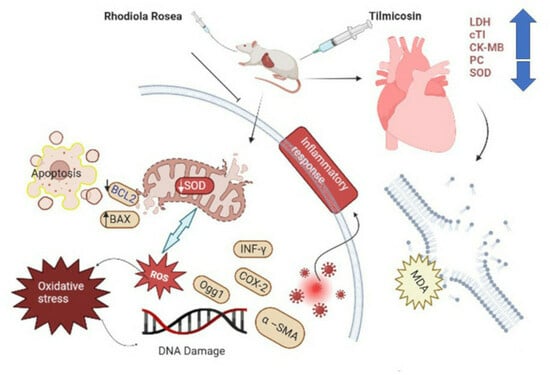
Exploration of Tilmicosin Cardiotoxicity in Rats and the Protecting Role of the Rhodiola rosea Extract: Potential Roles of Cytokines, Antioxidant, Apoptotic, and Anti-Fibrotic Pathways
Abstract
:1. Introduction
2. Materials and Methods
2.1. Drugs and Chemicals
2.2. Gas Chromatography–Mass Spectrometry (GC-MS) Analysis of Rhodiola rosea Extract
2.3. Animal Grouping and Treatment
2.4. Assessment of Body Weight and Cardiac Injury Biomarkers
2.5. Inflammatory and Anti-Inflammatory Cytokine Assessments
2.6. Oxidative Stress Biomarker (ROS, PC, MDA, TAC, 8-OHdG, and OGG1) Assessments in Cardiac Tissue Homogenate
2.7. qRT-PCR Analysis
2.8. Cardiac Histopathology and Immunohistochemistry
2.9. Western Blotting
2.10. Statistical Analysis
3. Results
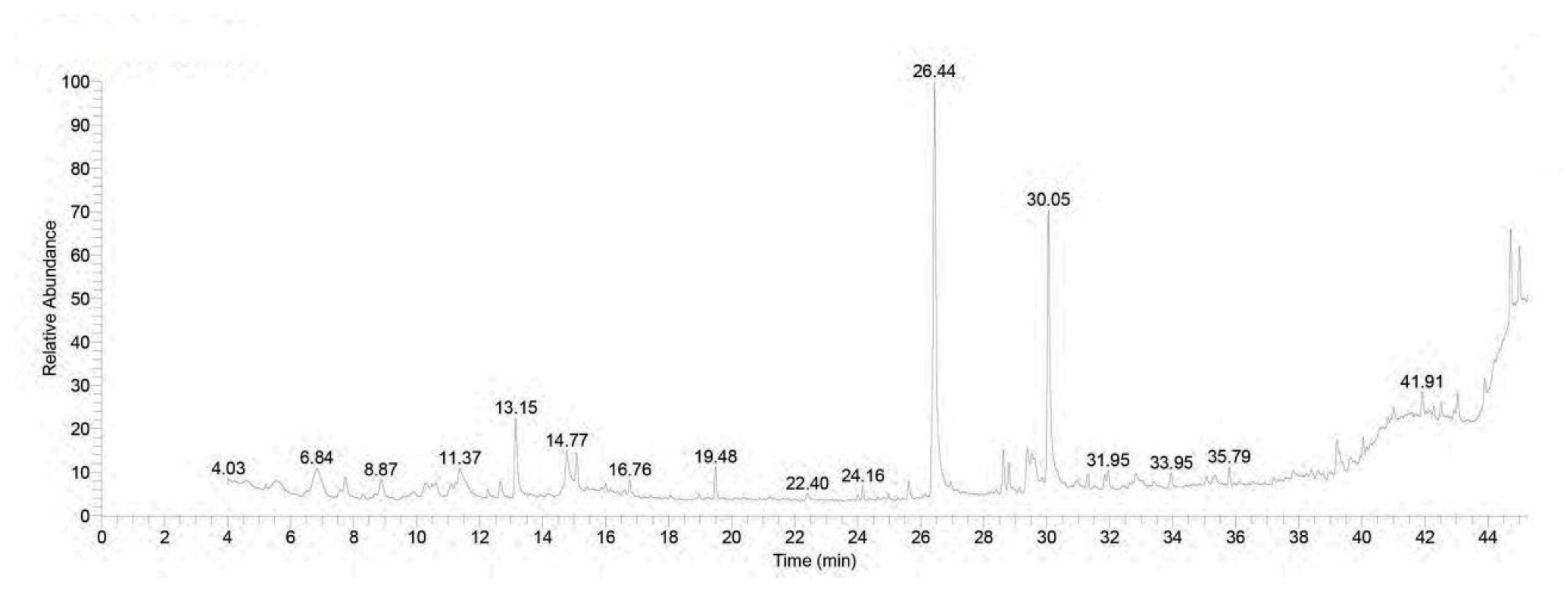
3.1. Chromatographic Pattern of GC-MS Spectral Analysis of Rhodiola rosea Extract
3.2. Effect of RHO and CARV Alone on Body Weight and Cardiac Injury Markers
3.3. Impacts of RHO and CARV Alone on TIL-Induced Changes in Inflammatory Cytokines
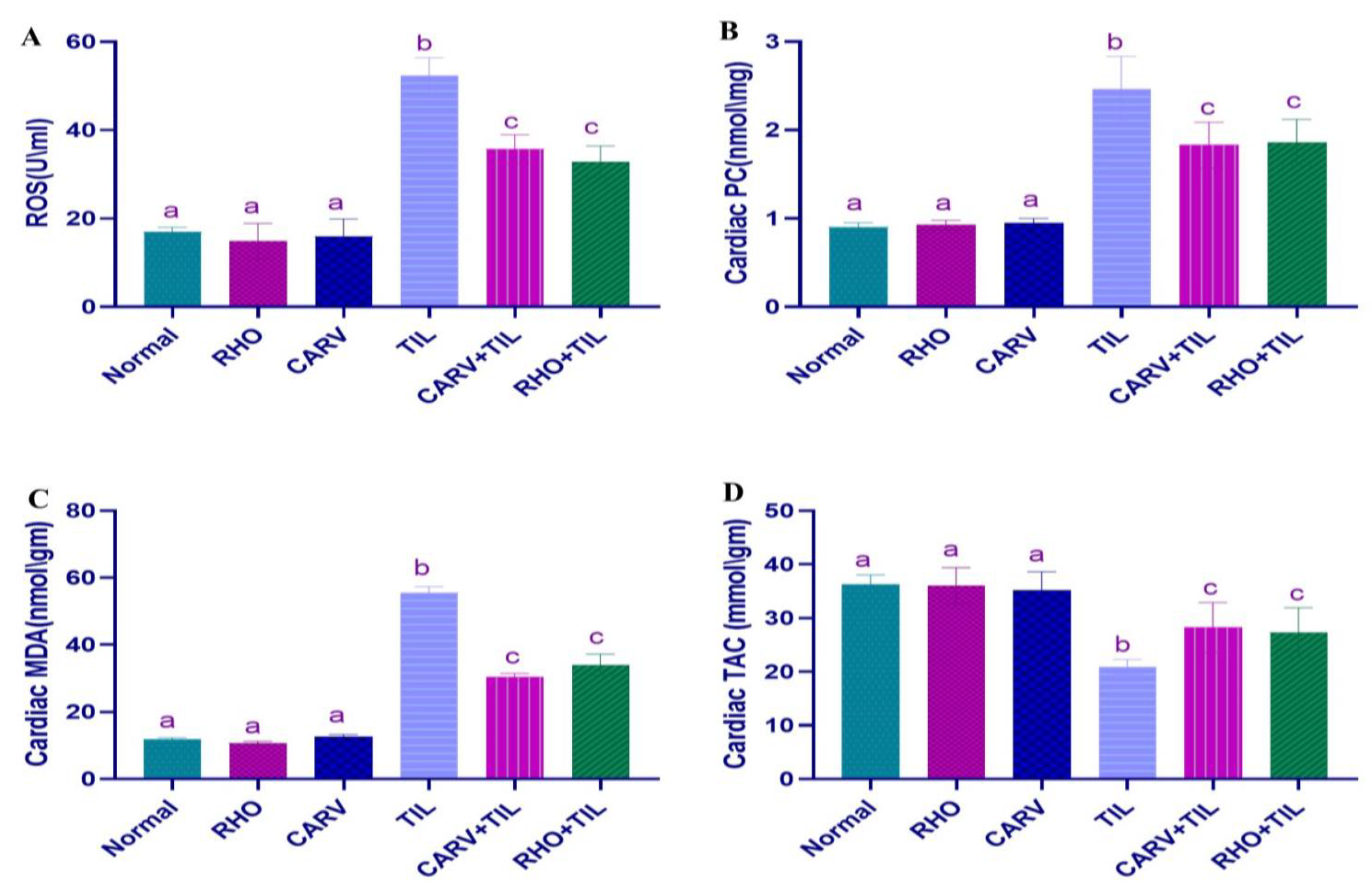
3.4. Effect of RHO and CARV Alone on Oxidative Stress Biomarkers (ROS, PC, MDA) and TAC in Cardiac Tissue Homogenate
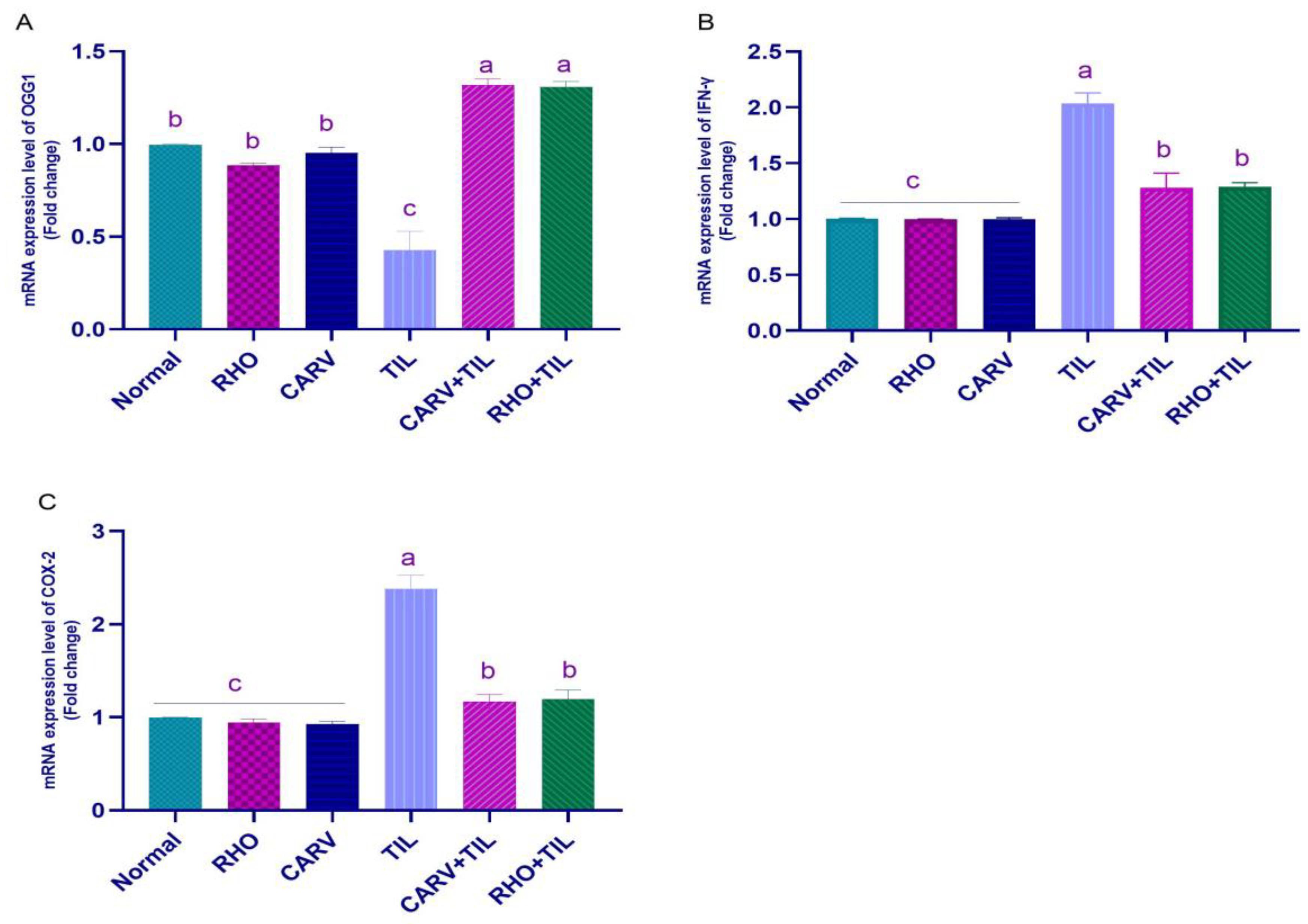
3.5. Impacts of RHO and CARV Alone against TIL-Induced Alteration in Cardiac mRNA Gene Expression
3.6. Protective Effect of RHO and CARV Alone against TIL-Induced Cardiac Muscle Histopathology
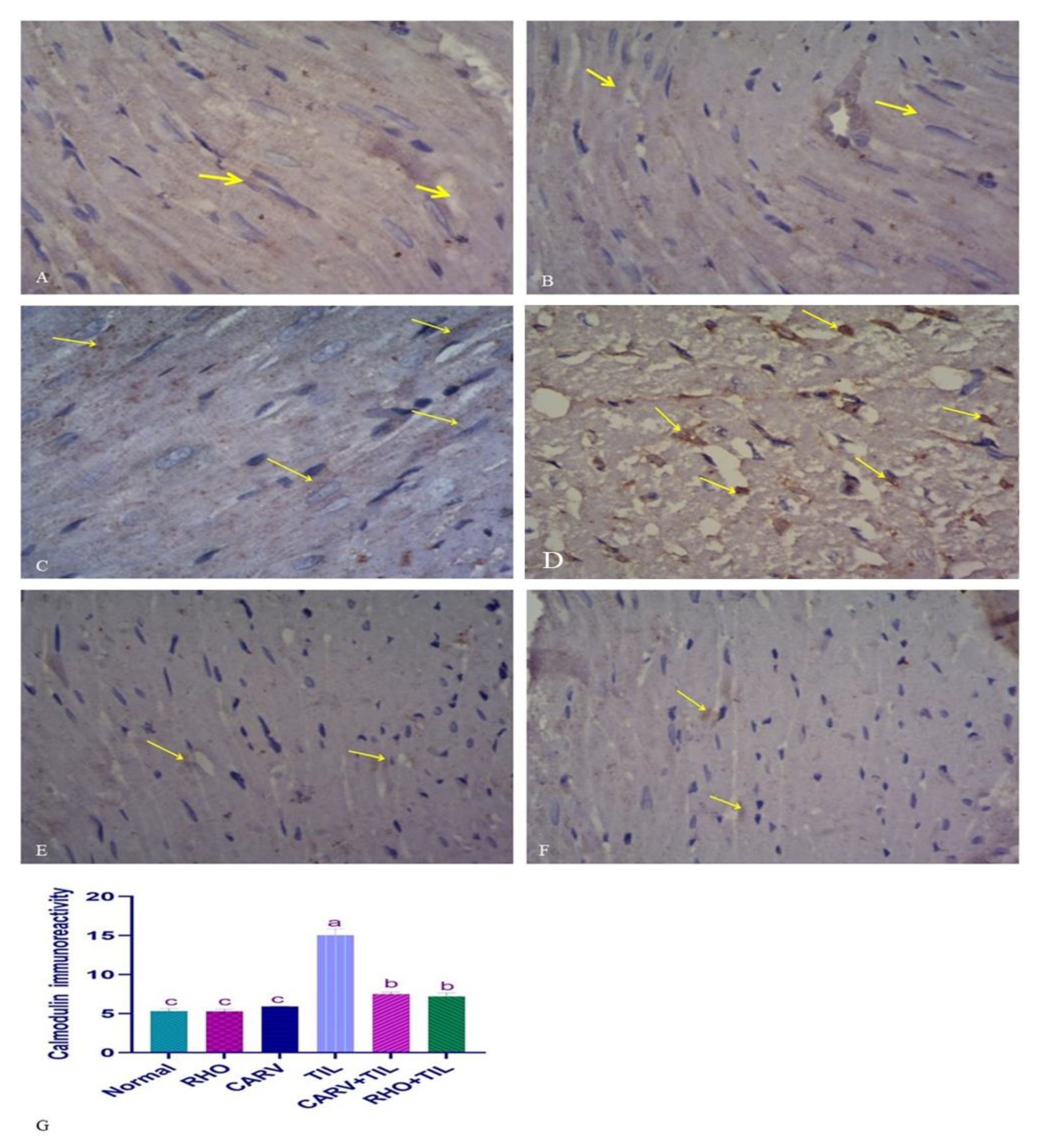
3.7. Immunohistochemical Analysis of the Protective Effect of RHO and CARV Alone in TIL-Induced Cardiac Muscle
4. Discussion
5. Conclusions
6. Limitations and Future Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Naccari, F.; Pellegrino, M.; Calò, M.; Licata, P.; Giofré, F.; Carli, S. Effectiveness and kinetic behaviour of tilmicosin in the treatment of respiratory infections in sheep. Vet. Rec. 2001, 148, 773–776. [Google Scholar] [CrossRef] [PubMed]
- Dingwell, R.; Duffield, T.; Leslie, K.; Keefe, G.P.; DesCoteaux, L.; Kelton, D.; Lissemore, K.; Schukken, Y.; Dick, P.; Bagg, R. The efficacy of intramammary tilmicosin at drying-off, and other risk factors for the prevention of new intramammary infections during the dry period. J. Dairy Sci. 2002, 85, 3250–3259. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Cao, X.; Xu, Q.; Su, J.; Li, X.; Zhou, W. Evaluation of the antibacterial activity of tilmicosin-SLN against Streptococcus agalactiae: In vitro and in vivo studies. Int. J. Nanomed. 2018, 13, 4747. [Google Scholar] [CrossRef] [PubMed]
- Khalil, S.R.; Abdel-Motal, S.M.; Abd-Elsalam, M.; Abd El-Hameed, N.E.; Awad, A. Restoring strategy of ethanolic extract of Moringa oleifera leaves against Tilmicosin-induced cardiac injury in rats: Targeting cell apoptosis-mediated pathways. Gene 2020, 730, 144272. [Google Scholar] [CrossRef]
- Gheith, I.; El-Mahmoudy, A.; Elmajdoub, A.; Awidat, S. Pharmacovigilance of tilmicosin in mice. Acta Sci. Vet. 2015, 43, 1318. [Google Scholar]
- Oztekin, E.; Sivrikaya, A.; Col, R.; Elmas, M.; Bas, A. Effects of different doses of tilmicosin on malondialdehyde and glutathione concentrations in mice. Acta Vet. Brno 2004, 73, 69–72. [Google Scholar]
- Youssef, M.A.; Ibrahim, H.M.; Farag, E.-S.M.; El-Khodery, S.A. Effects of tilmicosin phosphate administration on echocardiographic parameters in healthy donkeys (Equus asinus): An experimental study. J. Equine Vet. Sci. 2016, 38, 24–29. [Google Scholar] [CrossRef]
