Study on Dynamic Column Behavior and Complexation Mechanism of DBS-Modified Crown Ether-Based Silica to 90Sr
Abstract
:1. Introduction
2. Experiment and Calculation
2.1. Preparation of Material
2.2. Batch Experiments
2.3. Dynamic Column Operation
2.4. Mechanistic Model of Column Adsorption
2.5. Density Functional Theory Calculation
3. Results and Discussion
3.1. Static Batch Experiments
3.1.1. Influence of Coexisting Ions on Sr(II) Separation
3.1.2. Elution Behavior of Adsorbent with Different Eluents
3.2. Dynamic Column Adsorption and Simulation
3.2.1. Breakthrough Curves
3.2.2. Simulation of Breakthrough Curves
3.2.3. Dynamic Desorption Performance
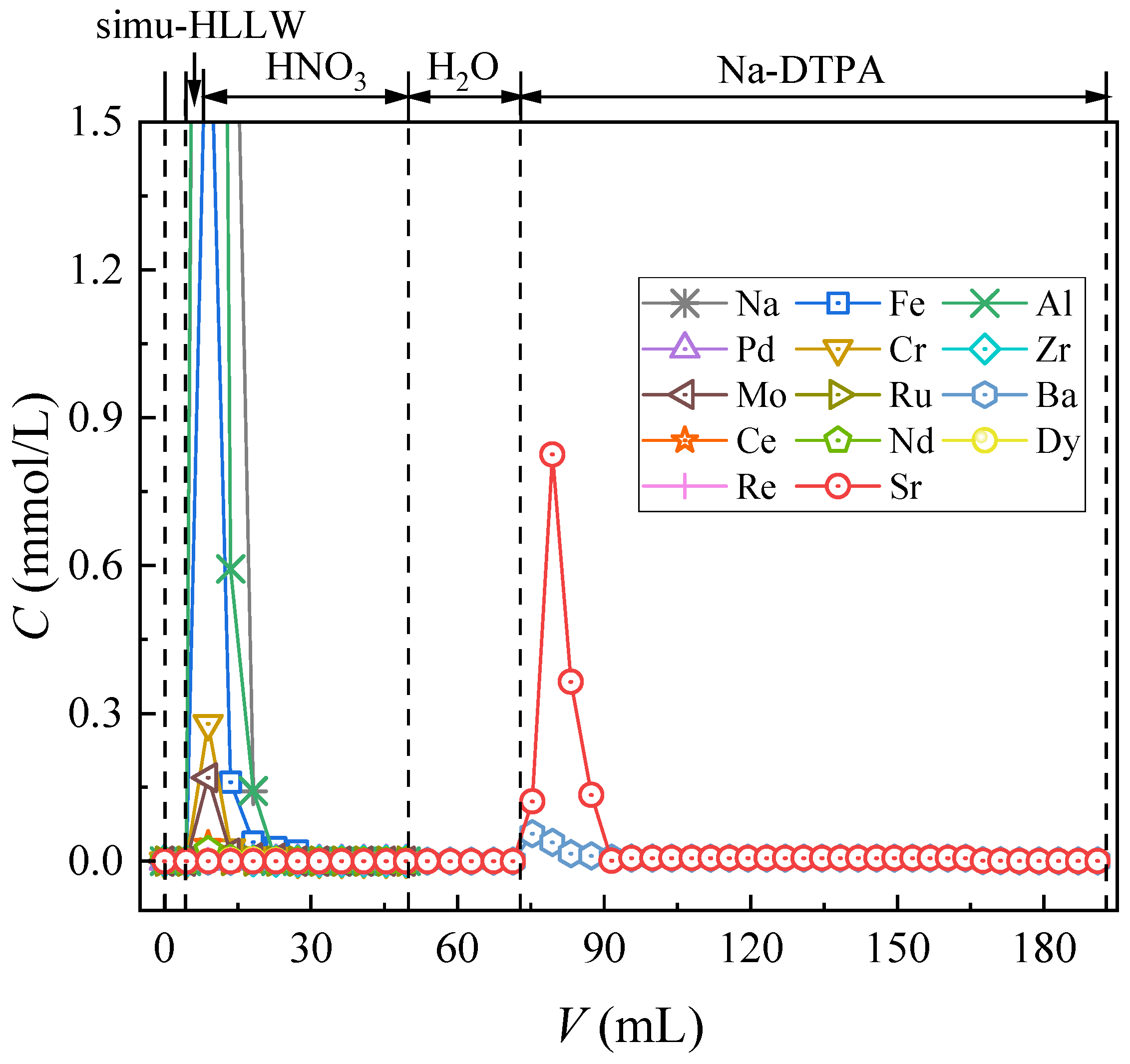
3.2.4. Column Separation of Simulated HLLW
3.3. Density Functional Theory Computational Results
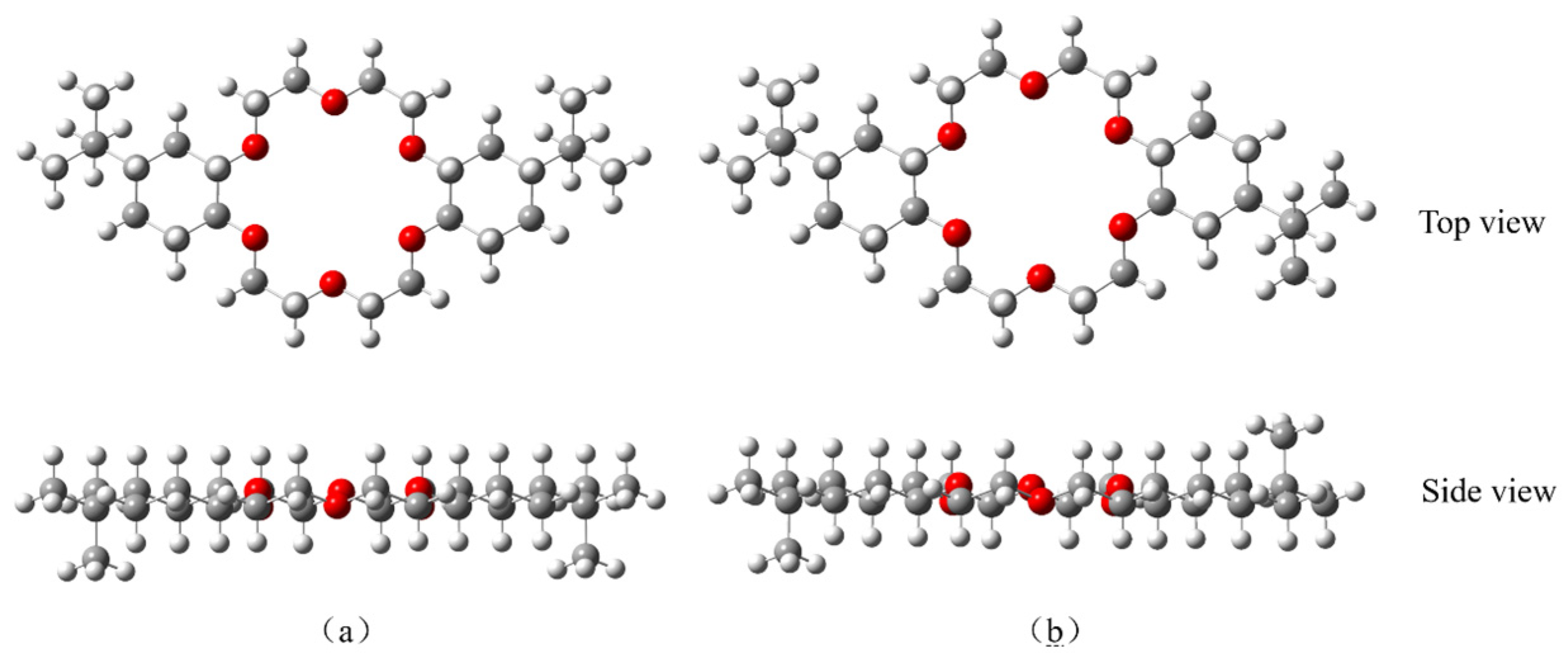
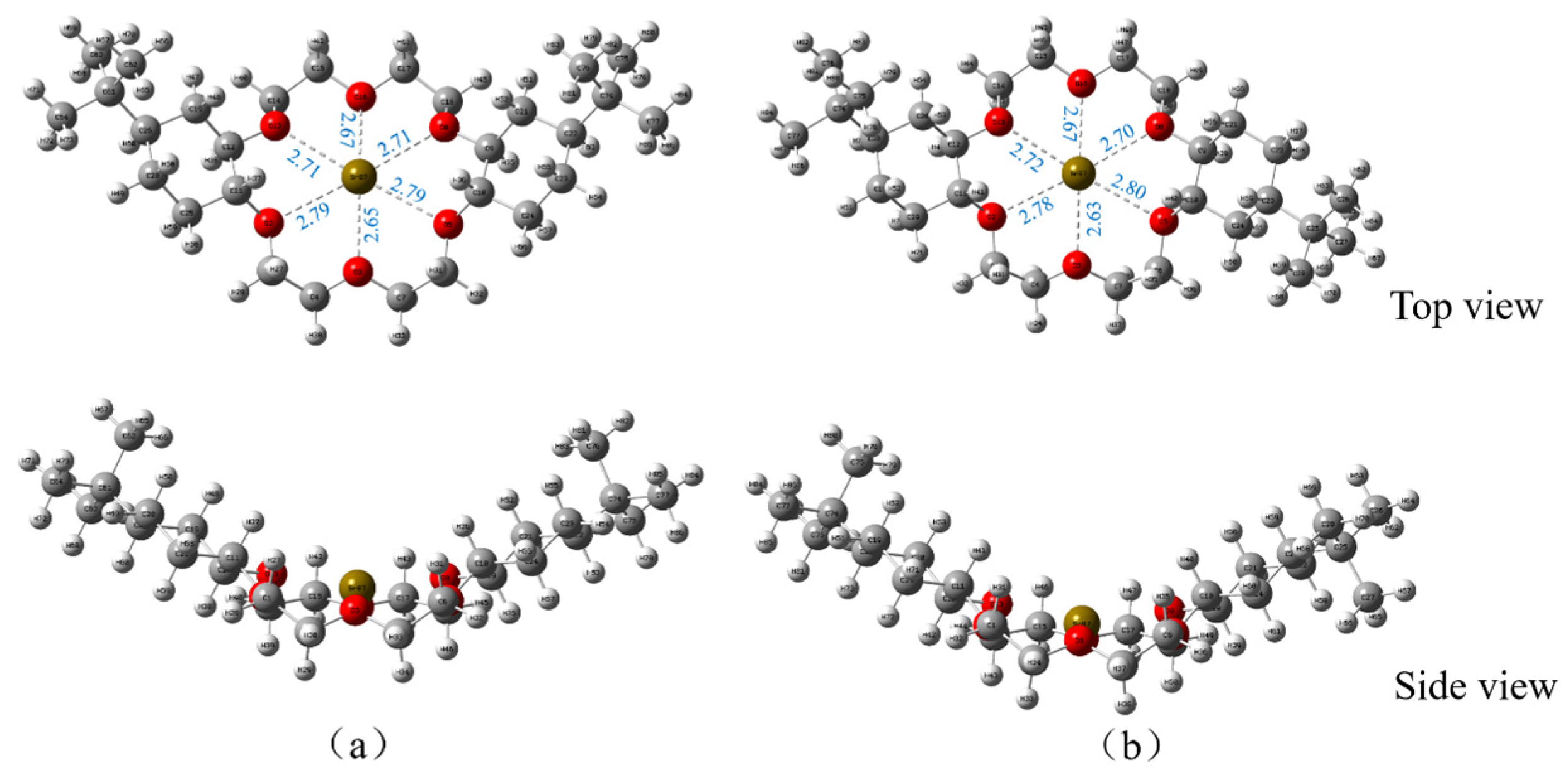
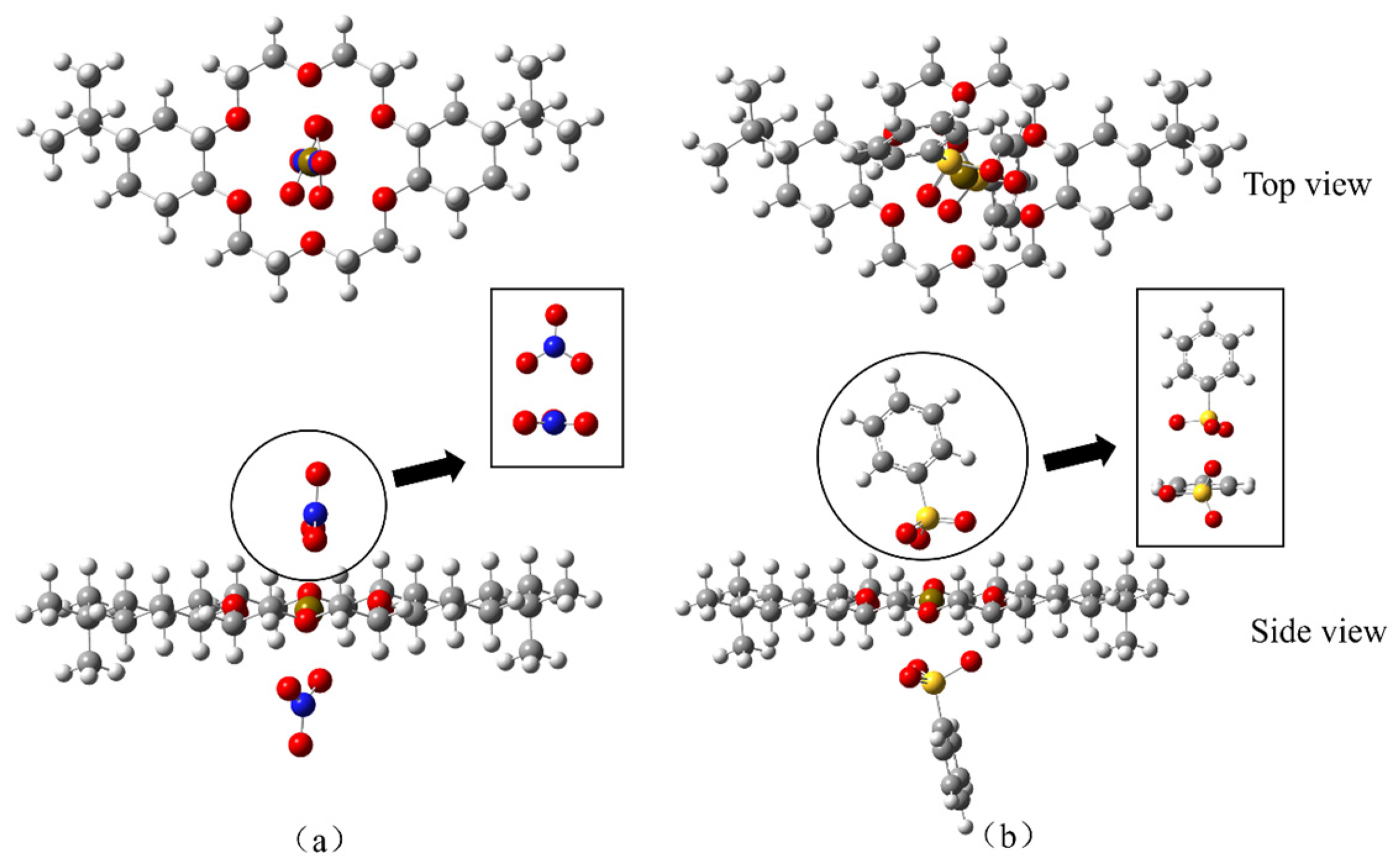
3.3.1. DtBuCH18C6-Sr(II) Complexation Model
3.3.2. Mulliken Charge Distribution
3.3.3. Binding Energy Analysis
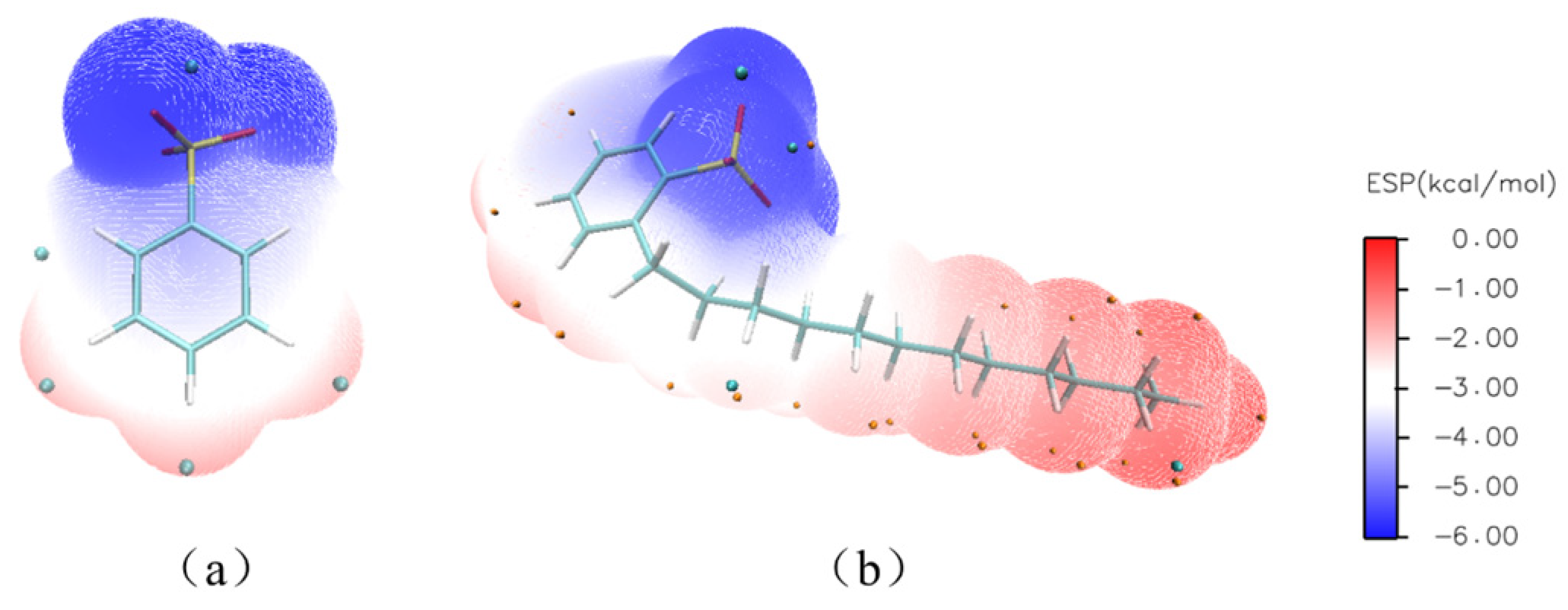
3.3.4. Anionic Coordination of DtBuCH18C6-Sr(II)
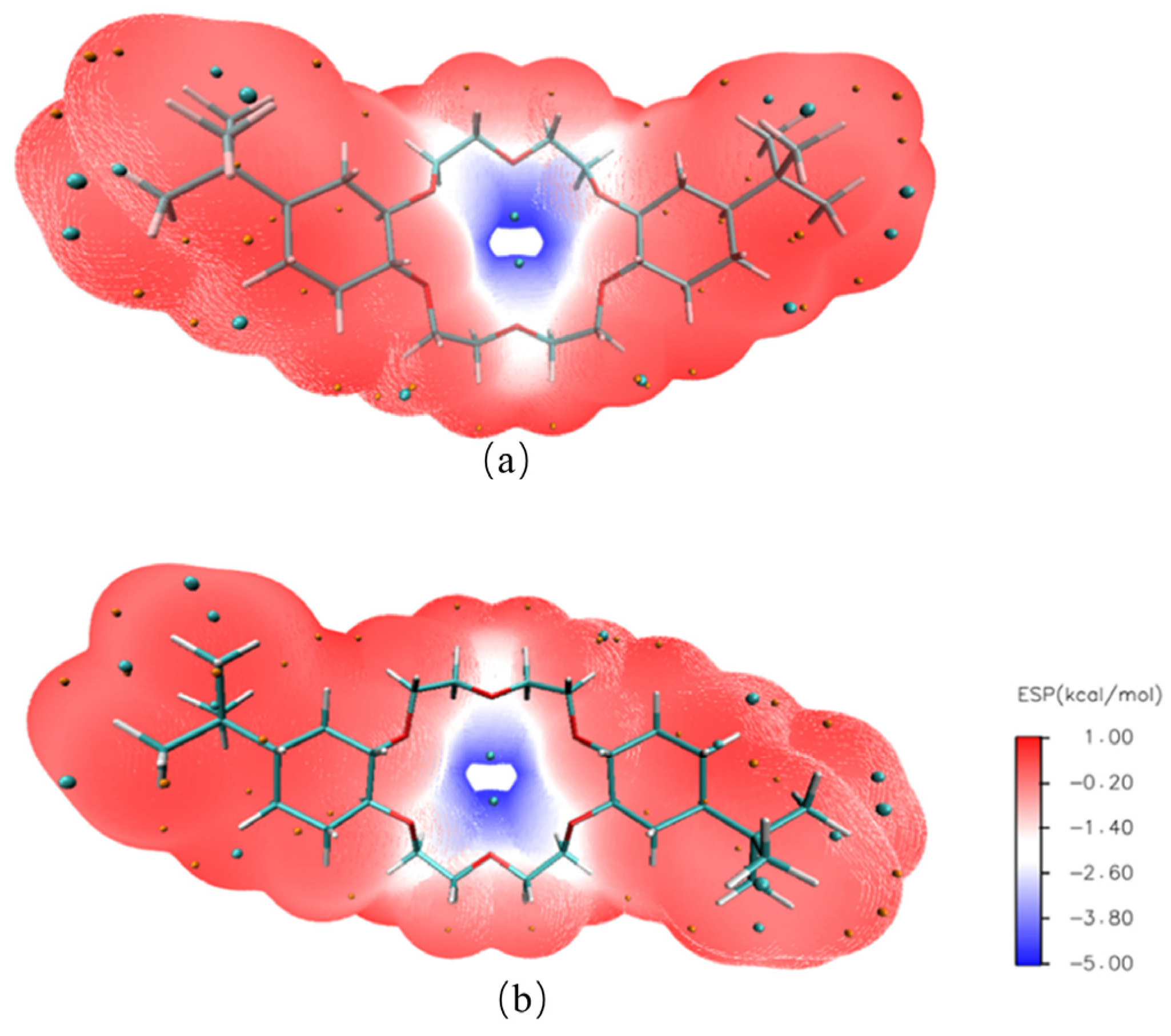
3.3.5. Interaction Force and Binding Energy Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wu, P.; Liu, H.; Sun, M.; Zeng, Y.; Ye, J.; Qin, S.; Cai, Y.; Feng, W.; Yuan, L. Covalent triazine frameworks for the selective sorption of palladium from highly acidic radioactive liquid wastes. J. Mater. Chem. A 2021, 9, 27320–27331. [Google Scholar] [CrossRef]
- Momen, M.A.; Dietz, M.L. Extraction chromatographic materials based on polysulfone microcapsules for the sorption of strontium from aqueous solution. React. Funct. Polym. 2021, 160, 104829. [Google Scholar] [CrossRef]
- Zhang, S.; Huang, Q.; Chen, L.; Yin, X.; Hamza, M.F.; Wei, Y.; Ning, S. Fast removal of strontium from nitric acid solution using an acid-resistant silica-based adsorbent. J. Environ. Chem. Eng. 2023, 11, 110529. [Google Scholar] [CrossRef]
- Schmidt, B.; Kegler, F.; Steinhauser, G.; Chyzhevskyi, I.; Dubchak, S.; Ivesic, C.; Koller-Peroutka, M.; Laarouchi, A.; Adlassnig, W. Uptake of Radionuclides by Bryophytes in the Chornobyl Exclusion Zone. Toxics 2023, 11, 218. [Google Scholar] [CrossRef]
