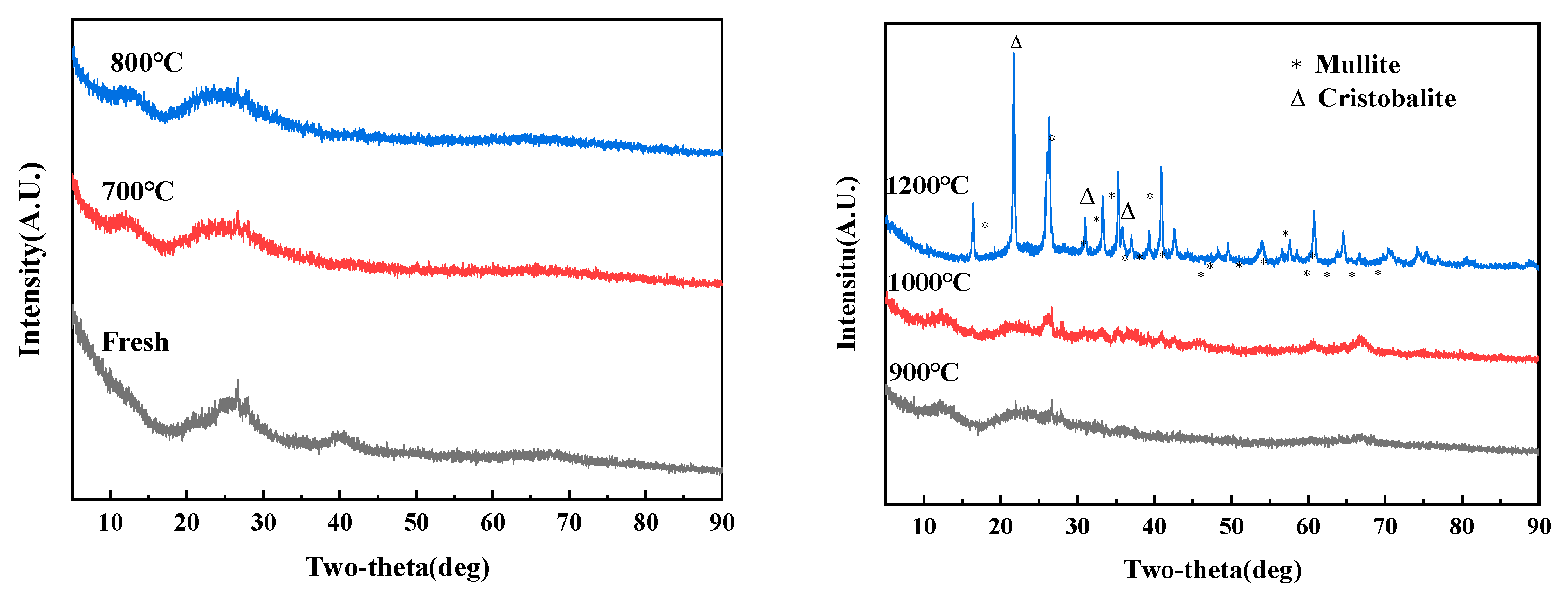
3.1. Characterization and Self-Sintering Behavior of Allophane
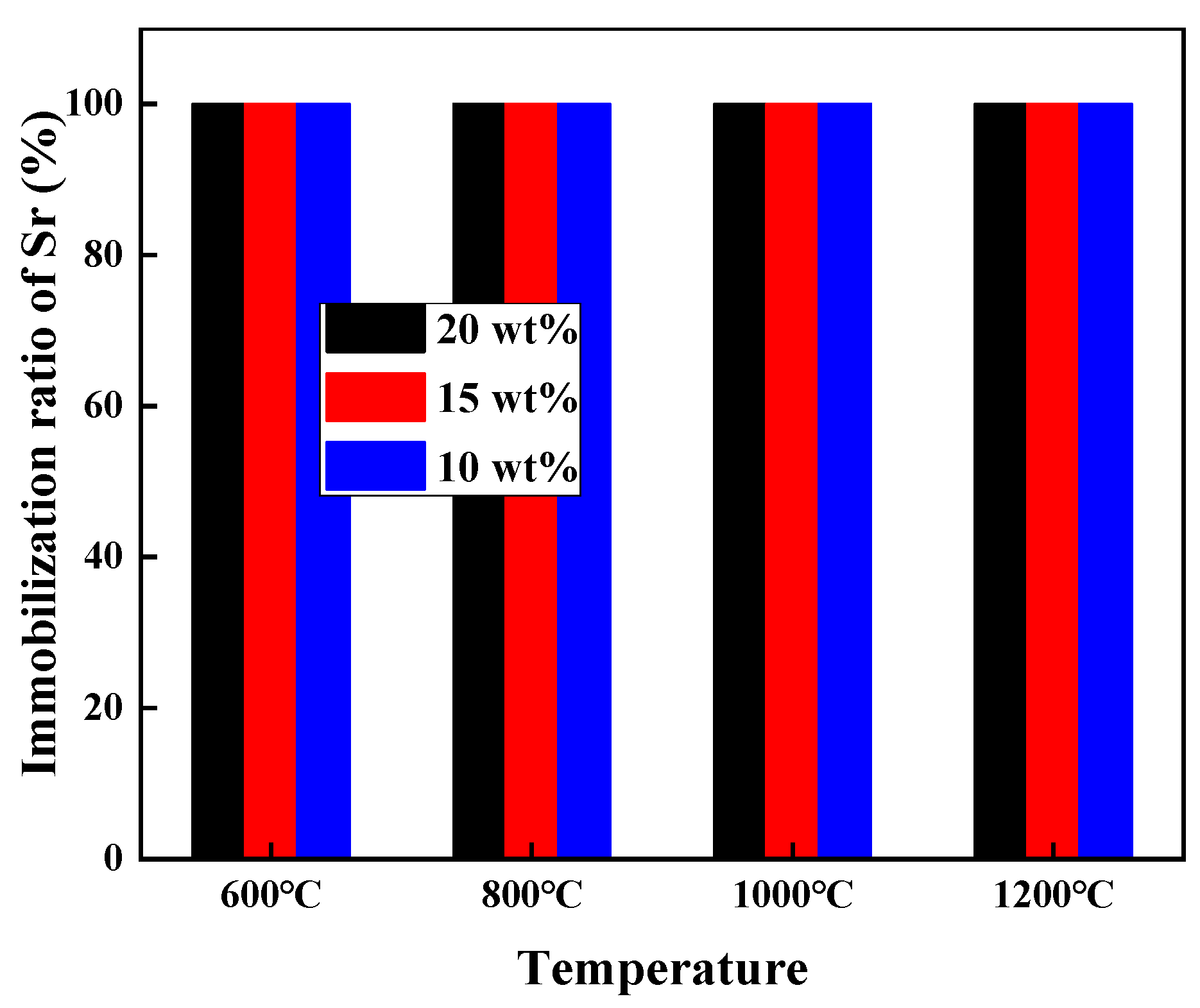
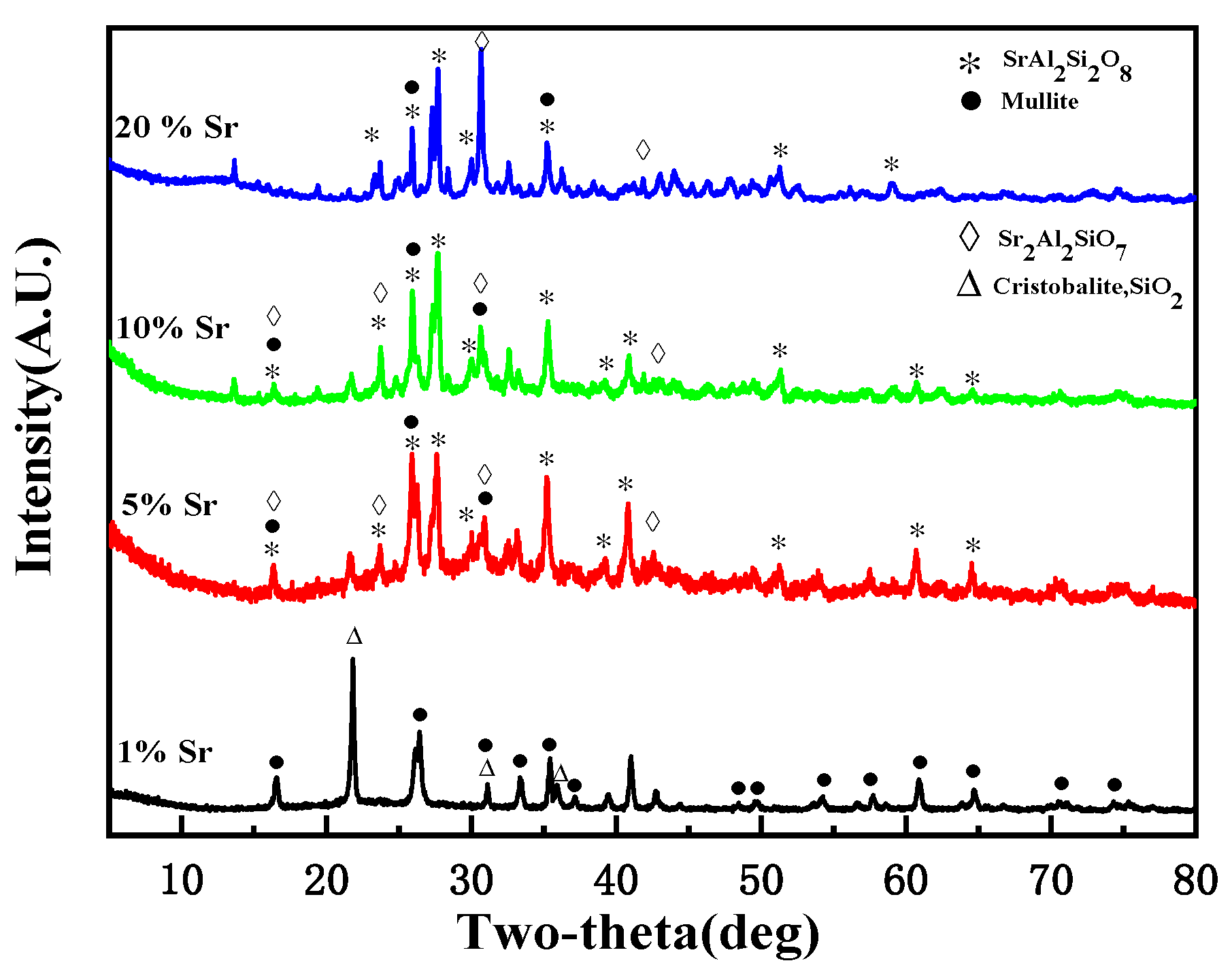
3.2. Sr Immobilization Ratio and Solidification
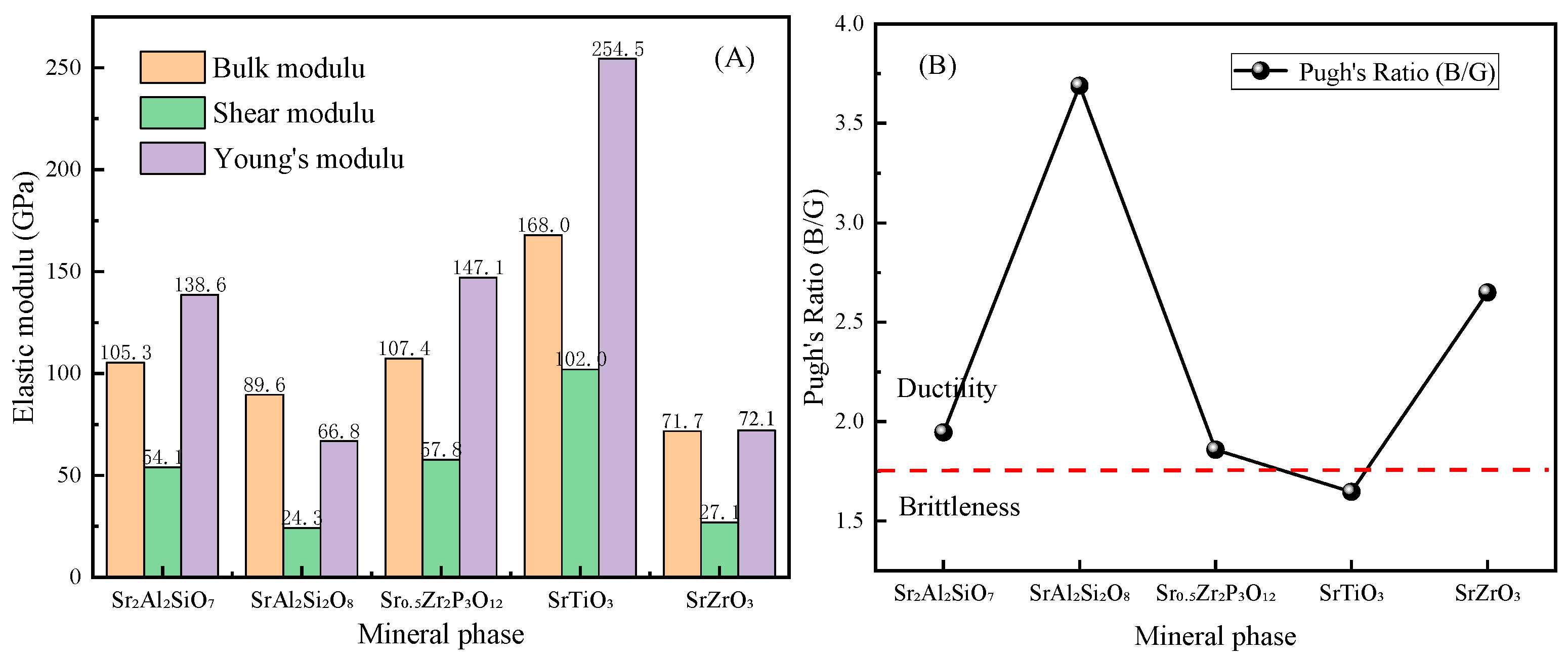
3.3. Calculation of Elastic Properties of Solidified Product
3.4. Simulation and Calculation of Sr/Ca Mixed-Crystal Structures
3.5. Analysis of Electron Localization Function (ELF)
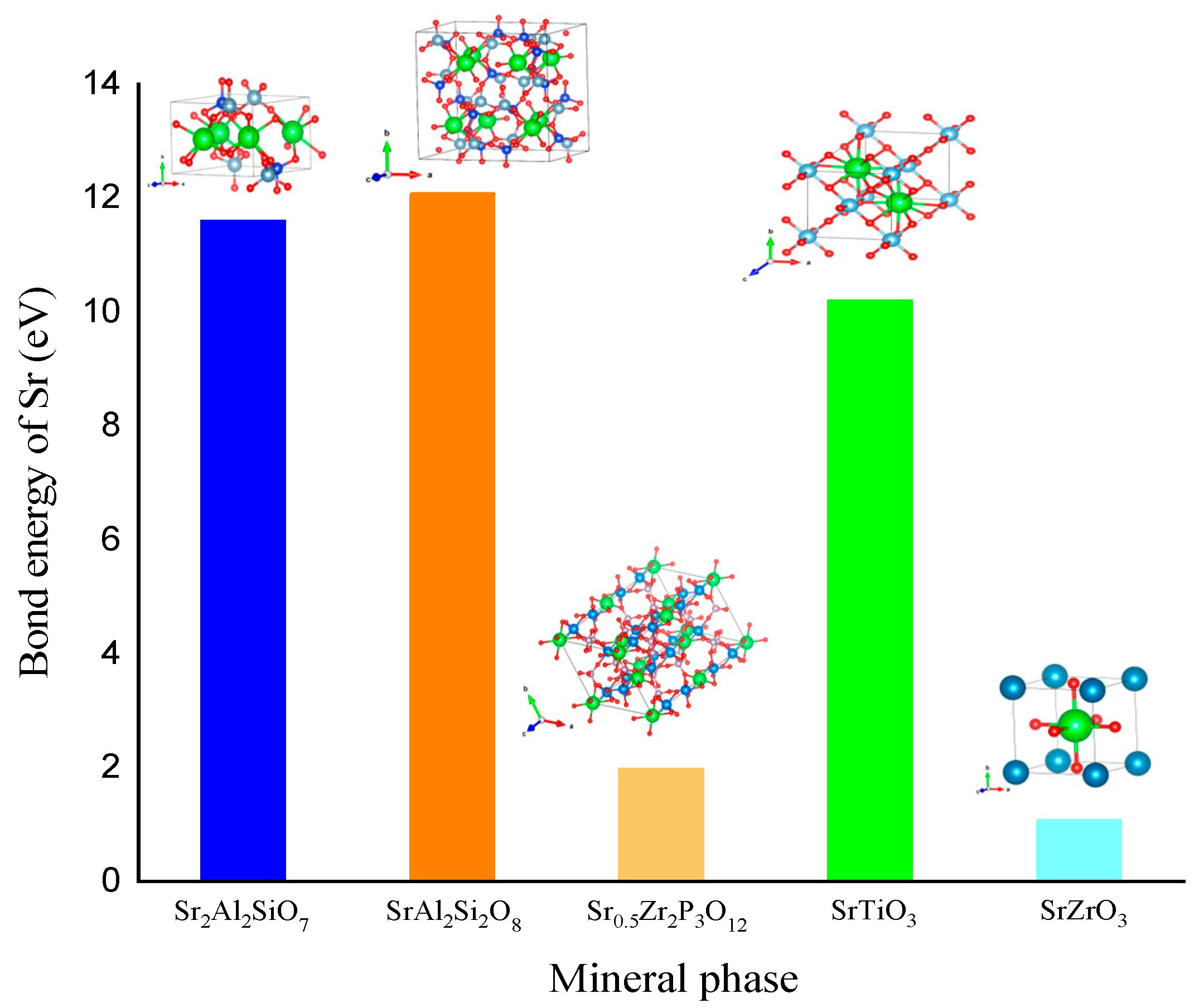
3.6. Comparison of Different Sr-Containing Ceramic Solidified Products
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shiner, M.E.; Klein-BenDavid, O.; L’Hôpital, E.; Dauzères, A.; Neji, M.; Teutsch, N.; Peled, A.; Bar-Nes, G. Retention of strontium in high- & low-pH cementitious matrices—OPC vs. model systems. Cem. Concr. Res. 2022, 152, 106659. [Google Scholar] [CrossRef]
- Wang, Y.; Wen, Y.; Mao, C.; Sang, H.; Wu, Y.; Li, H.; Wei, Y. Development of chromatographic process for the dynamic separation of 90Sr from high level liquid waste through breakthrough curve simulation and thermal analysis. Sep. Purif. Technol. 2022, 282, 120103. [Google Scholar] [CrossRef]
- Wu, Y.; Kim, S.-Y.; Tozawa, D.; Ito, T.; Tada, T.; Hitomi, K.; Kuraoka, E.; Yamazaki, H.; Ishii, K. Equilibrium and kinetic studies of selective adsorption and separation for strontium using DtBuCH18C6 loaded resin. J. Nucl. Sci. Technol. 2012, 49, 320–327. [Google Scholar] [CrossRef]
- Schmidt, B.; Kegler, F.; Steinhauser, G.; Chyzhevskyi, I.; Dubchak, S.; Ivesic, C.; Koller-Peroutka, M.; Laarouchi, A.; Adlassnig, W. Uptake of Radionuclides by Bryophytes in the Chornobyl Exclusion Zone. Toxics 2023, 11, 218. [Google Scholar] [CrossRef] [PubMed]