- Kart, A.; Karapehlivan, M.; Yapar, K.; Citil, M.; Akpinar, A. Protection through L-Carnitine on tissue oxidant status and sialic acid content in tilmicosin-induced alterations in BALB/c Mice. Acta Vet. Brno 2007, 76, 203–207. [Google Scholar] [CrossRef]
- Aboubakr, M.; Elsayd, F.; Soliman, A.; Fadl, S.E.; El-Shafey, A.; Abdelhiee, E.Y. L-Carnitine and vitamin E ameliorate cardiotoxicity induced by tilmicosin in rats. Environ. Sci. Pollut. Res. 2020, 27, 23026–23034. [Google Scholar] [CrossRef]
- Meissner, G.; Pasek, D.A.; Yamaguchi, N.; Ramachandran, S.; Dokholyan, N.V.; Tripathy, A. Thermodynamics of calmodulin binding to cardiac and skeletal muscle ryanodine receptor ion channels. Proteins Struct. Funct. Bioinform. 2009, 74, 207–211. [Google Scholar] [CrossRef]
- Meissner, G. Regulation of mammalian ryanodine receptors. Front. Biosci. Landmark 2002, 7, 2072–2080. [Google Scholar] [CrossRef] [PubMed]
- Shaddy, R.E.; Boucek, M.M.; Hsu, D.T.; Boucek, R.J.; Canter, C.E.; Mahony, L.; Ross, R.D.; Pahl, E.; Blume, E.D.; Dodd, D.A. Carvedilol for children and adolescents with heart failure: A randomized controlled trial. JAMA 2007, 298, 1171–1179. [Google Scholar] [CrossRef] [PubMed]
- Soma, K.; Yao, A.; Saito, A.; Inaba, T.; Ishikawa, Y.; Hirata, Y.; Komuro, I. Regular treatment strategy with a large amount of carvedilol for heart failure improves biventricular systolic failure in a patient with repaired tetralogy of Fallot. Int. Heart J. 2018, 59, 1169–1173. [Google Scholar] [CrossRef] [PubMed]
- Onay-Besikci, A.; Suzmecelik, E.; Ozcelikay, A.T. Carvedilol suppresses fatty acid oxidation and stimulates glycolysis in C2C12 cells. Can. J. Physiol. Pharmacol. 2012, 90, 1087–1093. [Google Scholar] [CrossRef] [PubMed]
- Khalil, S.R.; Salem, H.F.; Metwally, M.M.; Emad, R.M.; Elbohi, K.M.; Ali, S.A. Protective effect of Spirulina platensis against physiological, ultrastructural and cell proliferation damage induced by furan in kidney and liver of rat. Ecotoxicol. Environ. Saf. 2020, 192, 110256. [Google Scholar] [CrossRef]
- Pu, W.-L.; Zhang, M.-Y.; Bai, R.-Y.; Sun, L.-K.; Li, W.-H.; Yu, Y.-L.; Zhang, Y.; Song, L.; Wang, Z.-X.; Peng, Y.-F. Anti-inflammatory effects of Rhodiola rosea L.: A review. Biomed. Pharmacother. 2020, 121, 109552. [Google Scholar] [CrossRef]
- Kucinskaite, A.; Briedis, V.; Savickas, A. Experimental analysis of therapeutic properties of Rhodiola rosea L. and its possible application in medicine. Medicina 2004, 40, 614–619. [Google Scholar]
- Tolonen, A.; Pakonen, M.; Hohtola, A.; Jalonen, J. Phenylpropanoid glycosides from Rhodiola rosea. Chem. Pharm. Bull. 2003, 51, 467–470. [Google Scholar] [CrossRef]
- Mao, Y.; Li, Y.; Yao, N. Simultaneous determination of salidroside and tyrosol in extracts of Rhodiola L. by microwave assisted extraction and high-performance liquid chromatography. J. Pharm. Biomed. Anal. 2007, 45, 510–515. [Google Scholar] [CrossRef]
- Chou Lin, S.S.; Chin, L.W.; Chao, P.C.; Lai, Y.Y.; Lin, L.Y.; Chou, M.Y.; Chou, M.C.; Wei, J.C.C.; Yang, C.C. In vivo Th1 and Th2 cytokine modulation effects of Rhodiola rosea standardised solution and its major constituent, salidroside. Phytother. Res. 2011, 25, 1604–1611. [Google Scholar] [CrossRef]
- Abidov, M.; Grachev, S.; Seifulla, R.; Ziegenfuss, T. Extract of Rhodiola rosea radix reduces the level of C-reactive protein and creatinine kinase in the blood. Bull. Exp. Biol. Med. 2004, 138, 63–64. [Google Scholar] [CrossRef] [PubMed]