- Zhang, A.; Wei, Y.; Kumagai, M. Synthesis of a novel macroporous silica-based polymeric material containing 4, 4′,(5′)-di (tert-butylcyclohexano)-18-crown-6 functional group and its adsorption mechanism for strontium. React. Funct. Polym. 2004, 61, 191–202. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Z.; Liu, S.; Zhang, G.; Dong, L.; Gu, P.; Hou, L.A. Layered metal sulfide NMTS for rapid removal of radioactive strontium ions from aqueous solution. Sep. Purif. Technol. 2023, 310, 122887. [Google Scholar] [CrossRef]
- Mironyuk, I.; Tatarchuk, T.; Vasylyeva, H.; Naushad, M.; Mykytyn, I. Adsorption of Sr(II) cations onto phosphated mesoporous titanium dioxide: Mechanism, isotherm and kinetics studies. J. Environ. Chem. Eng. 2019, 7, 103430. [Google Scholar] [CrossRef]
- Liu, F.; Liu, Y.; Xu, Y.; Ni, L.; Meng, X.; Hu, Z.; Zhong, G.; Meng, M.; Wang, Y.; Han, J. Efficient static and dynamic removal of Sr(II) from aqueous solution using chitosan ion-imprinted polymer functionalized with dithiocarbamate. J. Environ. Chem. Eng. 2015, 3, 1061–1071. [Google Scholar] [CrossRef]
- Amesh, P.; Suneesh, A.; Venkatesan, K.; Maheswari, R.U.; Vijayalakshmi, S. Preparation and ion exchange studies of cesium and strontium on sodium iron titanate. Sep. Purif. Technol. 2020, 238, 116393. [Google Scholar] [CrossRef]
- Peng, L.; Zhang, X.; Guo, L.; Li, J.; Zhang, W.; Shi, B. Rapid removal and easy separation recovery of Cs+ and Sr2+ by zirconium molybdopyrophosphate-functionalized collagen fibers. J. Clean. Prod. 2023, 414, 137653. [Google Scholar] [CrossRef]
- Merceille, A.; Weinzaepfel, E.; Barré, Y.; Grandjean, A. The sorption behaviour of synthetic sodium nonatitanate and zeolite A for removing radioactive strontium from aqueous wastes. Sep. Purif. Technol. 2012, 96, 81–88. [Google Scholar] [CrossRef]
- Sharma, J.N.; Khan, P.N.; Dhami, P.S.; Jagasia, P.; Tessy, V.; Kaushik, C.P. Separation of strontium-90 from a highly saline high level liquid waste solution using 4,4′(5′)-[di-tert-butyldicyclohexano]-18-crown-6 + isodecyl alcohol/n-dodecane solvent. Sep. Purif. Technol. 2019, 229, 115502. [Google Scholar] [CrossRef]
