The Effects of Different Soil Component Couplings on the Methylation and Bioavailability of Mercury in Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Artificial Soil Preparation and Pot Experiment
2.2. Sample Collection
2.3. Chemical Analysis
2.4. Quality Assurance and Quality Control
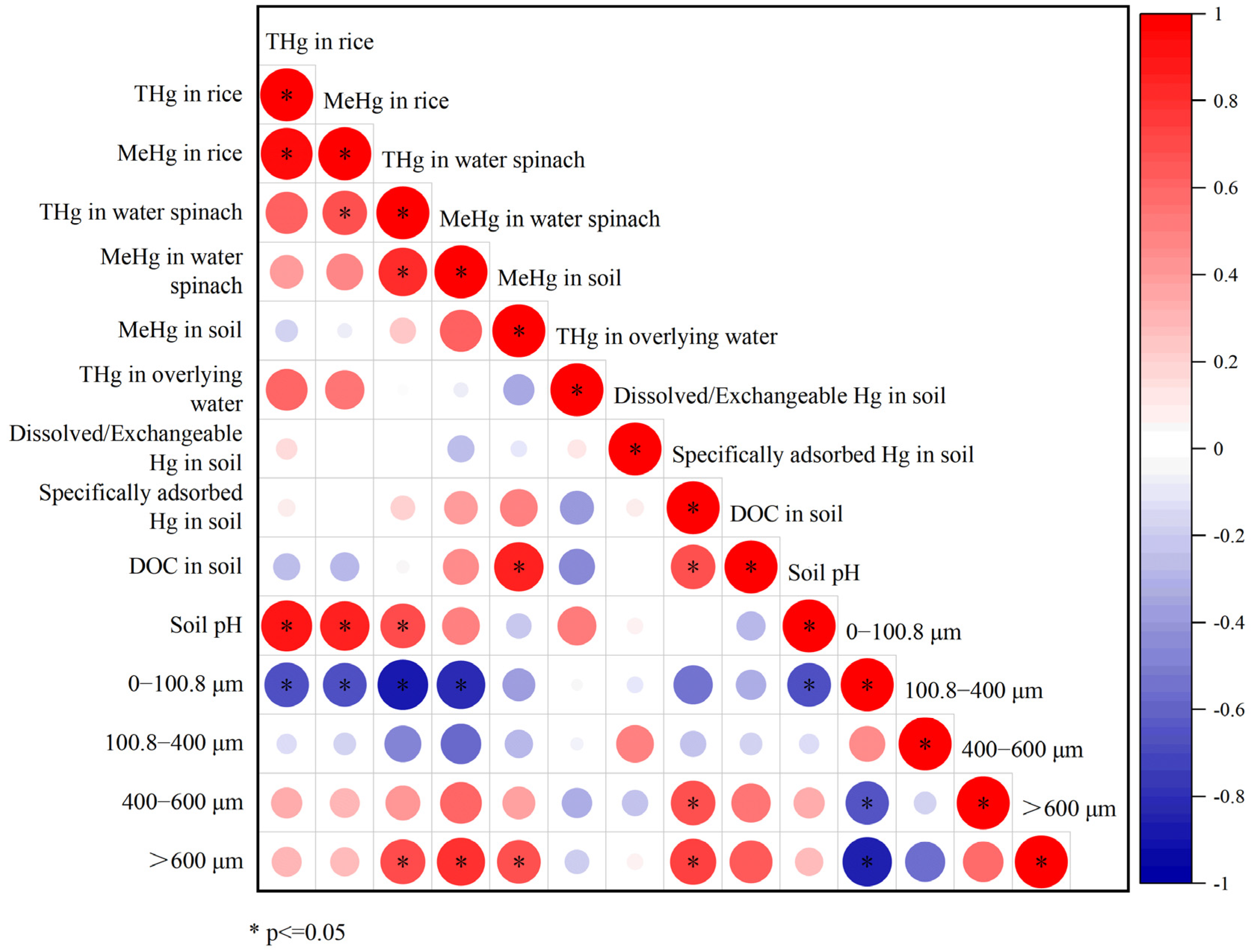
3. Results
3.1. Physicochemical Properties of the Soils
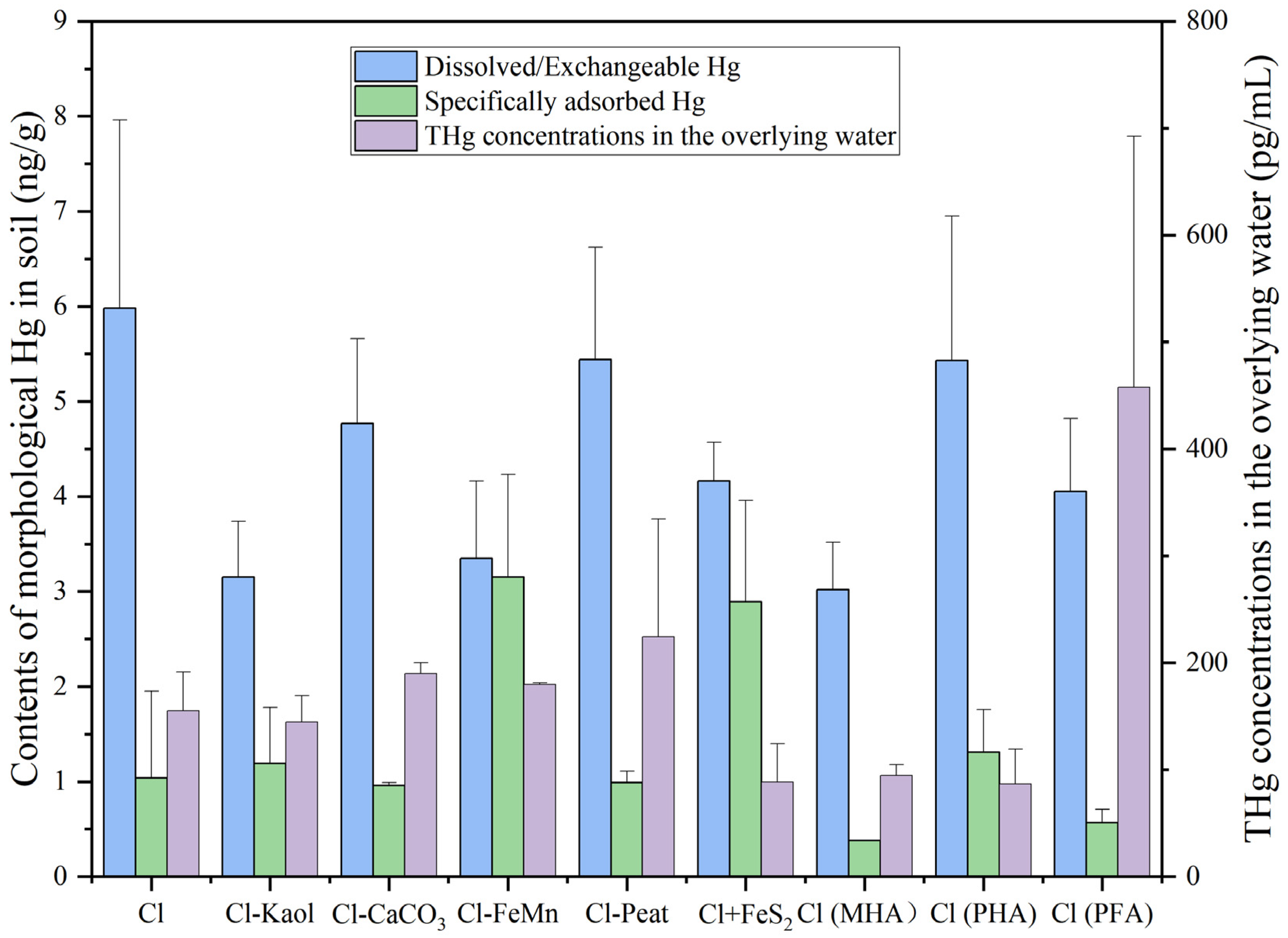
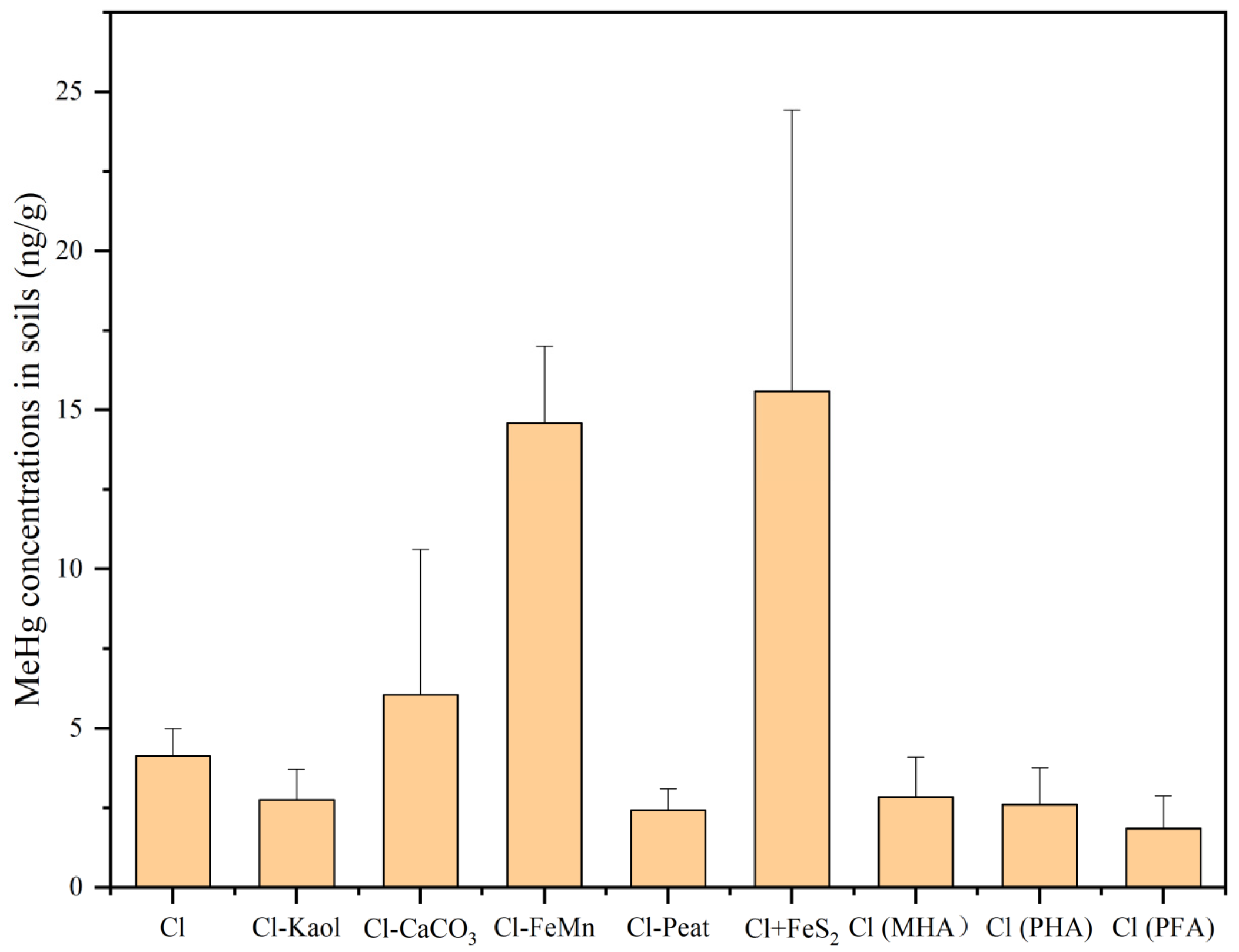
3.2. The Distributions of Hg and MeHg in the Soils
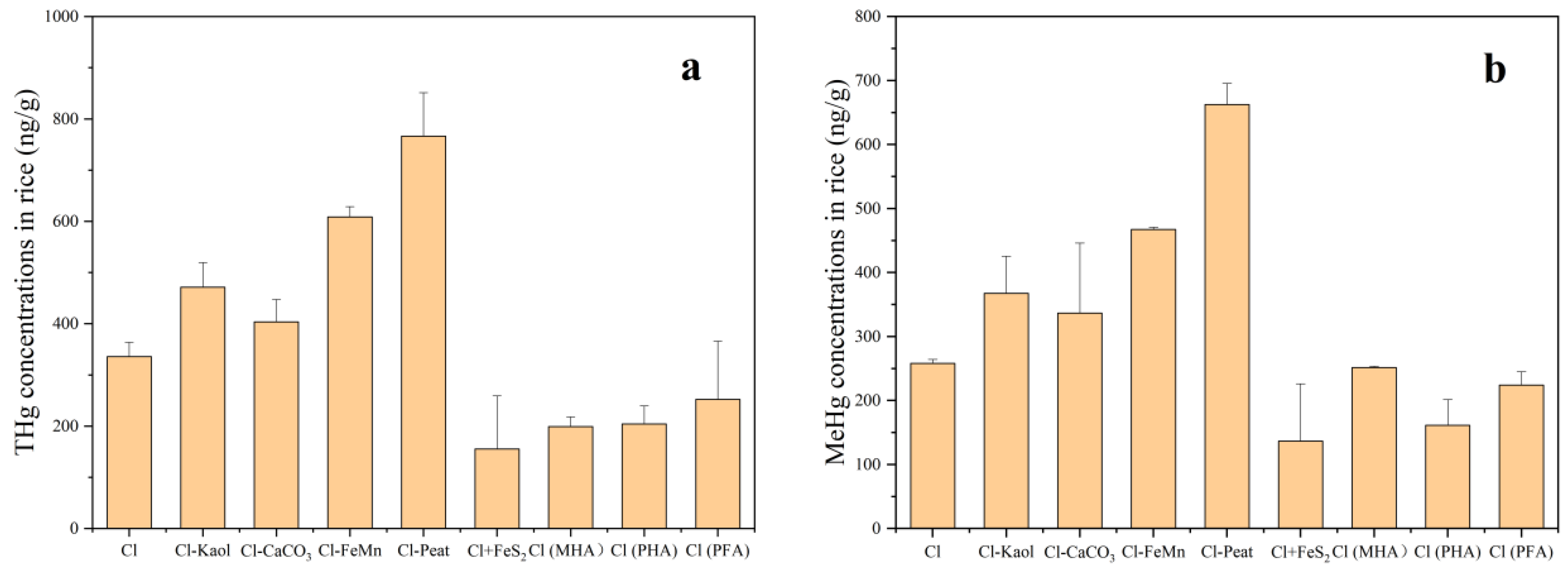
3.3. The Concentrations of THg and MeHg in Rice
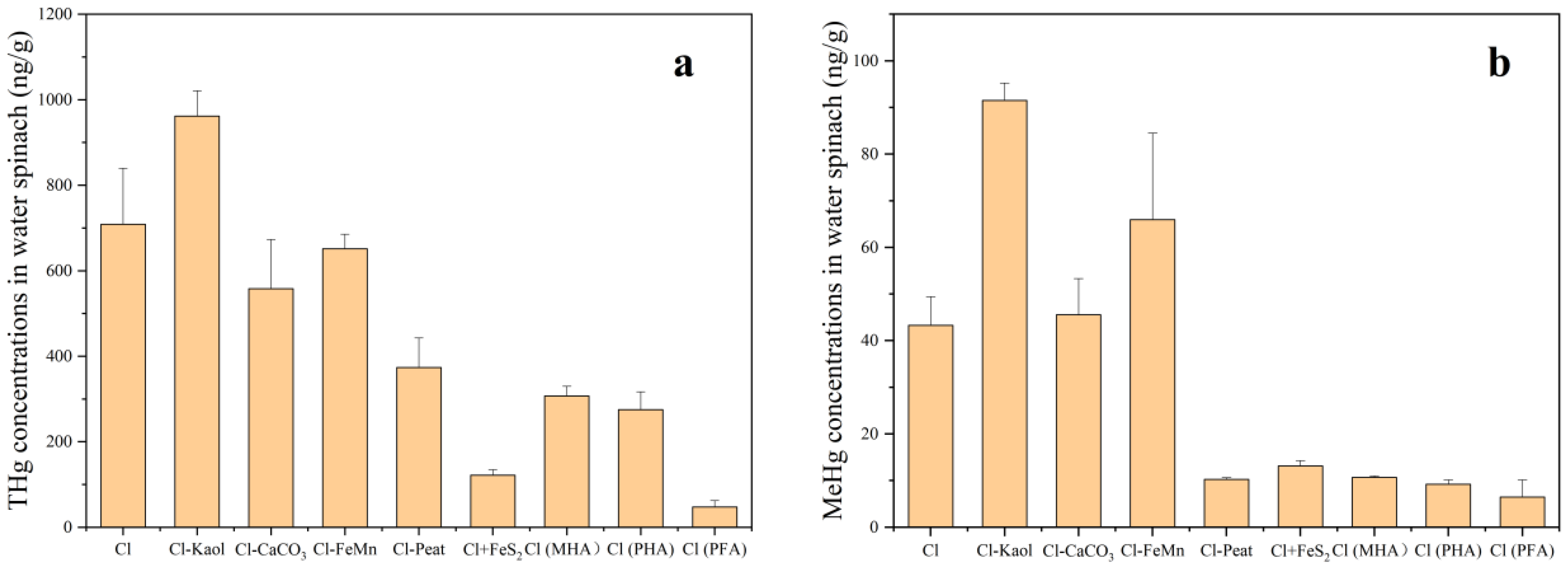
3.4. The Concentrations of THg and MeHg in Water Spinach
4. Discussion
4.1. The Effects of Soil Texture on Bioavailability of Hg in the Soil
4.2. The Effects of Soil Inorganic Components on the Methylation and Bioavailability of Hg in the Soil
4.3. The Effect of Organic Matter and Its Coupling with Inorganic Components on the Bioavailability of Mercury in Soil
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Y.; Yin, D.; Xiang, Y.; Xu, Q.; Zhang, C.; Xie, Q.; Wang, D. A Review of Studies on the Biogeochemical Behaviors of Mercury in the Three Gorges Reservoir, China. Bull. Environ. Contam. Toxicol. 2019, 102, 686–694. [Google Scholar] [PubMed]
- Beckers, F.; Rinklebe, J. Cycling of mercury in the environment: Sources, fate, and human health implications: A review. Crit. Rev. Environ. Sci. Technol. 2017, 47, 693–794. [Google Scholar]
- Meng, B.; Feng, X.; Qiu, G.; Anderson, C.W.N.; Wang, J.; Zhao, L. Localization and Speciation of Mercury in Brown Rice with Implications for Pan-Asian Public Health. Environ. Sci. Technol. 2014, 48, 7974–7981. [Google Scholar] [CrossRef]
- Qiu, G.; Feng, X.; Wang, S.; Shang, L. Mercury and methylmercury in riparian soil, sediments, mine-waste calcines, and moss from abandoned Hg mines in east Guizhou province, southwestern China. Appl. Geochem. 2005, 20, 627–638. [Google Scholar] [CrossRef]
- Tang, W.; Su, Y.; Gao, Y.; Zhong, H. Effects of Farming Activities on the Biogeochemistry of Mercury in Rice-Paddy Soil Systems. Bull. Environ. Contam. Toxicol. 2019, 102, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Ullrich, S.M.; Tanton, T.W.; Abdrashitova, S.A. Mercury in the aquatic environment: A review of factors affecting methylation. Crit. Rev. Environ. Sci. Technol. 2001, 31, 241–293. [Google Scholar] [CrossRef]
- Zhu, H.; Zhong, H.; Wu, J. Incorporating rice residues into paddy soils affects methylmercury accumulation in rice. Chemosphere 2016, 152, 259–264. [Google Scholar] [CrossRef]