- Mattioli, L.; Funari, C.; Perfumi, M. Effects of Rhodiola rosea L. extract on behavioural and physiological alterations induced by chronic mild stress in female rats. J. Psychopharmacol. 2009, 23, 130–142. [Google Scholar] [CrossRef]
- Abd El-Kareem, M.S.; Rabbih, M.A.E.F.; Selim, E.T.M.; Elsherbiny, E.A.E.-m.; El-Khateeb, A.Y. Application of GC/EIMS in combination with semi-empirical calculations for identification and investigation of some volatile components in basil essential oil. Int. J. Anal. Mass Spectrom. Chromatogr. 2016, 4, 14–25. [Google Scholar] [CrossRef]
- Mahamat, I.A.; Mehanna, E.S.E.; Oda, S.S.; Khafaga, A.F.; Gad El-Karim, D.R. Lead-Induced Hepato-Renal Toxicosis and the Ameliorative Role of Rhodiolarosea Extract in Albino Rats. Alex. J. Vet. Sci. 2021, 70, 76–85. [Google Scholar] [CrossRef]
- Kim, Y.-H.; Park, S.-M.; Kim, M.; Kim, S.H.; Lim, S.-Y.; Ahn, J.-C.; Song, W.-H.; Shim, W.-J. Cardioprotective effects of rosuvastatin and carvedilol on delayed cardiotoxicity of doxorubicin in rats. Toxicol. Mech. Methods 2012, 22, 488–498. [Google Scholar] [CrossRef] [PubMed]
- El-Demerdash, E.; Abdel-Sattar, S.A.; El-Bakly, W.M.; Mohamed, E.A. Antifibrotic effects of carvedilol and impact of liver fibrosis on carvedilol pharmacokinetics in a rat model. Eur. J. Drug Metab. Pharmacokinet. 2017, 42, 767–779. [Google Scholar] [CrossRef] [PubMed]
- Hamdy, N.M.; El-Demerdash, E. New therapeutic aspect for carvedilol: Antifibrotic effects of carvedilol in chronic carbon tetrachloride-induced liver damage. Toxicol. Appl. Pharmacol. 2012, 261, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Kart, A.; Yapar, K.; Karapehlivan, M.; Citil, M. The Possible Protective effect of L-carnitine on Tilmicosin-induced Cardiotoxicity in Mice. J. Vet. Med. Ser. A 2007, 54, 144–146. [Google Scholar] [CrossRef]
- Tao, H.; Wu, X.; Cao, J.; Peng, Y.; Wang, A.; Pei, J.; Xiao, J.; Wang, S.; Wang, Y. Rhodiola species: A comprehensive review of traditional use, phytochemistry, pharmacology, toxicity, and clinical study. Med. Res. Rev. 2019, 39, 1779–1850. [Google Scholar] [CrossRef]
- Chiang, H.-M.; Chen, H.-C.; Wu, C.-S.; Wu, P.-Y.; Wen, K.-C. Rhodiola plants: Chemistry and biological activity. J. Food Drug Anal. 2015, 23, 359–369. [Google Scholar] [CrossRef]
- Murthy, T.; Sowjanya, G. Development of discriminatory method for dissolution of carvedilol marketed formulations. Int. J. ChemTech Res. 2010, 2, 1047–1050. [Google Scholar]
- Elblehi, S.S.; El-Sayed, Y.S.; Soliman, M.M.; Shukry, M. Date palm pollen extract avert doxorubicin-induced cardiomyopathy fibrosis and associated oxidative/nitrosative stress, inflammatory cascade, and apoptosis-targeting Bax/Bcl-2 and Caspase-3 signaling pathways. Animals 2021, 11, 886. [Google Scholar] [CrossRef]
- Tsung, S.H. Creatine kinase activity and isoenzyme pattern in various normal tissues and neoplasms. Clin. Chem. 1983, 29, 2040–2043. [Google Scholar] [CrossRef] [PubMed]
- Lum, G.; Gambino, S.R. A comparison of serum versus heparinized plasma for routine chemistry tests. Am. J. Clin. Pathol. 1974, 61, 108–113. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Awad, A.; Khalil, S.R.; Hendam, B.M.; Abd El-Aziz, R.M.; Metwally, M.M.; Imam, T.S. Protective potency of Astragalus polysaccharides against tilmicosin-induced cardiac injury via targeting oxidative stress and cell apoptosis-encoding pathways in rat. Environ. Sci. Pollut. Res. 2020, 27, 20861–20875. [Google Scholar] [CrossRef]