- Li, Q.; Wang, X.; Song, L.; He, L.; Yu, Q.; Xiao, X.; Ding, S. Cyclohexyl substituted diglycolamide ligands for highly efficient separation of strontium: Synthesis, extraction and crystallography studies. J. Environ. Chem. Eng. 2023, 11, 110495. [Google Scholar] [CrossRef]
- Ye, Z.; Zhang, Y.; Hou, L.-a.; Zhang, M.; Zhu, Y.; Yang, Y. Preparation of a GO/PB-modified nanofiltration membrane for removal of radioactive cesium and strontium from water. Chem. Eng. J. 2022, 446, 137143. [Google Scholar] [CrossRef]
- Mishra, S.; Anand, P.V.; Patra, C.; Sinha, P.K.; Mukhopadhyay, C.; Ravi, J.; Sivakumar, D.; Desigan, N.; Rajesh, P.; Rajeev, R. Solvent wash studies for the removal of di-butyl phosphate from spent solvent under simulated PUREX condition. J. Radioanal. Nucl. Chem. 2023, 1–11, 343–353. [Google Scholar] [CrossRef]
- Eom, H.H.; Kim, Y.; Harbottle, D.; Lee, J.W. Immobilization of KTS-3 on an electrospun fiber membrane for efficient removal of Cs+ and Sr2+. J. Environ. Chem. Eng. 2021, 9, 105991. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, X.; Kim, S.-Y.; Wei, Y. Simultaneous separation and recovery of Cs (I) and Sr (II) using a hybrid macrocyclic compounds loaded adsorbent. Kinetic, equilibrium and dynamic adsorption studies. J. Nucl. Sci. Technol. 2016, 53, 1968–1977. [Google Scholar] [CrossRef]
- Pin, C.; Briot, D.; Bassin, C.; Poitrasson, F. Concomitant separation of strontium and samarium-neodymium for isotopic analysis in silicate samples, based on specific extraction chromatography. Anal. Chim. Acta 1994, 298, 209–217. [Google Scholar] [CrossRef]
- De Muynck, D.; Huelga-Suarez, G.; Van Heghe, L.; Degryse, P.; Vanhaecke, F. Systematic evaluation of a strontium-specific extraction chromatographic resin for obtaining a purified Sr fraction with quantitative recovery from complex and Ca-rich matrices. J. Anal. At. Spectrom. 2009, 24, 1498–1510. [Google Scholar] [CrossRef]
- Li, C.-F.; Wang, X.-C.; Guo, J.-H.; Chu, Z.-Y.; Feng, L.-J. Rapid separation scheme of Sr, Nd, Pb, and Hf from a single rock digest using a tandem chromatography column prior to isotope ratio measurements by mass spectrometry. J. Anal. At. Spectrom. 2016, 31, 1150–1159. [Google Scholar] [CrossRef]