- Froidevaux, P.; Pittet, P.-A.; Bühlmann, D.; Bochud, F.; Straub, M. Ion-imprinted resin for use in an automated solid phase extraction system for determining 90Sr in environmental and human samples. J. Radioanal. Nucl. Chem. 2021, 330, 797–804. [Google Scholar] [CrossRef]
- Pant, A.D.; Ruhela, R.; Tomar, B.S.; Anilkumar, S. Determination of 90Sr in environmental samples using solid phase extraction chromatography. J. Radioanal. Nucl. Chem. 2019, 322, 49–55. [Google Scholar] [CrossRef]
- Prekajski Đorđević, M.; Maletaškić, J.; Stanković, N.; Babić, B.; Yoshida, K.; Yano, T.; Matović, B. In-situ immobilization of Sr radioactive isotope using nanocrystalline hydroxyapatite. Ceram. Int. 2018, 44, 1771–1777. [Google Scholar] [CrossRef]
- Tian, Q.; Sasaki, K. Application of fly ash-based materials for stabilization/solidification of cesium and strontium. Environ. Sci. Pollut. Res. 2019, 26, 23542–23554. [Google Scholar] [CrossRef]
- Lin, S.-L.; Lai, J.S.; Chian, E.S.K. Modifications of sulfur polymer cement (SPC) stabilization and solidification (S/S) process. Waste Manag. 1995, 15, 441–447. [Google Scholar] [CrossRef]
- Kuenzel, C.; Cisneros, J.F.; Neville, T.P.; Vandeperre, L.J.; Simons, S.J.R.; Bensted, J.; Cheeseman, C.R. Encapsulation of Cs/Sr contaminated clinoptilolite in geopolymers produced from metakaolin. J. Nucl. Mater. 2015, 466, 94–99. [Google Scholar] [CrossRef]