- Lee, J.C.; Cho, Y.J.; Kim, J.; Kim, N.; Kang, B.G.; Cha, C.I.; Joo, K.M. Region-specific changes in the immunoreactivity of vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide receptors (VPAC2, and PAC1 receptor) in the aged rat brains. Brain Res. 2010, 1351, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Bancroft, J.D.; Layton, C. The hematoxylins and eosin. Bancroft’s Theory Pract. Histol. Tech. 2012, 7, 173–186. [Google Scholar]
- Tamargo, J.; De Miguel, B.; Tejerina, M. A comparison of josamycin with macrolides and related antibiotics on isolated rat atria. Eur. J. Pharmacol. 1982, 80, 285–293. [Google Scholar] [CrossRef]
- Jin, P.; Li, L.-H.; Shi, Y.; Hu, N.-B. Salidroside inhibits apoptosis and autophagy of cardiomyocyte by regulation of circular RNA hsa_circ_0000064 in cardiac ischemia-reperfusion injury. Gene 2021, 767, 145075. [Google Scholar] [CrossRef] [PubMed]
- Aparna, V.; Dileep, K.V.; Mandal, P.K.; Karthe, P.; Sadasivan, C.; Haridas, M. Anti-inflammatory property of n-hexadecanoic acid: Structural evidence and kinetic assessment. Chem. Biol. Drug Des. 2012, 80, 434–439. [Google Scholar] [CrossRef]
- Paniagua-Pérez, R.; Flores-Mondragón, G.; Reyes-Legorreta, C.; Herrera-López, B.; Cervantes-Hernández, I.; Madrigal-Santillán, O.; Morales-González, J.A.; Alvarez-González, I.; Madrigal-Bujaidar, E. Evaluation of the anti-inflammatory capacity of beta-sitosterol in rodent assays. Afr. J. Tradit. Complement. Altern. Med. 2017, 14, 123–130. [Google Scholar] [CrossRef]
- Khatri, P.; Rana, J.; Jamdagni, P.; Sindhu, A. Phytochemical screening, GC-MS and FT-IR analysis of methanolic extract leaves of Elettaria cardamomum. Int. J. Res. 2017, 5, 213–224. [Google Scholar] [CrossRef]
- de Souza, C.O.; Vannice, G.K.; Rosa Neto, J.C.; Calder, P.C. Is palmitoleic acid a plausible nonpharmacological strategy to prevent or control chronic metabolic and inflammatory disorders? Mol. Nutr. Food Res. 2018, 62, 1700504. [Google Scholar] [CrossRef]
- Hernández-Saavedra, D.; Stanford, K.I. The regulation of lipokines by environmental factors. Nutrients 2019, 11, 2422. [Google Scholar] [CrossRef]
- Ibrahim, K.A.; Abdelgaid, H.A.; El-Desouky, M.A.; Fahmi, A.A.; Abdel-Daim, M.M. Linseed ameliorates renal apoptosis in rat fetuses induced by single or combined exposure to diesel nanoparticles or fenitrothion by inhibiting transcriptional activation of p21/p53 and caspase-3/9 through pro-oxidant stimulus. Environ. Toxicol. 2021, 36, 958–974. [Google Scholar] [CrossRef] [PubMed]
- Ola, O.S.; Sofolahan, T.A. A monoterpene antioxidant, linalool, mitigates benzene-induced oxidative toxicities on hematology and liver of male rats. Egypt. J. Basic Appl. Sci. 2021, 8, 39–53. [Google Scholar] [CrossRef]
- Ibrahim, A.E.; Abdel-Daim, M.M. Modulating effects of Spirulina platensis against tilmicosin-induced cardiotoxicity in mice. Cell J. 2015, 17, 137. [Google Scholar]
- Nepali, S.; Ki, H.-H.; Lee, J.-H.; Cha, J.-Y.; Lee, Y.-M.; Kim, D.-K. Triticum aestivum sprout-derived polysaccharide exerts hepatoprotective effects against ethanol-induced liver damage by enhancing the antioxidant system in mice. Int. J. Mol. Med. 2017, 40, 1243–1252. [Google Scholar] [CrossRef]
- Chen, L.; Liu, P.; Feng, X.; Ma, C. Salidroside suppressing LPS-induced myocardial injury by inhibiting ROS-mediated PI 3K/Akt/mTOR pathway in vitro and in vivo. J. Cell. Mol. Med. 2017, 21, 3178–3189. [Google Scholar] [CrossRef] [PubMed]