- Takahashi, T.; Ito, T.; Kim, S.-Y. Extraction behavior of Sr (II) from high-level liquid waste using ionic liquid extraction system with DtBuCH18C6. Energy Procedia 2017, 131, 170–177. [Google Scholar] [CrossRef]
- Wu, Y.; Kim, S.-Y.; Tozawa, D.; Ito, T.; Tada, T.; Hitomi, K.; Kuraoka, E.; Yamazaki, H.; Ishii, K. Equilibrium and kinetic studies of selective adsorption and separation for strontium using DtBuCH18C6 loaded resin. J. Nucl. Sci. Technol. 2012, 49, 320–327. [Google Scholar] [CrossRef]
- Wang, Y.; Wen, Y.; Mao, C.; Sang, H.; Wu, Y.; Li, H.; Wei, Y. Development of chromatographic process for the dynamic separation of 90Sr from high level liquid waste through breakthrough curve simulation and thermal analysis. Sep. Purif. Technol. 2022, 282, 120103. [Google Scholar] [CrossRef]
- Kudo, Y.; Koide, T.; Zhao, Y.; Katsuta, S.; Takeda, Y. Ion-pair Formation between Cd (II), Na (I), and Ag (I) complex ions with 18-crown-6 ether derivatives and various pairing anions in water: An understanding of the ion-pair formation based on the HSAB principle. Anal. Sci. 2011, 27, 1207–1211. [Google Scholar] [CrossRef]
- Nisola, G.M.; Parohinog, K.J.; Cho, M.K.; Burnea, F.K.B.; Lee, J.Y.; Seo, J.G.; Lee, S.-P.; Chung, W.-J. Covalently decorated crown ethers on magnetic graphene oxides as bi-functional adsorbents with tailorable ion recognition properties for selective metal ion capture in water. Chem. Eng. J. 2020, 389, 123421. [Google Scholar] [CrossRef]
- Deb, A.S.; Manju, M.; Sengupta, A.; Ali, S.M. Efficient separation of strontium ions from aqueous solution by dibenzo-18-crown-6 functionalized resin: Static and dynamic adsorption studies with computational DFT insights. Chem. Eng. J. Adv. 2022, 11, 100308. [Google Scholar] [CrossRef]
- Boda, A.; Ali, S.M.; Shenoi, M.R.; Rao, H.; Ghosh, S.K. DFT modeling on the suitable crown ether architecture for complexation with Cs+ and Sr2+ metal ions. J. Mol. Model. 2011, 17, 1091–1108. [Google Scholar] [CrossRef]
- Pathak, S.; Jayabun, S.; Boda, A.; Ali, S.M.; Sengupta, A. Experimental and theoretical insight into the extraction mechanism, kinetics, thermodynamics, complexation and radiolytic stability of novel calix crown ether in ionic liquid with Sr2+. J. Mol. Liq. 2020, 316, 113864. [Google Scholar] [CrossRef]