- Meng, B.; Feng, X.; Qiu, G.; Cai, Y.; Wang, D.; Li, P.; Shang, L.; Sommar, J. Distribution patterns of inorganic mercury and methylmercury in tissues of rice (Oryza sativa L.) plants and possible bioaccumulation pathways. J. Agric. Food Chem. 2010, 58, 4951–4958. [Google Scholar] [CrossRef]
- Jing, Y.D.; He, Z.L.; Yang, X.E. Effects of pH, organic acids, and competitive cations on mercury desorption in soils. Chemosphere 2007, 69, 1662–1669. [Google Scholar] [CrossRef]
- Parks, J.M.; Johs, A.; Podar, M.; Bridou, R.; Hurt, R.A.; Smith, S.D.; Tomanicek, S.J.; Qian, Y.; Brown, S.D.; Brandt, C.C.; et al. The Genetic Basis for Bacterial Mercury Methylation. Science 2013, 339, 1332–1335. [Google Scholar]
- Tang, Z.; Fan, F.; Deng, S.; Wang, D. Mercury in rice paddy fields and how does some agricultural activities affect the translocation and transformation of mercury—A critical review. Ecotoxicol. Environ. Saf. 2020, 202, 110950. [Google Scholar] [CrossRef]
- Zhao, L.; Meng, B.; Feng, X. Mercury methylation in rice paddy and accumulation in rice plant: A review. Ecotoxicol. Environ. Saf. 2020, 195, 110462. [Google Scholar] [CrossRef] [PubMed]
- Yin, D.; He, T.; Yin, R.; Zeng, L. Effects of soil properties on production and bioaccumulation of methylmercury in rice paddies at a mercury mining area, China. J. Environ. Sci. 2018, 68, 194–205. [Google Scholar] [CrossRef]
- Sipkova, A.; Szakova, J.; Hanc, A.; Tlustos, P. Mobility of mercury in soil as affected by soil physicochemical properties. J. Soils Sediments 2016, 16, 2234–2241. [Google Scholar] [CrossRef]
- Chen, T.C.; Huang, G.; Liu, C.; Chen, C.; Chuang, S.; Huang, Y. Novel effective waste iron oxide-coated magnetic adsorbent for phosphate adsorption. Desalin. Water Treat. 2014, 52, 766–774. [Google Scholar] [CrossRef]
- Li, S.; Wang, M.; Zhao, Z.; Ma, C.; Chen, S. Adsorption and Desorption of Cd by Soil Amendment: Mechanisms and Environmental Implications in Field-Soil Remediation. Sustainability 2018, 10, 2337. [Google Scholar] [CrossRef]
- Yin, H.; Zhu, J. In situ remediation of metal contaminated lake sediment using naturally occurring, calcium-rich clay mineral-based low-cost amendment. Chem. Eng. J. 2016, 285, 112–120. [Google Scholar] [CrossRef]
- Liu, J.; Valsaraj, K.T.; Delaune, R.D. Inhibition of Mercury Methylation by Iron Sulfides in an Anoxic Sediment. Environ. Eng. Sci. 2009, 26, 833–840. [Google Scholar] [CrossRef]
- Tang, W.; Hintelmann, H.; Gu, B.; Feng, X.; Liu, Y.; Gao, Y.; Zhao, J.; Zhu, H.; Lei, P.; Zhong, H. Increased Methylmercury Accumulation in Rice after Straw Amendment. Environ. Sci. Technol. 2019, 53, 6144–6153. [Google Scholar] [CrossRef]