- Oku, H.; Nakazato, H.; Horikawa, T.; Tsuruta, Y.; Suzuki, R. Pirfenidone suppresses tumor necrosis factor-α, enhances interleukin-10 and protects mice from endotoxic shock. Eur. J. Pharmacol. 2002, 446, 167–176. [Google Scholar] [CrossRef]
- Dunn, G.P.; Ikeda, H.; Bruce, A.T.; Koebel, C.; Uppaluri, R.; Bui, J.; Chan, R.; Diamond, M.; Michael White, J.; Sheehan, K.C. Interferon-γ and cancer immunoediting. Immunol. Res. 2005, 32, 231–245. [Google Scholar] [CrossRef] [PubMed]
- Kak, G.; Raza, M.; Tiwari, B.K. Interferon-gamma (IFN-γ): Exploring its implications in infectious diseases. Biomol. Concepts 2018, 9, 64–79. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.-Y.; Dong, M.; Shen, J.-Z.; Wu, B.-B.; Wu, C.-M.; Du, X.-D.; Wang, Z.; Qi, Y.-T.; Li, B.-Y. Tilmicosin and tylosin have anti-inflammatory properties via modulation of COX-2 and iNOS gene expression and production of cytokines in LPS-induced macrophages and monocytes. Int. J. Antimicrob. Agents 2006, 27, 431–438. [Google Scholar] [CrossRef]
- Pan, J.; Zhang, L.; Xu, S.; Cheng, X.; Yu, H.; Bao, J.; Lu, R. Induction of apoptosis in human papillary-thyroid-carcinoma BCPAP cells by diallyl trisulfide through activation of the MAPK signaling pathway. J. Agric. Food Chem. 2018, 66, 5871–5878. [Google Scholar] [CrossRef]
- Lin, S.-Y.; Xu, D.; Du, X.-X.; Ran, C.-L.; Xu, L.; Ren, S.-J.; Tang, Z.-T.; Yin, L.-Z.; He, C.-L.; Yuan, Z.-X. Protective effects of salidroside against carbon tetrachloride (CCl4)-induced liver injury by initiating mitochondria to resist oxidative stress in mice. Int. J. Mol. Sci. 2019, 20, 3187. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, Y.-J.; Liu, W.-W.; Shi, A.-W.; Gu, N. Salidroside suppresses HUVECs cell injury induced by oxidative stress through activating the Nrf2 signaling pathway. Molecules 2016, 21, 1033. [Google Scholar] [CrossRef]
- Erickson, J.R.; He, B.J.; Grumbach, I.M.; Anderson, M.E. CaMKII in the cardiovascular system: Sensing redox states. Physiol. Rev. 2011, 91, 889–915. [Google Scholar] [CrossRef]
- Kohlhaas, M.; Liu, T.; Knopp, A.; Zeller, T.; Ong, M.F.; Böhm, M.; O’Rourke, B.; Maack, C. Elevated cytosolic Na+ increases mitochondrial formation of reactive oxygen species in failing cardiac myocytes. Circulation 2010, 121, 1606–1613. [Google Scholar] [CrossRef]
- Cappetta, D.; Esposito, G.; Coppini, R.; Piegari, E.; Russo, R.; Ciuffreda, L.P.; Rivellino, A.; Santini, L.; Rafaniello, C.; Scavone, C. Effects of ranolazine in a model of doxorubicin-induced left ventricle diastolic dysfunction. Br. J. Pharmacol. 2017, 174, 3696–3712. [Google Scholar] [CrossRef] [PubMed]
- Tscheschner, H.; Meinhardt, E.; Schlegel, P.; Jungmann, A.; Lehmann, L.H.; Müller, O.J.; Most, P.; Katus, H.A.; Raake, P.W. CaMKII activation participates in doxorubicin cardiotoxicity and is attenuated by moderate GRP78 overexpression. PLoS ONE 2019, 14, e0215992. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, Y.-W.; Tsai, Y.-N.; Huang, Y.-T.; Liu, S.-H.; Lin, Y.-J.; Lo, L.-W.; Hu, Y.-F.; Chung, F.-P.; Lin, S.-F.; Chang, S.-L. Rhodiola crenulata reduces ventricular arrhythmia through mitigating the activation of IL-17 and inhibiting the MAPK signaling pathway. Cardiovasc. Drugs Ther. 2021, 35, 889–900. [Google Scholar] [CrossRef] [PubMed]