- Jensen, M.P.; Dzielawa, J.A.; Rickert, P.; Dietz, M.L. EXAFS Investigations of the Mechanism of Facilitated Ion Transfer into a Room-Temperature Ionic Liquid. J. Amer. Chem. Soc. 2002, 124, 10664–10665. [Google Scholar] [CrossRef]
- Mu, W.; Du, S.; Yu, Q.; Li, X.; Wei, H.; Yang, Y.; Peng, S. Highly efficient removal of radioactive 90Sr based on sulfonic acid-functionalized α-zirconium phosphate nanosheets. Chem. Eng. J. 2019, 361, 538–546. [Google Scholar] [CrossRef]
- Chen, Z.; Wu, Y.; Wei, Y. Adsorption characteristics and radiation stability of a silica-based DtBuCH18C6 adsorbent for Sr (II) separation in HNO3 medium. J. Radioanal. Nucl. Chem. 2014, 299, 485–491. [Google Scholar] [CrossRef]
- Ruthven, D.M. Adsorption (Chemical Engineering). In Encyclopedia of Physical Science and Technology, 3rd ed.; Meyers, R.A., Ed.; Academic Press: New York, NY, USA, 2003; pp. 251–271. [Google Scholar]
- Frisch, M.E.; Trucks, G.; Schlegel, H.B.; Scuseria, G.; Robb, M.; Cheeseman, J.; Scalmani, G.; Barone, V.; Petersson, G.; Nakatsuji, H. Gaussian 16; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef]
- Jing, Z.; Wang, G.; Zhou, Y.; Pang, D.; Zhu, F.; Liu, H. Selectivity of 18-crown-6 ether to alkali ions by density functional theory and molecular dynamics simulation. J. Mol. Liq. 2020, 311, 113305. [Google Scholar] [CrossRef]
- Zhang, A.; Xiao, C.; Liu, Y.; Hu, Q.; Chen, C.; Kuraoka, E. Preparation of macroporous silica-based crown ether materials for strontium separation. J. Porous Mater. 2010, 17, 153–161. [Google Scholar] [CrossRef]
- Hundal, G.; Hundal, M.S.; Obrai, S.; Poonia, N.; Kumar, S. Metal Complexes of Tetrapodal Ligands: Synthesis, Spectroscopic and Thermal Studies, and X-ray Crystal Structure Studies of Na (I), Ca (II), Sr (II), and Ba (II) Complexes of Tetrapodal Ligands N, N, N ‘, N ‘-Tetrakis (2-hydroxypropyl) ethylenediamine and N, N, N ‘, N ‘-Tetrakis (2-hydroxyethyl) ethylenediamine. Inorg. Chem. 2002, 41, 2077–2086. [Google Scholar] [CrossRef]
- Esbelin, E. Study of Molybdenum (VI) Complexation and Precipitation by Zirconium (IV) in Strongly Acid Medium. Application to Nuclear Spent Fuel Dissolution; U.S. Department of Energy: Oak Ridge, TN, USA, 1999.