- He, M.; Tian, L.; Braaten, H.F.V.; Wu, Q.; Luo, J.; Cai, L.; Meng, J.; Lin, Y. Mercury-Organic Matter Interactions in Soils and Sediments: Angel or Devil? Bull. Environ. Contam. Toxicol. 2019, 102, 621–627. [Google Scholar] [CrossRef]
- Yu, G.; Wu, H.; Qing, C.; Jiang, X.; Zhang, J. Bioavailability of Humic Substance-Bound Mercury to Lettuce and its Relationship with Soil Properties. Commun. Soil Sci. Plant Anal. 2004, 35, 1123–1139. [Google Scholar] [CrossRef]
- Chai, X.; Liu, G.; Wu, J.; Tong, H.; Ji, R.; Zhao, Y. Effects of fulvic substances on the distribution and migration of Hg in landfill leachate. J. Environ. Monit. 2011, 13, 1464. [Google Scholar]
- Liu, Y.; Zhi, L.; Zhou, S.; Xie, F. Effects of mercury binding by humic acid and humic acid resistance on mercury stress in rice plants under high Hg/humic acid concentration ratios. Environ. Sci. Pollut. Res. 2020, 27, 18650–18660. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.Y.; Qing, C.L.; Guo, T.Y.; Guo, Y.J. Effects of humic acid on transport and transformation of mercury in soil-plant systems. Water Air Soil Pollut. 1997, 95, 35–43. [Google Scholar] [CrossRef]
- Yao, A.; Qing, C.; Mu, S.; Reardon, E.J. Effects of humus on the environmental activity of mineral-bound Hg: Influence on Hg volatility. Appl. Geochem. 2006, 21, 446–454. [Google Scholar]
- Ran, S.; He, T.; Zhou, X.; Yin, D. Effects of fulvic acid and humic acid from different sources on Hg methylation in soil and accumulation in rice. J. Environ. Sci. 2022, 119, 93–105. [Google Scholar] [CrossRef]
- Cheshire, M.V.; Dumat, C.; Fraser, A.R.; Hillier, S.; Staunton, S. The interaction between soil organic matter and soil clay minerals by selective removal and controlled addition of organic matter. Eur. J. Soil. Sci. 2000, 51, 497–509. [Google Scholar] [CrossRef]
- Wei, C.; Xie, D.; Li, B. Progress in resaerch on soil ogano-mineral complexes. Adv. Earth Sci. 2003, 18, 221–227. (In Chinese) [Google Scholar]
- Tombácz, E.; Libor, Z.; Illés, E.; Majzik, A.; Klumpp, E. The role of reactive surface sites and complexation by humic acids in the interaction of clay mineral and iron oxide particles. Org. Geochem. 2004, 35, 257–267. [Google Scholar] [CrossRef]
- Zhuang, J.; Yu, G. Effects of surface coatings on electrochemical properties and contaminant sorption of clay minerals. Chemosphere 2002, 49, 619–628. [Google Scholar] [CrossRef]
- Brogowski, Z.; Glinski, J.; Wilgat, M. distribution of some trace elements in size fractions of two profiles of soils formed from boulder loams. Zesz Probl Postep. Nauk. Roln 1977, 197, 309–318. [Google Scholar]
- Leinweber, P.; Paetsch, C.; Schulten, H.R. Heavy metal retention by organo-mineral particle-size fractions from soils in long-term agricultural experiments. Arch. Agron. Soil Sci. 1995, 39, 271–285. [Google Scholar] [CrossRef]