- Oda, S.S.; Derbalah, A.E. Impact of diclofenac sodium on tilmicosin-induced acute cardiotoxicity in rats (tilmicosin and diclofenac cardiotoxicity). Cardiovasc. Toxicol. 2018, 18, 63–75. [Google Scholar] [CrossRef]
- Shinde, A.V.; Humeres, C.; Frangogiannis, N.G. The role of α-smooth muscle actin in fibroblast-mediated matrix contraction and remodeling. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2017, 1863, 298–309. [Google Scholar] [CrossRef]
- Umbarkar, P.; Ejantkar, S.; Tousif, S.; Lal, H. Mechanisms of fibroblast activation and myocardial fibrosis: Lessons learned from FB-specific conditional mouse models. Cells 2021, 10, 2412. [Google Scholar] [CrossRef]
- Kong, P.; Christia, P.; Frangogiannis, N.G. The pathogenesis of cardiac fibrosis. Cell. Mol. Life Sci. 2014, 71, 549–574. [Google Scholar] [CrossRef]
- Yang, S.; Pei, T.; Wang, L.; Zeng, Y.; Li, W.; Yan, S.; Xiao, W.; Cheng, W. Salidroside Alleviates Renal Fibrosis in SAMP8 Mice by Inhibiting Ferroptosis. Molecules 2022, 27, 8039. [Google Scholar] [CrossRef]
- Pillai, V.B.; Bindu, S.; Sharp, W.; Fang, Y.H.; Kim, G.; Gupta, M.; Samant, S.; Gupta, M.P. Sirt3 protects mitochondrial DNA damage and blocks the development of doxorubicin-induced cardiomyopathy in mice. Am. J. Physiol. Heart Circ. Physiol. 2016, 310, H962–H972. [Google Scholar] [CrossRef]
- Cooke, M.S.; Olinski, R.; Evans, M.D. Does measurement of oxidative damage to DNA have clinical significance? Clin. Chim. Acta 2006, 365, 30–49. [Google Scholar] [CrossRef]
- Evans, M.D.; Dizdaroglu, M.; Cooke, M.S. Oxidative DNA damage and disease: Induction, repair and significance. Mutat. Res./Rev. Mutat. Res. 2004, 567, 1–61. [Google Scholar]
- Sun, P.; Song, S.Z.; Jiang, S.; Li, X.; Yao, Y.L.; Wu, Y.L.; Lian, L.H.; Nan, J.X. Salidroside Regulates Inflammatory Response in Raw 264.7 Macrophages via TLR4/TAK1 and Ameliorates Inflammation in Alcohol Binge Drinking-Induced Liver Injury. Molecules 2016, 21, 1490. [Google Scholar] [CrossRef]
- Zheng, T.; Yang, X.; Li, W.; Wang, Q.; Chen, L.; Wu, D.; Bian, F.; Xing, S.; Jin, S. Salidroside Attenuates High-Fat Diet-Induced Nonalcoholic Fatty Liver Disease via AMPK-Dependent TXNIP/NLRP3 Pathway. Oxidative Med. Cell. Longev. 2018, 2018, 8597897. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Zhao, M.; Feng, L.; Wang, P.; Li, Y.; Li, W. Salidroside attenuates acute lung injury via inhibition of inflammatory cytokine production. Biomed. Pharmacother. Biomed. Pharmacother. 2021, 142, 111949. [Google Scholar] [CrossRef] [PubMed]
- Abou-Zeid, S.M.; Ahmed, A.I.; Awad, A.; Mohammed, W.A.; Metwally, M.M.; Almeer, R.; Abdel-Daim, M.M.; Khalil, S.R. Moringa oleifera ethanolic extract attenuates tilmicosin-induced renal damage in male rats via suppression of oxidative stress, inflammatory injury, and intermediate filament proteins mRNA expression. Biomed. Pharmacother. 2021, 133, 110997. [Google Scholar] [CrossRef] [PubMed]
- Tsai-Turton, M.; Luong, B.T.; Tan, Y.; Luderer, U. Cyclophosphamide-induced apoptosis in COV434 human granulosa cells involves oxidative stress and glutathione depletion. Toxicol. Sci. Off. J. Soc. Toxicol. 2007, 98, 216–230. [Google Scholar] [CrossRef]
- Farag, M.R.; Elhady, W.M.; Ahmed, S.Y.A.; Taha, H.S.A.; Alagawany, M. Astragalus polysaccharides alleviate tilmicosin-induced toxicity in rats by inhibiting oxidative damage and modulating the expressions of HSP70, NF-kB and Nrf2/HO-1 pathway. Res. Vet. Sci. 2019, 124, 137–148. [Google Scholar] [CrossRef]