- Takeuchi, M.; Aihara, H.; Nakahara, M.; Tanaka, K. Simulation study of sludge precipitation in spent fuel reprocessing. Procedia Chem. 2016, 21, 182–189. [Google Scholar] [CrossRef]
- Surrao, A.; Smith, S.W.; Foerster, E.; Spitz, H.B.; Graczyk, D.G.; Landero-Figueroa, J.A.; McLain, D.R.; Connick, W.B.; Steeb, J.L. Improving the separation of strontium and barium with Sr Resin using chelating eluent solutions. J. Radioanal. Nucl. Chem. 2019, 319, 1185–1192. [Google Scholar] [CrossRef]
- Fujie, K.; Kikuchi, T.; Kubota, H. Adsorption Separation Process: A Method for Prediction of the Breakthrough Time by Using the Short Column Test. Kagaku Kogaku Ronbunshu 2009, 35, 47–54. [Google Scholar] [CrossRef]
- Dennington, R.; Keith, T.A.; Millam, J.M. GaussView 6.0. 16; Semichem Inc.: Shawnee Mission, KS, USA, 2016. [Google Scholar]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Sun, P.; Liu, Y.; Mo, F.; Wu, M.; Xiao, Y.; Xiao, X.; Wang, W.; Dong, X. Efficient photocatalytic degradation of high-concentration moxifloxacin over dodecyl benzene sulfonate modified graphitic carbon nitride: Enhanced photogenerated charge separation and pollutant enrichment. J. Clean. Prod. 2023, 393, 136320. [Google Scholar] [CrossRef]
- Ruan, X.; Chen, Y.; Chen, H.; Qian, G.; Frost, R.L. Sorption behavior of methyl orange from aqueous solution on organic matter and reduced graphene oxides modified Ni–Cr layered double hydroxides. Chem. Eng. J. 2016, 297, 295–303. [Google Scholar] [CrossRef]
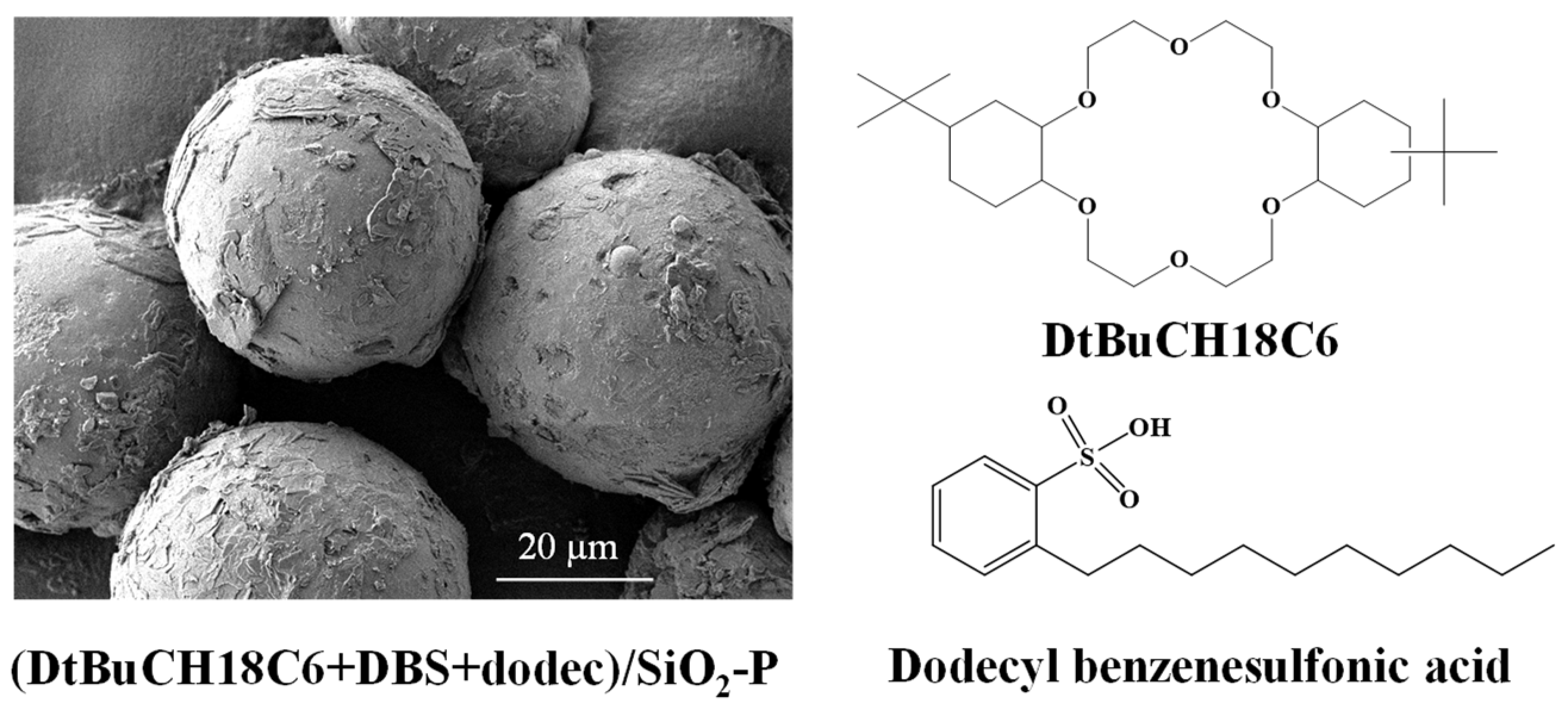
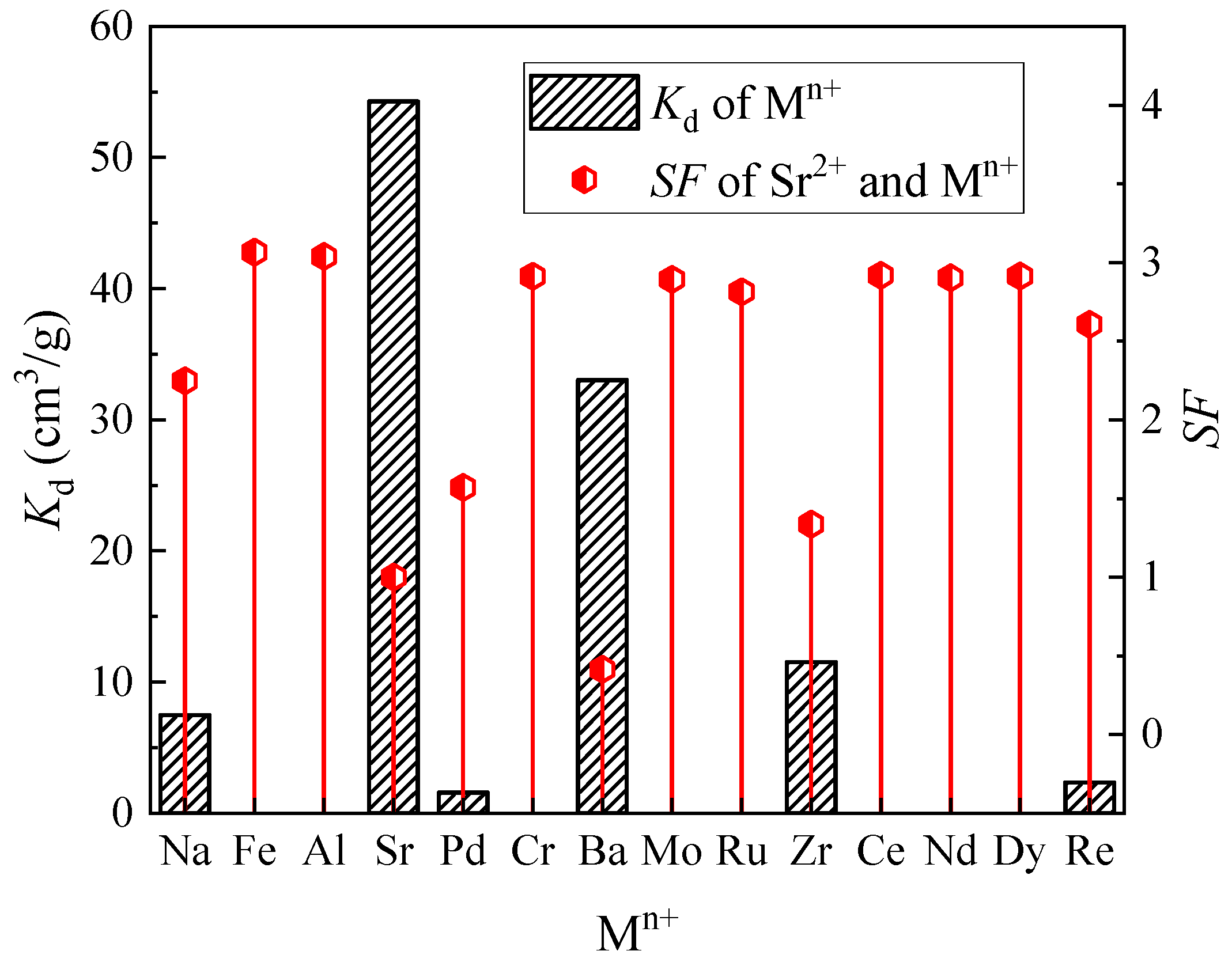


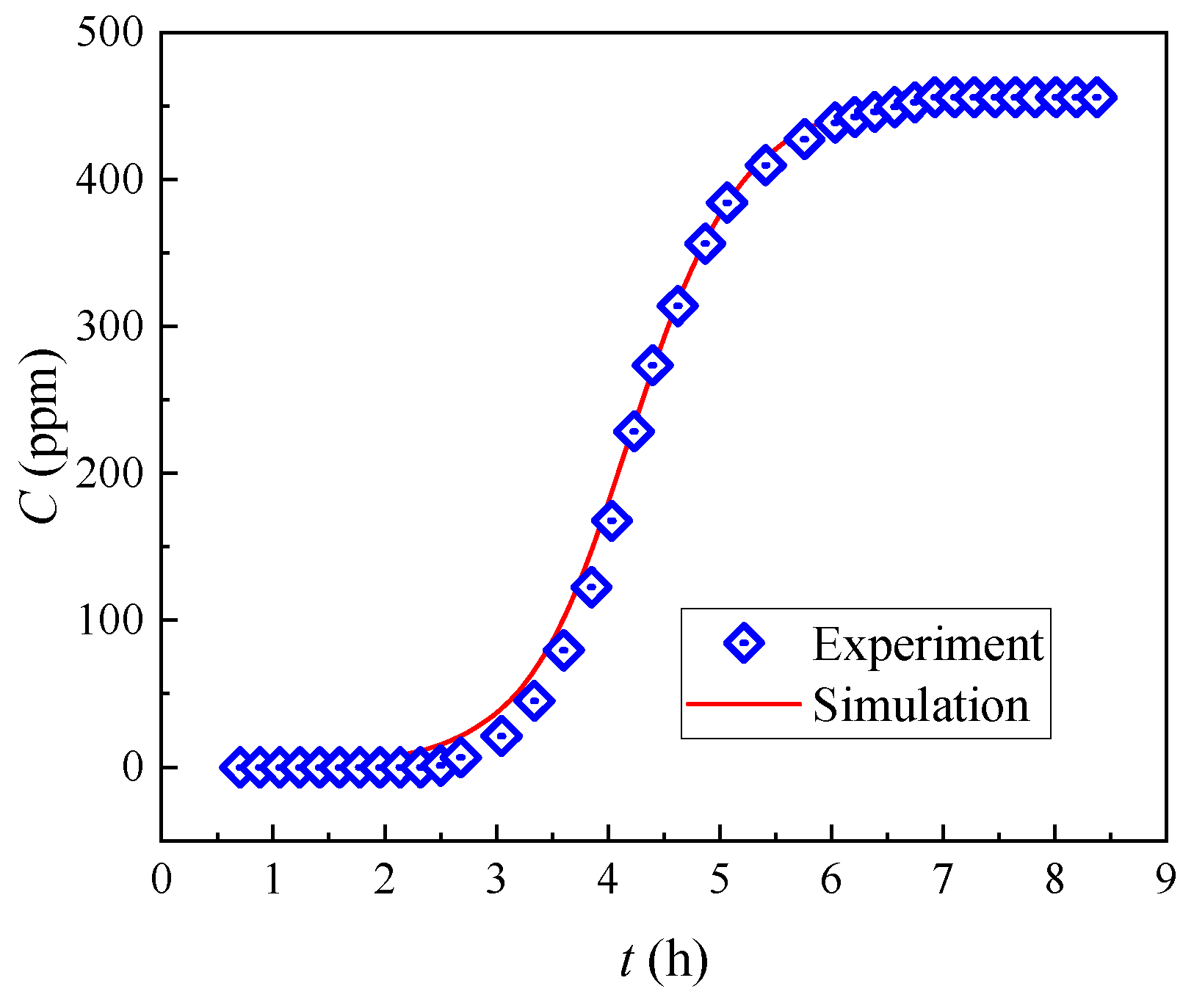

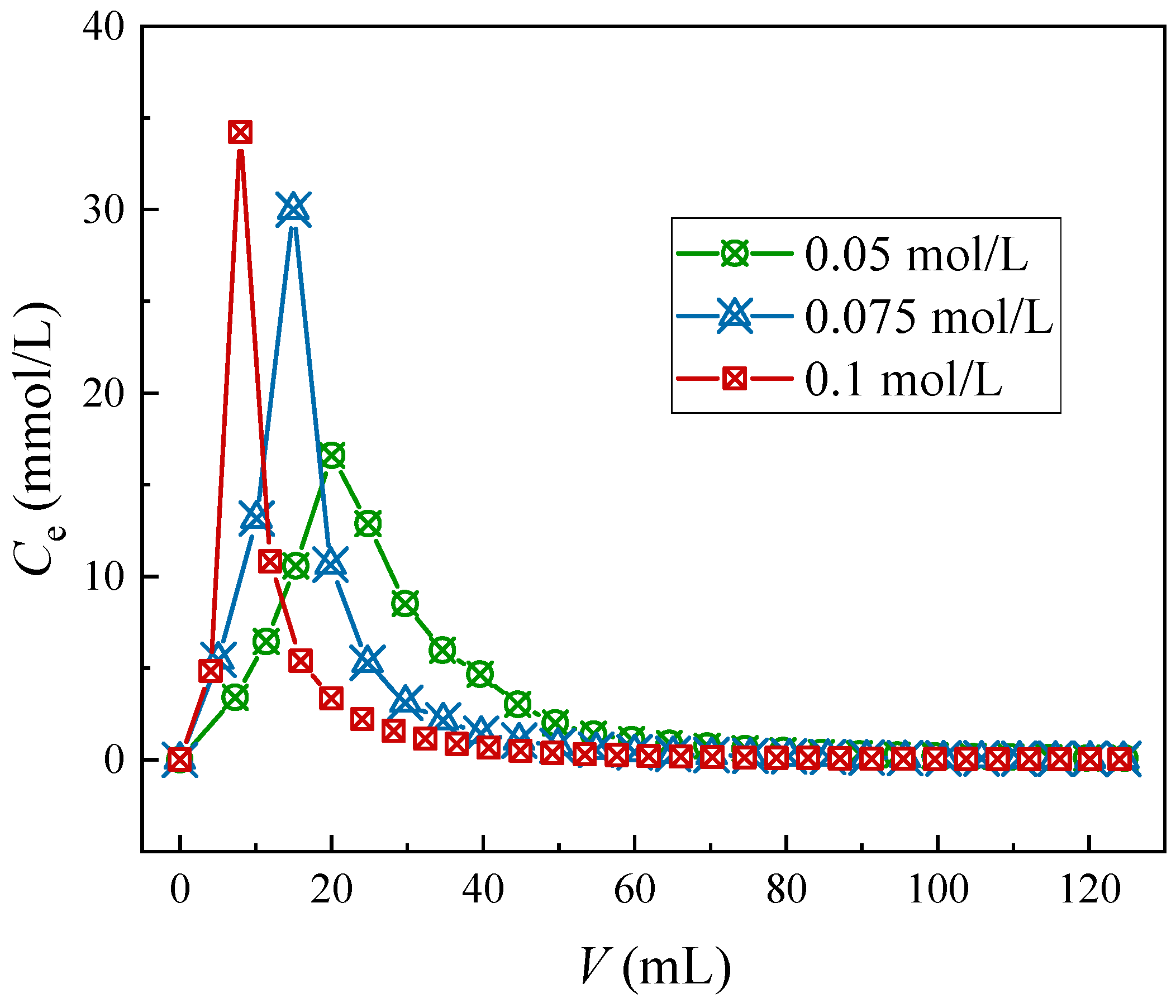