- Tian, Q.; Sasaki, K. Application of fly ash-based geopolymer for removal of cesium, strontium and arsenate from aqueous solutions: Kinetic, equilibrium and mechanism analysis. Water Sci. Technol. 2019, 79, 2116–2125. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Jiang, Z.; Wu, D.; Peng, X.; Xu, Y.; Li, N.; Qi, Y.; Li, P. Immobilization of strontium-loaded zeolite A by metakaolin based-geopolymer. Ceram. Int. 2017, 43, 4434–4439. [Google Scholar] [CrossRef]
- Abdelrahman, E.A.; Abou El-Reash, Y.G.; Youssef, H.M.; Kotp, Y.H.; Hegazey, R.M. Utilization of rice husk and waste aluminum cans for the synthesis of some nanosized zeolite, zeolite/zeolite, and geopolymer/zeolite products for the efficient removal of Co(II), Cu(II), and Zn(II) ions from aqueous media. J. Hazard. Mater. 2021, 401, 123813. [Google Scholar] [CrossRef]
- Wang, L.; Geddes, D.A.; Walkley, B.; Provis, J.L.; Mechtcherine, V.; Tsang, D.C.W. The role of zinc in metakaolin-based geopolymers. Cem. Concr. Res. 2020, 136, 106194. [Google Scholar] [CrossRef]
- Tian, Q.; Pan, Y.; Bai, Y.; Sasaki, K. Immobilization of strontium in geopolymers activated by different concentrations of sodium silicate solutions. Environ. Sci. Pollut. Res. 2022, 29, 24298–24308. [Google Scholar] [CrossRef]
- Tian, Q.; Sasaki, K. Structural characterizations of fly ash-based geopolymer after adsorption of various metal ions. Environ. Technol. 2021, 42, 941–951. [Google Scholar] [CrossRef]
- Sang, H.; Mao, C.; Ming, F.; Xu, L.; Wei, Y.; Wu, Y. Selective separation and immobilization process of 137Cs from high-level liquid waste based on silicon-based heteropoly salt and natural minerals. Chem. Eng. J. 2022, 449, 137842. [Google Scholar] [CrossRef]