- Cetin, N.; Boyraz, U.; Cetin, E. Ghrelin alleviates tilmicosin-induced myocardial oxidative stress in rats. J. Anim. Vet. Adv. 2011, 10, 2038–2042. [Google Scholar] [CrossRef]
- Seddik, M.; El-Borai, N.; El-Bialy, B.; Elsabbagh, H. The Potential Teratogenic Effect of Tilmicosin in Rats: Visceral Malformations and Histomorphological Alterations in Fetal Internal Organs. J. Curr. Vet. Res. 2021, 3, 50–62. [Google Scholar] [CrossRef]
- Lu, H.; Lei, X.; Zhang, Q. Moderate activation of IKK2-NF-kB in unstressed adult mouse liver induces cytoprotective genes and lipogenesis without apparent signs of inflammation or fibrosis. BMC Gastroenterol. 2015, 15, 94. [Google Scholar] [CrossRef]



| Gene | Direction | Primer Sequence | Annealing Temp. | (bp) | Accession Number |
|---|---|---|---|---|---|
| OGG1 | F: | ATCTGTTCTTCCAACAACAAC | 58 °C | 212 | NM_030870 |
| R: | GCCAGCATAAGGTCCCCACAG | ||||
| IFN-γ | F: | TGTCATCGAATCGCACCTGATC | 57 °C | 185 | NM_138880 |
| R: | GACTCCTTTTCCGCTTCCTTAG | ||||
| Bax | F: | GGCGAATTGGCGATGAACTG | 57 °C | 167 | NM_017059.2 |
| R: | ATGGTTCTGATCAGCTCGGG | ||||
| Bcl-2 | F: | GATTGTGGCCTTCTTTGAGT | 57 °C | 172 | NM_016993.1 |
| R: | ATAGTTCCACAAAGGCATCC | ||||
| SOD | F: | AGGATTAACTGAAGGCGAGCAT | 59 °C | 173 | NM_017050.1 |
| R: | TCTACAGTTAGCAGGCCAGCAG | ||||
| Caspase-3 | F: | TGTCAGCTACTCCCAGGTTG | 57 °C | 146 | NM_012922 |
| R: | TCAAGAAGGTGGTGAAGCAG | ||||
| COX-2 | F: | CATGGGAGTTGGGCAGTC | 56 °C | 70 | AF159101 |
| R: | TCAATCTCGGGTGGCTGAACG | ||||
| GAPDH | F: | AGGTGGAAGAATGGGAGTTG | 55 °C | 197 | NM_017008.4 |
| R: | GCTTTCTCCAACCTCTCCTACTACA |
| No. | Retention Time (min) | (Chemical Compound) | Area% | MF | Molecular Formula | Molecular Weight |
|---|---|---|---|---|---|---|
| 1 | 6.85 | Linalool | 3.62 | 797 | C10H18O | 154 |
| 2 | 6.85 | 1,6-Octadien-3-ol, 3,7-dimethyl | 3.62 | 833 | C10H18O | 154 |
| 3 | 8.88 | Benzene, 1-methoxy-4-(1-propenyl)- | 1.41 | 784 | C10H12O | 148 |
| 4 | 8.88 | Estragole | 1.41 | 866 | C10H12O | 148 |
| 5 | 12.65 | Guaiacol, 6-propenyl- | 1.17 | 831 | C10H12O2 | 164 |
| 6 | 12.65 | Phenol, 2-methoxy-6-(1-propenyl)- | 1.17 | 827 | C10H12O2 | 164 |
| 7 | 13.15 | 2-Propenoic acid, 3-phenyl-, methyl ester | 5.11 | 897 | C10H10O2 | 162 |
| 8 | 15.08 | Trans-à-Bergamotene | 1.77 | 919 | C15H24 | 204 |
| 9 | 16.76 | á-Copaene | 0.92 | 839 | C15H24 | 204 |
| 10 | 24.16 | Ethanol, 2-(9-octadecenyloxy)-, (Z)- | 0.73 | 776 | C20H40O2 | 312 |
| 11 | 25.62 | pentadecanoic acid, 14-methyl-, methyl ester | 1.16 | 798 | C17H34O2 | 270 |
| 12 | 25.62 | Palmitic Acid methyl ester | 1.16 | 787 | C17H34O2 | 270 |
| 13 | 25.62 | Hexadecanoic acid, methyl ester | 1.16 | 789 | C17H34O2 | 270 |
| 14 | 26.44 | n-Hexadecanoic acid | 26.47 | 921 | C16H32O2 | 256 |
| 15 | 28.62 | 7,10-Octadecadienoic acid, methyl ester | 2.50 | 876 | C19H34O2 | 294 |
| 16 | 28.80 | 9-Octadecenoic acid (z)-, methyl ester | 1.55 | 877 | C19H36O2 | 296 |
| 17 | 29.53 | 9-Octadecenoic acid (z)- | 0.93 | 819 | C18H34O2 | 282 |
| 18 | 29.63 | Oleic Acid | 1.15 | 781 | C18H34O2 | 282 |
| 19 | 29.63 | cis-Vaccenic acid | 1.15 | 792 | C18H34O2 | 282 |
| 20 | 29.63 | 9-Octadecenoic acid (z)- | 1.15 | 809 | C18H34O2 | 282 |
| 21 | 29.63 | trans-13-Octadecenoic acid | 1.15 | 796 | C18H34O2 | 282 |
| 22 | 31.94 | Alanine | 1.16 | 665 | C14H14F3NO4 | 317 |
| 23 | 35.79 | 4H-1-Benzopyran-4-one, 2-(3,4-dihydroxyphenyl)-6,8-di-á-d glucopyranosyl-5,7-dihydroxy | 1.04 | 708 | C27H30O16 | 610 |