- Davies, K.S.; Shaw, G. Fixation of 137Cs by soils and sediments in the Esk Estuary, Cumbria, UK. Sci. Total Environ. 1993, 132, 71–92. [Google Scholar] [CrossRef]
- Cho, K.; Kang, J.; Kim, S.; Purev, O.; Myung, E.; Kim, H.; Choi, N. Effect of inorganic carbonate and organic matter in thermal treatment of mercury-contaminated soil. Environ. Sci. Pollut. Res. 2021, 28, 48184–48193. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Drosos, M.; Hu, H.; He, X.; Wang, G.; Zhang, H.; Hu, Z.; Xi, B. Organic amendments affect dissolved organic matter composition and mercury dissolution in pore waters of mercury-polluted paddy soil. Chemosphere 2019, 232, 356–365. [Google Scholar] [CrossRef]
- Li, Y.; He, X.; Wang, Y.; Guan, J.; Guo, J.; Xu, B.; Chen, Y.; Wang, G. Organic fertilizer amendment increases methylmercury accumulation in rice plants. Chemosphere 2020, 249, 126166. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Zhang, Q.; Hu, W.; Zhao, J.; Chen, Y.; Zhong, H.; Wang, G.; Zhang, Z.; Gao, Y. Elemental sulfur amendment enhance methylmercury accumulation in rice (Oryza sativa L.) grown in Hg mining polluted soil. J. Hazard. Mater. 2019, 379, 120701. [Google Scholar] [CrossRef]
- Liu, G.; Wang, J.; Liu, X.; Liu, X.; Li, X.; Ren, Y.; Wang, J.; Dong, L. Partitioning and geochemical fractions of heavy metals from geogenic and anthropogenic sources in various soil particle size fractions. Geoderma 2018, 312, 104–113. [Google Scholar] [CrossRef]
- Tang, Z.; Fan, F.; Wang, X.; Shi, X.; Deng, S.; Wang, D. Mercury in rice (Oryza sativa L.) and rice-paddy soils under long-term fertilizer and organic amendment. Ecotoxicol. Environ. Saf. 2018, 150, 116–122. [Google Scholar] [CrossRef]
- Tao, S.; Qing, X.; Chuxian, L.; Jinyong, H.; Caipeng, Y.; Xuejie, Z.; Dingyong, W. Inorganic versus organic fertilizers: How do they lead to methylmercury accumulation in rice grains. Environ. Pollut. 2022, 314, 120341. [Google Scholar]
- Wang, Y.; Chen, L.; Chen, Y.; Xue, Y.; Liu, G.; Zheng, X.; Zhou, L.; Zhong, H. Effects of varying amounts of different biochars on mercury methylation in paddy soils and methylmercury accumulation in rice (Oryza sativa L.). Sci. Total Environ. 2023, 874, 162459. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, C.; Shi, X.; Lin, T.; Wang, D. Effect of organic matter and pH on mercury release from soils. J. Environ. Sci. 2007, 19, 1349–1354. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Dai, J.; Wang, R.; Li, F.; Wang, W. Adsorption and desorption of divalent mercury (Hg2+) on humic acids and fulvic acids extracted from typical soils in China. Colloid Surf. A 2009, 335, 194–201. [Google Scholar] [CrossRef]