- Ma, J.; Fang, Z.; Yang, X.; Wang, B.; Luo, F.; Zhao, X.; Wang, X.; Yang, Y. Investigating hollandite–perovskite composite ceramics as a potential waste form for immobilization of radioactive cesium and strontium. J. Mater. Sci. 2021, 56, 9644–9654. [Google Scholar] [CrossRef]
- Keskar, M.; Patkare, G.; Shafeeq, M.; Phatak, R.A.; Kannan, S. Structural and thermal study of Sr(Th1−xUx)(PO4)2 compounds. J. Solid State Chem. 2021, 300, 122228. [Google Scholar] [CrossRef]
- Yang, J.; Shu, X.; Luo, F.; Wang, L.; Gu, Y.; Wu, J.; Lu, X. Solubility of Sr2+ in the Gd2Zr2O7 ceramics via appropriate occupation designs. J. Alloys Compd. 2019, 808, 151563. [Google Scholar] [CrossRef]
- Mao, X.; Li, Z.; Yi, F.; Wei, L.; Zhou, Y. Chemical durability of strontium-contaminated soil vitrified by microwave sintering. J. Radioanal. Nucl. Chem. 2023, 332, 435–445. [Google Scholar] [CrossRef]
- Papynov, E.K.; Shichalin, O.O.; Buravlev, I.Y.; Belov, A.A.; Portnyagin, A.S.; Fedorets, A.N.; Azarova, Y.A.; Tananaev, I.G.; Sergienko, V.I. Spark plasma sintering-reactive synthesis of SrWO4 ceramic matrices for 90Sr immobilization. Vacuum 2020, 180, 109628. [Google Scholar] [CrossRef]
- Papynov, E.K.; Belov, A.A.; Shichalin, O.O.; Buravlev, I.Y.; Azon, S.A.; Golub, A.V.; Gerasimenko, A.V.; Parotkina, Y.A.; Zavjalov, A.P.; Tananaev, I.G.; et al. SrAl2Si2O8 ceramic matrices for 90Sr immobilization obtained via spark plasma sintering-reactive synthesis. Nucl. Eng. Technol. 2021, 53, 2289–2294. [Google Scholar] [CrossRef]