| Element | Conc. (g/L) | Element | Conc. (g/L) |
|---|---|---|---|
| Na | 31.2 g/L | Pd | 0.05 g/L |
| Nd | 0.7 g/L | Re | 0.2 g/L |
| Ce | 0.8 g/L | Ru | 1.2 g/L |
| Cr | 1.1 g/L | Sr | 0.9 g/L |
| Dy | 0.1 g/L | Zr | 0.1 g/L |
| Mo | 1.2 g/L | Al | 5.3 g/L |
| Fe | 4.8 g/L | Ba | 1.1 g/L |
| Flow Rate (mL/min) | 5% B. P. (BV) | 5% B. Cap. (mmol/g) | 100% B. P. (BV) | T. Cap. (mmol/g) | Utilization (%) |
|---|---|---|---|---|---|
| 0.3 | 13 | 0.22 | 25 | 0.24 | 88.17 |
| DtBuCH18C6 + DBS + Dodec | Silica Support | ||||
|---|---|---|---|---|---|
| Kfa1 | KF1 | b1 | Kfa2 | KF2 | b2 |
| 142.5 | 3.1011 | 0.3818 | 10 | 0.055 | 0.2 |
| Breakthrough Points | 5% BP | 100% BP | ||
|---|---|---|---|---|
| Flow rate (mL/min) | 0.3 | 0.5 [23] | 0.3 | 0.5 [23] |
| Simulation result (h) | 2.72 | 2.03 | 6.83 | 5.20 |
| Experiment data (h) | 3.05 | 2.56 | 6.92 | 4.53 |
| Elements | Recovery (%) | Elements | Recovery (%) | Elements | Recovery (%) |
|---|---|---|---|---|---|
| Sr | >99 | Pd | >99 | Ce | >99 |
| Ba | >99 | Cr | >99 | Nd | >99 |
| Na | 82.35 | Zr | 98.88 | Dy | >99 |
| Fe | >99 | Mo | >99 | Re | >99 |
| Al | >99 | Ru | 96.42 |
| Atoms | 4′,4″ | 4′,5″ | ||
|---|---|---|---|---|
| Gas | Water | Gas | Water | |
| Sr | 1.474 | 1.670 | 1.474 | 1.669 |
| O2 | −0.480 | −0.427 | −0.449 | −0.429 |
| O3 | −0.466 | −0.433 | −0.460 | −0.438 |
| O5 | −0.480 | −0.427 | −0.455 | −0.427 |
| O8 | −0.454 | −0.453 | −0.480 | −0.453 |
| O13 | −0.455 | −0.453 | −0.484 | −0.452 |
| O16 | −0.460 | −0.434 | −0.466 | −0.432 |
| Energy | 4′,4″ | 4′,5″ |
|---|---|---|
| Ecomplex (Hartree) | −1577.5760 | −1577.5758 |