- Zhong, S.; Qiu, G.; Feng, X.; Lin, C.; Bishop, K. Sulfur and iron influence the transformation and accumulation of mercury and methylmercury in the soil-rice system. J. Soils Sediments 2018, 18, 578–585. [Google Scholar] [CrossRef]
- Yao, C.; He, T. Effect of peat and thiol-modified peat application on mercury (im)mobilization in mercury-polluted paddy soil. Ecotoxicol. Environ. Saf. 2023, 254, 114743. [Google Scholar] [CrossRef]
- Zhuang, S. Preliminary Study on Enhanced Phytoremediation of Mercury-Contaminated Farmland Soils. Ph.D. Thesis, Shanghai Normal University, Shanghai, China, 2018. [Google Scholar]
- Peng, G. Regulating Effects of Humic Acids on the Speciation and Bioavailability of Mercury in Soils and Its Mechanisms. Ph.D. Thesis, Southwest University, Chongqing, China, 2012. [Google Scholar]
- Zhong, H.; Wang, W. Effects of sediment composition on inorganic mercury partitioning, speciation and bioavailability in oxic surficial sediments. Environ. Pollut. 2008, 151, 222–230. [Google Scholar] [CrossRef]
- Gondar, D.; Lopez, R.; Fiol, S.; Antelo, J.M.; Arce, F. Characterization and acid–base properties of fulvic and humic acids isolated from two horizons of an ombrotrophic peat bog. Geoderma 2005, 126, 367–374. [Google Scholar] [CrossRef]
- Bloom, N. Determination of Picogram Levels of Methylmercury by Aqueous Phase Ethylation, Followed by Cryogenic Gas Chromatography with Cold Vapour Atomic Fluorescence Detection. Can. J. Fish. Aquat. Sci. 1989, 46, 1131–1140. [Google Scholar] [CrossRef]
- He, T.; Feng, X.; Guo, Y.; Qiu, G.; Li, Z.; Liang, L.; Lu, J. The impact of eutrophication on the biogeochemical cycling of mercury species in a reservoir: A case study from Hongfeng Reservoir, Guizhou, China. Environ. Pollut. 2008, 154, 56–67. [Google Scholar] [CrossRef]
- Liang, L.; Horvat, M.; Cernichiari, E.; Gelein, B.; Balogh, S. Simple solvent extraction technique for elimination of matrix interferences in the determination of methylmercury in environmental and biological samples by ethylation-gas chromatography-cold vapor atomic fluorescence spectrometry. Talanta 1996, 43, 1883–1888. [Google Scholar] [CrossRef]
- Feng, X.; Li, P.; Qiu, G.; Wang, S.; Li, G.; Shang, L.; Meng, B.; Jiang, H.; Bai, W.; Li, Z.; et al. Human exposure to methylmercury through rice intake in mercury mining areas, Guizhou province, China. Environ. Sci. Technol. 2008, 42, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Horvat, M.; Feng, X.; Shang, L.; Li, H.; Pang, P. Re-evaluation of distillation and comparison with HNO3 leaching/solvent extraction for isolation of methylmercury compounds from sediment/soil samples. Appl. Organomet. Chem. 2004, 18, 264–270. [Google Scholar] [CrossRef]