- Papynov, E.K.; Belov, A.A.; Shichalin, O.O.; Buravlev, I.Y.; Azon, S.A.; Gridasova, E.A.; Parotkina, Y.A.; Yagofarov, V.Y.; Drankov, A.N.; Golub, A.V.; et al. Synthesis of Perovskite-Like SrTiO3 Ceramics for Radioactive Strontium Immobilization by Spark Plasma Sintering-Reactive Synthesis. Russ. J. Inorg. Chem. 2021, 66, 645–653. [Google Scholar] [CrossRef]
- Baldermann, A.; Grießbacher, A.C.; Baldermann, C.; Purgstaller, B.; Letofsky-Papst, I.; Kaufhold, S.; Dietzel, M. Removal of Barium, Cobalt, Strontium, and Zinc from Solution by Natural and Synthetic Allophane Adsorbents. Geosciences 2018, 8, 309. [Google Scholar] [CrossRef]
- Wu, Y.; Lee, C.-P.; Mimura, H.; Zhang, X.; Wei, Y. Stable solidification of silica-based ammonium molybdophosphate by allophane: Application to treatment of radioactive cesium in secondary solid wastes generated from fukushima. J. Hazard. Mater. 2018, 341, 46–54. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, Y.; Sang, H.; Wu, Y.; Wei, Y. Study on the immobilization of cesium absorbed by copper ferrocyanide using allophane through pressing/sintering method. J. Nucl. Mater. 2020, 532, 152008. [Google Scholar] [CrossRef]
- Xu, M.; Wu, Y.; Wei, Y. Stable solidification of silica-based ammonium molybdophosphate absorbing cesium using allophane: Mechenical property and leaching studies. J. Radioanal. Nucl. Chem. 2018, 316, 1313–1321. [Google Scholar] [CrossRef]
- Dinsdale, A.; Fang, C.; Que, Z.; Fan, Z. Understanding the Thermodynamics and Crystal Structure of Complex Fe Containing Intermetallic Phases Formed on Solidification of Aluminium Alloys. JOM 2019, 71, 1731–1736. [Google Scholar] [CrossRef]