| 24 | 40.03 | 17-Pentatriacontene | 1.01 | 677 | C35H70 | 490 |
| 25 | 41.00 | 4H-1-Benzopyran-4-one, 2-(3,4-dimethoxyphenyl)-3,5-dihydroxy-7-methoxy | 0.99 | 733 | C18H16O7 | 344 |
| 26 | 41.00 | Dasycarpidan-1-methanol, acetate (ester) | 0.99 | 730 | C20H26N2O2 | 320 |
| 27 | 41.91 | Oleic acid, eicosyl ester | 1.56 | 667 | C38H74O2 | 562 |
| 28 | 43.04 | Isochiapin B | 1.60 | 717 | C19H22O6 | 346 |
| 29 | 43.04 | 9,12-octadecadienoic acid (z,z) | 1.60 | 731 | C27H54O4Si2 | 498 |
| 30 | 43.89 | .Psi.,.Psi.-Carotene, 1,1′,2,2′-tetrahydro-1,1′-dimet hoxy | 1.45 | 672 | C42H64O2 | 600 |
| 31 | 44.72 | á-Sitosterol | 5.39 | 786 | C29H50O | 414 |
| 32 | 45.00 | Isochiapin B | 3.11 | 725 | C19H22O6 | 346 |
| Normal | RHO | CARV | TIL | CARV + TIL | RHO + TIL | |
|---|---|---|---|---|---|---|
| B.W (gm) | 195.6 ± 3.1 a | 199.1 ± 5.1 a | 198.0 ± 5.0 a | 154.0 ± 4.8 c | 170.1 ± 3.5 b | 168.6 ± 3.1 b |
| LDH (U/L) | 707.50 ± 12.6 b | 721.83 ± 13.2 b | 717.16 ± 11.7 b | 1427.33 ± 39.2 a | 600.16 ± 11.1 c | 601.83 ± 10.9 c |
| CK-MB (U/L) | 701.50 ± 13.03 b | 697.66 ± 19.38 b | 700.33 ± 12.19 b | 1205.33 ± 28.71 a | 571.16 ± 8.841 c | 572.33 ± 8.71 c |
| Cardiac cTI (Pg/mL) | 0.31 ± 0.04 c | 0.31 ± 0.05 c | 0.32 ± 0.06 c | 1.8367 ± 0.27 a | 1.060 ± 0.08 b | 1.06 ± 0.08 b |
| Normal | RHO | CARV | TIL | CARV + TIL | RHO + TIL | |
|---|---|---|---|---|---|---|
| TNF (pg/mL) | 100.16 ± 2.04 c | 105.33 ± 2.16 c | 103.00 ± 2.36 c | 202.66 ± 6.28 a | 160.33 ± 2.92 b | 165.02 ± 3.02 b |
| IL-1 B (pg/mL) | 119.16 ± 2.85 c | 121.83 ± 2.92 c | 120.66 ± 3.07 c | 230.5 ± 4.27 a | 177.66 ± 5.92 b | 176.00 ± 5.79 b |
| IL-10 (pg/mL) | 303.33 ± 5.04 a | 306.83 ± 4.83 a | 308.33 ± 4.96 a | 149.33 ± 2.94 c | 208.83 ± 4.35 b | 202.33 ± 5.31 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elgendy, S.A.; Soliman, M.M.; Ghamry, H.I.; Shukry, M.; Mohammed, L.A.; Nasr, H.E.; Alotaibi, B.S.; Jafri, I.; Sayed, S.; Osman, A.; et al. Exploration of Tilmicosin Cardiotoxicity in Rats and the Protecting Role of the Rhodiola rosea Extract: Potential Roles of Cytokines, Antioxidant, Apoptotic, and Anti-Fibrotic Pathways. Toxics 2023, 11, 857. https://doi.org/10.3390/toxics11100857
Elgendy SA, Soliman MM, Ghamry HI, Shukry M, Mohammed LA, Nasr HE, Alotaibi BS, Jafri I, Sayed S, Osman A, et al. Exploration of Tilmicosin Cardiotoxicity in Rats and the Protecting Role of the Rhodiola rosea Extract: Potential Roles of Cytokines, Antioxidant, Apoptotic, and Anti-Fibrotic Pathways. Toxics. 2023; 11(10):857. https://doi.org/10.3390/toxics11100857
Chicago/Turabian StyleElgendy, Salwa A., Mohamed Mohamed Soliman, Heba I. Ghamry, Mustafa Shukry, Lina Abdelhady Mohammed, Hend Elsayed Nasr, Badriyah S. Alotaibi, Ibrahim Jafri, Samy Sayed, Amira Osman, and et al. 2023. "Exploration of Tilmicosin Cardiotoxicity in Rats and the Protecting Role of the Rhodiola rosea Extract: Potential Roles of Cytokines, Antioxidant, Apoptotic, and Anti-Fibrotic Pathways" Toxics 11, no. 10: 857. https://doi.org/10.3390/toxics11100857
APA StyleElgendy, S. A., Soliman, M. M., Ghamry, H. I., Shukry, M., Mohammed, L. A., Nasr, H. E., Alotaibi, B. S., Jafri, I., Sayed, S., Osman, A., & Elnoury, H. A. (2023). Exploration of Tilmicosin Cardiotoxicity in Rats and the Protecting Role of the Rhodiola rosea Extract: Potential Roles of Cytokines, Antioxidant, Apoptotic, and Anti-Fibrotic Pathways. Toxics, 11(10), 857. https://doi.org/10.3390/toxics11100857