- Wang, J.; Feng, X.; Anderson, C.W.N.; Qiu, G.; Ping, L.; Bao, Z. Ammonium thiosulphate enhanced phytoextraction from mercury contaminated soil—Results from a greenhouse study. J. Hazard. Mater. 2011, 186, 119–127. [Google Scholar] [CrossRef]
- Huang, B.; Yuan, Z.; Li, D.; Zheng, M.; Nie, X.; Liao, Y. Effects of soil particle size on the adsorption, distribution, and migration behaviors of heavy metal(loid)s in soil: A review. Environ. Sci. Process. Impacts 2020, 22, 1596–1615. [Google Scholar] [CrossRef]
- Ajmone-Marsan, F.; Biasioli, M.; Kralj, T.; Grčman, H.; Davidson, C.M.; Hursthouse, A.S.; Madrid, L.; Rodrigues, S. Metals in particle-size fractions of the soils of five European cities. Environ. Pollut. 2008, 152, 73–81. [Google Scholar] [CrossRef]
- Tian, Z.; Pan, Y.; Chen, M.; Zhang, S.; Chen, Y. The relationships between fractal parameters of soil particle size and heavy-metal content on alluvial-proluvial fan. J. Contam. Hydrol. 2023, 254, 104140. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Wang, J.J.; Xiao, R.; Pensky, S.M.; Kongchum, M.; DeLaune, R.D.; Seo, D. Mercury adsorption in the Mississippi River deltaic plain freshwater marsh soil of Louisiana Gulf coastal wetlands. Chemosphere 2018, 195, 455–462. [Google Scholar] [CrossRef]
- Wang, Y.; He, T.; Yin, D.; Han, Y.; Zhou, X.; Zhang, G.; Tian, X. Modified clay mineral: A method for the remediation of the mercury-polluted paddy soil. Ecotoxicol. Environ. Saf. 2020, 204, 111121. [Google Scholar] [CrossRef]
- Ran, S.; He, T.; Li, S.; Yin, D.; Wu, P.; Xu, Y.; Zhao, J. Selenium/sulfur-modified montmorillonite materials mitigate mercury pollution in farmland. Environ. Pollut. 2023, 329, 121719. [Google Scholar] [CrossRef]
- Lu, X.; Huangfu, X.; Zhang, X.; Wang, Y.; Ma, J. Removal of trace mercury (II) from aqueous solution by in situ MnO(x) combined with poly-aluminum chloride. J. Water Health 2015, 13, 383–393. [Google Scholar] [CrossRef]
- Feng, X.H.; Zhai, L.M.; Tan, W.F.; Liu, F.; He, J.Z. Adsorption and redox reactions of heavy metals on synthesized Mn oxide minerals. Environ. Pollut. 2007, 147, 366–373. [Google Scholar] [CrossRef]
- Hu, Z.; Zhu, Y.; Li, M.; Zhang, L.; Cao, Z.; Smith, F.A. Sulfur (S)-induced enhancement of iron plaque formation in the rhizosphere reduces arsenic accumulation in rice (Oryza sativa L.) seedlings. Environ. Pollut. 2007, 147, 387–393. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, J.; Zhang, B.; Liu, Y.; Xu, X.; Li, Y.; Li, B.; Gao, Y.; Chai, Z. The influence of iron plaque on the absorption, translocation and transformation of mercury in rice (Oryza sativa L.) seedlings exposed to different mercury species. Plant Soil 2016, 398, 87–97. [Google Scholar] [CrossRef]