- Li, P.; Zhao, F.; Xiao, H.; Zhang, H.; Gong, H.; Zhang, S.; Liu, Z.; Zu, X. First-Principles Study of Thermo-Physical Properties of Pu-Containing Gd2Zr2O7. Nanomaterials 2019, 9, 196. [Google Scholar] [CrossRef]
- Zhao, F.A.; Xiao, H.Y.; Liu, Z.J.; Li, S.; Zu, X.T. A DFT study of mechanical properties, thermal conductivity and electronic structures of Th-doped Gd2Zr2O7. Acta Mater. 2016, 121, 299–309. [Google Scholar] [CrossRef]
- Zhao, F.A.; Xiao, H.Y.; Jiang, M.; Liu, Z.J.; Zu, X.T. A DFT+U study of Pu immobilization in Gd2Zr2O7. J. Nucl. Mater. 2015, 467, 937–948. [Google Scholar] [CrossRef]
- Sun, Q.; Liu, C.; Fan, T.; Cheng, H.; Cui, P.; Gu, X.; Chen, L.; Ata-Ul-Karim, S.T.; Zhou, D.; Wang, Y. A molecular level understanding of antimony immobilization mechanism on goethite by the combination of X-ray absorption spectroscopy and density functional theory calculations. Sci. Total Environ. 2023, 865, 161294. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Duan, H.; Wei, D.; Cui, B.; Wang, X. Stability of diethyl dithiocarbamate chelates with Cu(II), Zn(II) and Mn(II). J. Mol. Struct. 2019, 1184, 375–381. [Google Scholar] [CrossRef]
- Pfrommer, B.G.; Côté, M.; Louie, S.G.; Cohen, M.L. Relaxation of Crystals with the Quasi-Newton Method. J. Comput. Phys. 1997, 131, 233–240. [Google Scholar] [CrossRef]
- Liao, M.; Liu, Y.; Min, L.; Lai, Z.; Han, T.; Yang, D.; Zhu, J. Alloying effect on phase stability, elastic and thermodynamic properties of Nb-Ti-V-Zr high entropy alloy. Intermetallics 2018, 101, 152–164. [Google Scholar] [CrossRef]