| EDtBuCH18C6 (Hartree) | −1546.9517 | −1546.9517 |
| ESr(II) (Hartree) | −30.5711 | −30.5711 |
| EBSSE (Hartree) | 0.0014 | 0.0014 |
| ΔE (kJ·mol−1) | −135.9013 | −135.5275 |
| DBS | BSA | |
|---|---|---|
| HOMO–LUMO gap/eV | 7.866 | 7.665 |
| ADCH atomic charges/Angstrom | ||
| S | 0.666 | 0.661 |
| O1 | −0.463 | −0.512 |
| O2 | −0.446 | −0.512 |
| O3 | −0.508 | −0.494 |
| Energy | NO3− | C6H5O3S− |
|---|---|---|
| Ecomplex (Hartree) | −2137.8550 | −3287.3322 |
| EDtBuCH18C6 (Hartree) | −1546.9517 | −1546.9517 |
| ESr(II) (Hartree) | −30.5687 | −30.5687 |
| Eanion (Hartree) | −279.4531 | −854.7269 |
| ΔE (kJ·mol−1) | −861.5328 | −189.7551 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Sang, H.; Zheng, J.; Xu, L.; Liu, T.; Wei, Y. Study on Dynamic Column Behavior and Complexation Mechanism of DBS-Modified Crown Ether-Based Silica to 90Sr. Toxics 2023, 11, 919. https://doi.org/10.3390/toxics11110919
Wu Y, Sang H, Zheng J, Xu L, Liu T, Wei Y. Study on Dynamic Column Behavior and Complexation Mechanism of DBS-Modified Crown Ether-Based Silica to 90Sr. Toxics. 2023; 11(11):919. https://doi.org/10.3390/toxics11110919
Chicago/Turabian StyleWu, Yan, Hongji Sang, Jiawei Zheng, Lejin Xu, Tong Liu, and Yuezhou Wei. 2023. "Study on Dynamic Column Behavior and Complexation Mechanism of DBS-Modified Crown Ether-Based Silica to 90Sr" Toxics 11, no. 11: 919. https://doi.org/10.3390/toxics11110919
APA StyleWu, Y., Sang, H., Zheng, J., Xu, L., Liu, T., & Wei, Y. (2023). Study on Dynamic Column Behavior and Complexation Mechanism of DBS-Modified Crown Ether-Based Silica to 90Sr. Toxics, 11(11), 919. https://doi.org/10.3390/toxics11110919