- Skyllberg, U.; Drott, A. Competition between disordered iron sulfide and natural organic matter associated thiols for mercury(II)-an EXAFS study. Environ. Sci. Technol. 2010, 44, 1254–1259. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Dang, F.; Zhao, J.; Zhong, H. Selenium inhibits sulfate-mediated methylmercury production in rice paddy soil. Environ. Pollut. 2016, 213, 232–239. [Google Scholar] [CrossRef]
- Campbell, P.; Lewis, A.G.; Chapman, P.M.; Crowder, A.A.; Fletcher, W.K.; Imber, B.; Luoma, S.N.; Stokes, P.M.; Winfrey, M. Biologically Available Metals in Sediments (NRCC No 27694); National Research Council of Canada, Ottawa: Ottawa, ON, Canada, 1988. [Google Scholar]
- Meng, X. Peat resource of China and progress and prospect of its applicative exploitation. Humic Acid 2004, 5, 24–29. [Google Scholar]
- Li, S.; Yao, C.; He, T.; Yin, D.; Wang, Y. Study on the Remediation Effect of Modified Peat Soil on Mercury Contaminated Paddy Soil. J. Southwest Univ. Nat. Sci. 2022, 44, 1–8. (In Chinese) [Google Scholar]
- Ghori, N.H.; Ghori, T.; Hayat, M.Q.; Imadi, S.R.; Gul, A.; Altay, V.; Ozturk, M. Heavy metal stress and responses in plants. Int. J. Environ. Sci. Technol. 2019, 16, 1807–1828. [Google Scholar] [CrossRef]
- Wang, X.; Wang, W.; Yang, L.; Jin, L.; Song, Y.; Jiang, S.; Qin, L. Transport pathways of cadmium (Cd) and its regulatory mechanisms in plant. Acta Ecol. Sin. 2015, 35, 7921–7929. (In Chinese) [Google Scholar]
- Nevo, Y.; Nelson, N. The NRAMP family of metal-ion transporters. Acta (BBA)-Mol. Cell Res. 2006, 1763, 609–620. [Google Scholar] [CrossRef]
- Guerinot, M.L. The ZIP family of metal transporters. Acta (BBA)-Biomembr. 2000, 1465, 190–198. [Google Scholar] [CrossRef]
- Qiao, X.; Dechao, D.; Qiongyao, C.; Jiyan, S. Influence of Humic Acid on Pb Uptake and Accumulation in Tea Plants. J. Agric. Food Chem. 2018, 66, 12327–12334. [Google Scholar]
- Epstein, E.; Jefferies, R.L. The genetic basis of selective ion transport in plants. Annu. Rev. Plant Physiol. 1964, 15, 169–184. [Google Scholar] [CrossRef]
- Li, H. Study on Extraction and Characterization of HA and FA. Ph.D. Thesis, Huazhong University of Science and Technology, Wuhan, China, 2012. [Google Scholar]
- Yao, A.; Qing, C.; Mu, S. Complex stability characteristics of humus with Hg and its environmental significance. Chin. J. Eco-Agric. 2006, 14, 138–140. (In Chinese) [Google Scholar]
- Yu, G.; Qing, C.; Mou, S.; Wei, S. Characteristics of mercury adsorption and desorption on humic acids. Circumstantiae 2001, 21, 601–606. (In Chinese) [Google Scholar]