- Liu, K.-C.; Thomas, G.; Caballero, A.; Moya, J.S.; de Aza, S. Time-Temperature-Transformation Curves for Kaolinite-α-Alumina. J. Am. Ceram. Soc. 1994, 77, 1545–1552. [Google Scholar] [CrossRef]
- Han, L.-F.; Xu, Z.-L.; Cao, Y.; Wei, Y.-M.; Xu, H.-T. Preparation, characterization and permeation property of Al2O3, Al2O3–SiO2 and Al2O3–kaolin hollow fiber membranes. J. Membr. Sci. 2011, 372, 154–164. [Google Scholar] [CrossRef]
- Ravindran, P.; Fast, L.; Korzhavyi, P.A.; Johansson, B.; Wills, J.; Eriksson, O. Density functional theory for calculation of elastic properties of orthorhombic crystals: Application to TiSi2. J. Appl. Phys. 1998, 84, 4891–4904. [Google Scholar] [CrossRef]
- Dobson, P.J. Physical Properties of Crystals—Their Representation by Tensors and Matrices. Phys. Bull. 1985, 36, 506. [Google Scholar] [CrossRef]
- Li, H.; Sang, H.; Mao, C.; Wang, Y.; Xu, L.; Wu, Y. Study on stable solidification of silica-based ammonium molybdophosphate adsorbing cesium: Micromechanics and density functional theory modeling. J. Nucl. Mater. 2022, 560, 153501. [Google Scholar] [CrossRef]
- Koumpouras, K.; Larsson, J.A. Distinguishing between chemical bonding and physical binding using electron localization function (ELF). J. Phys. Condens. Matter 2020, 32, 315502. [Google Scholar] [CrossRef] [PubMed]

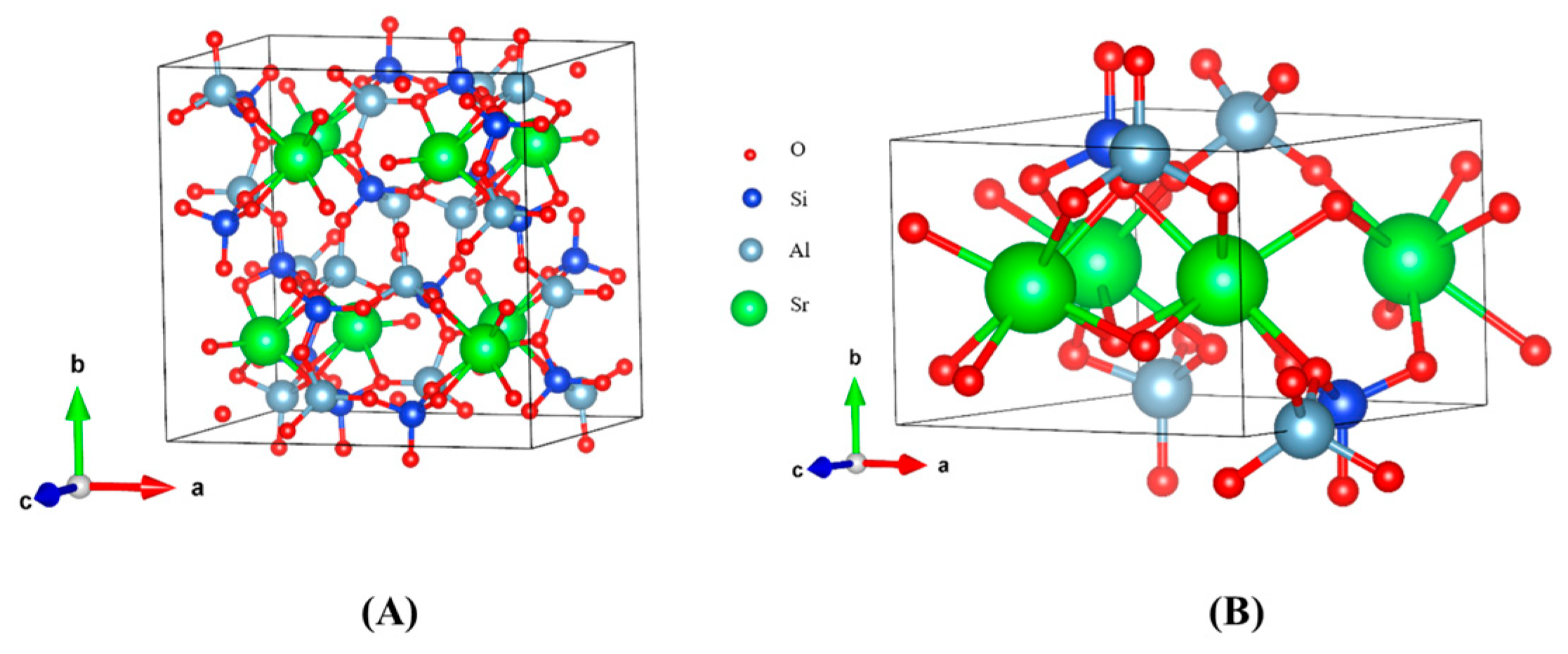

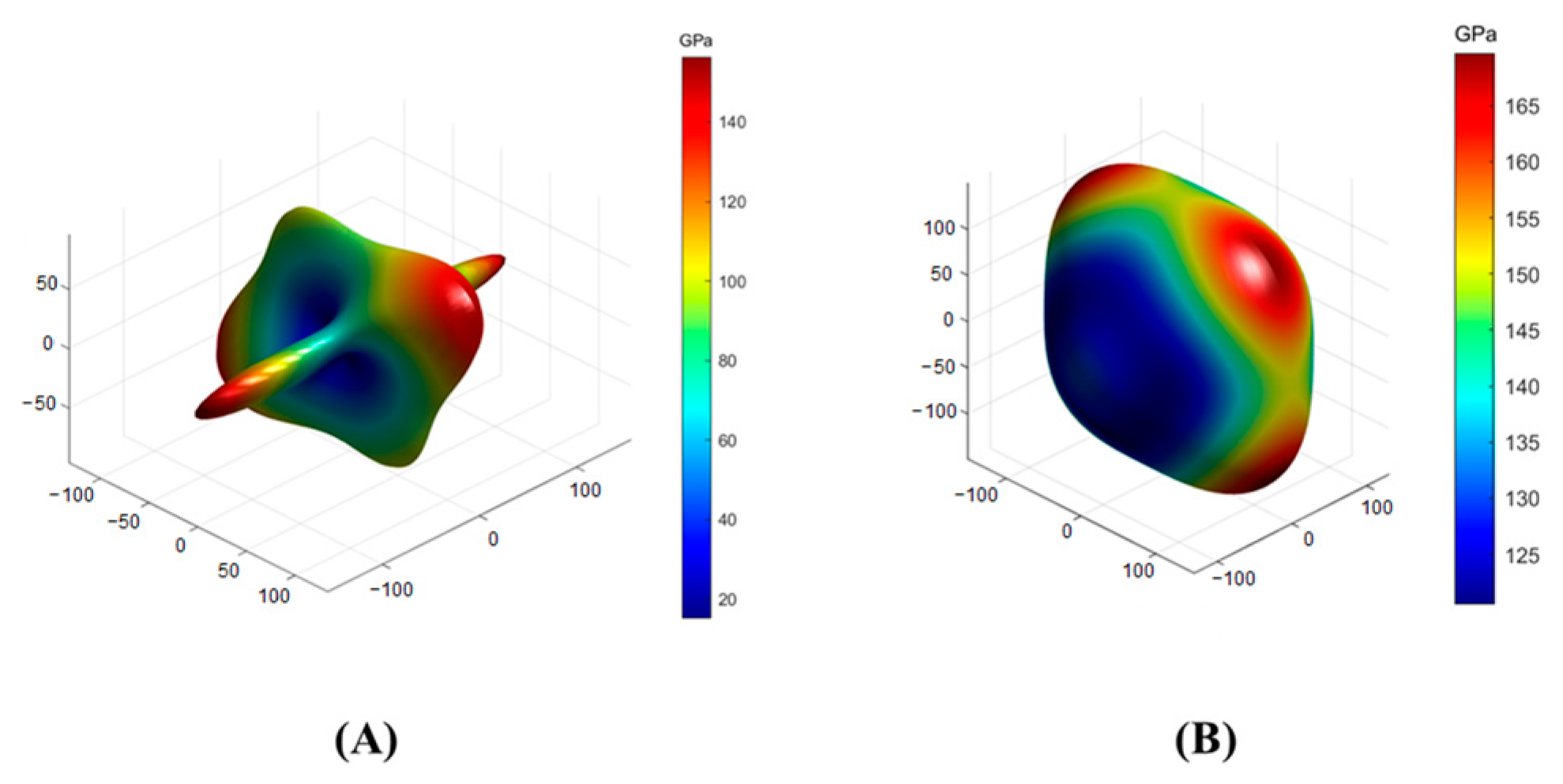
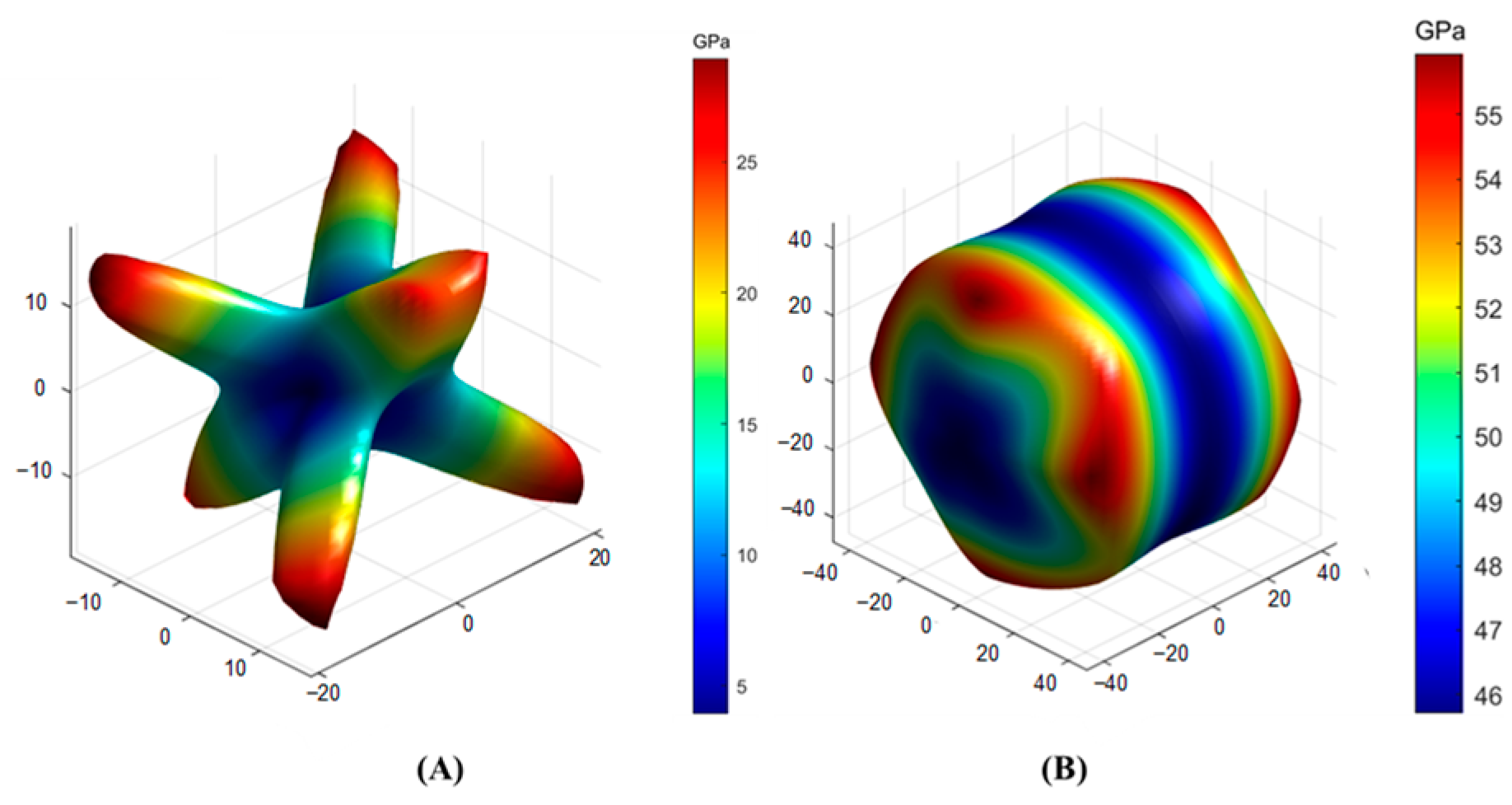




| Crystal | Crystal System | Space Group | Lattice Parameter | |||||
|---|---|---|---|---|---|---|---|---|
| a (Å) | b (Å) | c (Å) | α | β | γ | |||
| SrAl2Si2O8 | Monoclinic | C2/c | 9.385 | 9.385 | 9.650 | 81.056° | 81.056° | 74.481° |
| Sr2Al2SiO7 | Triclinic | P1 | 7.837 | 5.264 | 7.848 | 90.231° | 90.000° | 90.000° |
| Crystal | Stiffness Tensor Cij/GPa | ||||||
|---|---|---|---|---|---|---|---|
| SrAl2Si2O8 | C11 | C12 | C13 | C15 | C22 | C23 | C25 |
| 154.742 | 43.414 | 72.793 | 13.905 | 187.346 | 52.2 | −16.89 | |
| C33 | C35 | C44 | C46 | C55 | C66 | ||
| 129.94 | −0.01 | 14.429 | 9.931 | 45.872 | 13.305 | ||
| Sr2Al2SiO7 | C11 | C12 | C13 | C14 | C15 | C16 | C22 |
| 193.271 | 63.599 | 78.3 | 1.878 | 0 | 0 | 160.477 | |
| C23 | C24 | C25 | C26 | C33 | C34 | C35 | |
| 62.674 | 0.001 | 0 | 0 | 191.154 | −1.141 | 0 | |
| C36 | C44 | C45 | C46 | C55 | C56 | C66 | |
| 0 | 46.079 | 0 | 0 | 68.894 | −0.546 | 45.721 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Sang, H.; Zheng, J.; Yang, S.; Gu, Z.; Wu, H.; Wei, Y. Preparation and Density Functional Theory Studies of Aluminosilicate-Based Ceramic Solidified Products for Sr Immobilization. Toxics 2023, 11, 850. https://doi.org/10.3390/toxics11100850
Wu Y, Sang H, Zheng J, Yang S, Gu Z, Wu H, Wei Y. Preparation and Density Functional Theory Studies of Aluminosilicate-Based Ceramic Solidified Products for Sr Immobilization. Toxics. 2023; 11(10):850. https://doi.org/10.3390/toxics11100850
Chicago/Turabian StyleWu, Yan, Hongji Sang, Jiawei Zheng, Shuyi Yang, Zhengcheng Gu, Hao Wu, and Yuezhou Wei. 2023. "Preparation and Density Functional Theory Studies of Aluminosilicate-Based Ceramic Solidified Products for Sr Immobilization" Toxics 11, no. 10: 850. https://doi.org/10.3390/toxics11100850