- Schellekens, J.; Buurman, P.; Kalbitz, K.; Zomeren, A.V.; Vidal-Torrado, P.; Cerli, C.; Comans, R.N.J. Molecular Features of Humic Acids and Fulvic Acids from Contrasting Environments. Environ. Sci Technol. 2017, 51, 1330–1339. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, H.; Wang, Y.; Peng, Y. Compositional characteristics of dissolved organic matter during coal liquefaction wastewater treatment and its environmental implications. Sci Total Environ. 2020, 704, 135409. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhang, F. The Advances in Rhizosphere Microbiology. Soils 2006, 38, 113–121. (In Chinese) [Google Scholar]
- Liu, T.; Wang, J.; Feng, X.; Zhang, H.; Zhu, Z.; Cheng, S. Spectral insight into thiosulfate-induced mercury speciation transformation in a historically polluted soil. Sci. Total Environ. 2018, 657, 938–944. [Google Scholar] [CrossRef]

| Code | Quartzite (kg) | Kaolinite (kg) | Calcium Carbonate (kg) | Iron Oxide (kg) | Manganese Oxide (kg) | Peat (kg) | Humic Acid/Fulvic Acid (kg) | FeS2 (kg) |
|---|---|---|---|---|---|---|---|---|
| Cl | 7.00 | 4.20 | 1.40 | 0.56 | 1.40 | 1.50 | - | - |
| Cl-Kaol | 11.20 | - | 1.40 | 0.56 | 1.40 | 1.50 | - | - |
| Cl-CaCO3 | 8.40 | 4.20 | - | 0.56 | 1.40 | 1.50 | - | - |
| Cl-FeMn | 7.70 | 4.20 | 1.40 | - | - | 1.50 | - | - |
| Cl-Peat | 7.70 | 4.20 | 1.40 | 0.56 | 1.40 | - | - | - |
| Cl+FeS2 | 6.92 | 4.20 | 1.40 | 0.56 | 1.40 | 1.50 | - | 0.08 |
| Cl (MHA) | 7.00 | 4.20 | 1.40 | 0.56 | 1.40 | - | 0.70 | - |
| Cl (PHA) | 7.00 | 4.20 | 1.40 | 0.56 | 1.40 | - | 0.70 | - |
| Cl (PFA) | 4.36 | 2.40 | 0.80 | 0.32 | 0.08 | - | 0.04 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, A.; Ran, S.; He, T.; Yin, D.; Xu, Y. The Effects of Different Soil Component Couplings on the Methylation and Bioavailability of Mercury in Soil. Toxics 2023, 11, 942. https://doi.org/10.3390/toxics11110942
Qin A, Ran S, He T, Yin D, Xu Y. The Effects of Different Soil Component Couplings on the Methylation and Bioavailability of Mercury in Soil. Toxics. 2023; 11(11):942. https://doi.org/10.3390/toxics11110942
Chicago/Turabian StyleQin, Aming, Shu Ran, Tianrong He, Deliang Yin, and Yiyuan Xu. 2023. "The Effects of Different Soil Component Couplings on the Methylation and Bioavailability of Mercury in Soil" Toxics 11, no. 11: 942. https://doi.org/10.3390/toxics11110942
APA StyleQin, A., Ran, S., He, T., Yin, D., & Xu, Y. (2023). The Effects of Different Soil Component Couplings on the Methylation and Bioavailability of Mercury in Soil. Toxics, 11(11), 942. https://doi.org/10.3390/toxics11110942




