Ecotoxicology of Polymetallic Nodule Seabed Mining: The Effects of Cobalt and Nickel on Phytoplankton Growth and Pigment Concentration
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Design of Experiments
2.1.1. Strains
2.1.2. Design of Experiments
2.2. Parameters
2.2.1. Cell Abundance
2.2.2. Chlorophyll a
2.2.3. Pigments
2.2.4. Protein and Antioxidant Enzyme Markers
2.2.5. Metal Concentration
2.3. Data Analysis
3. Results
3.1. NOEC and LOEC
3.2. Cobalt Toxic Effects
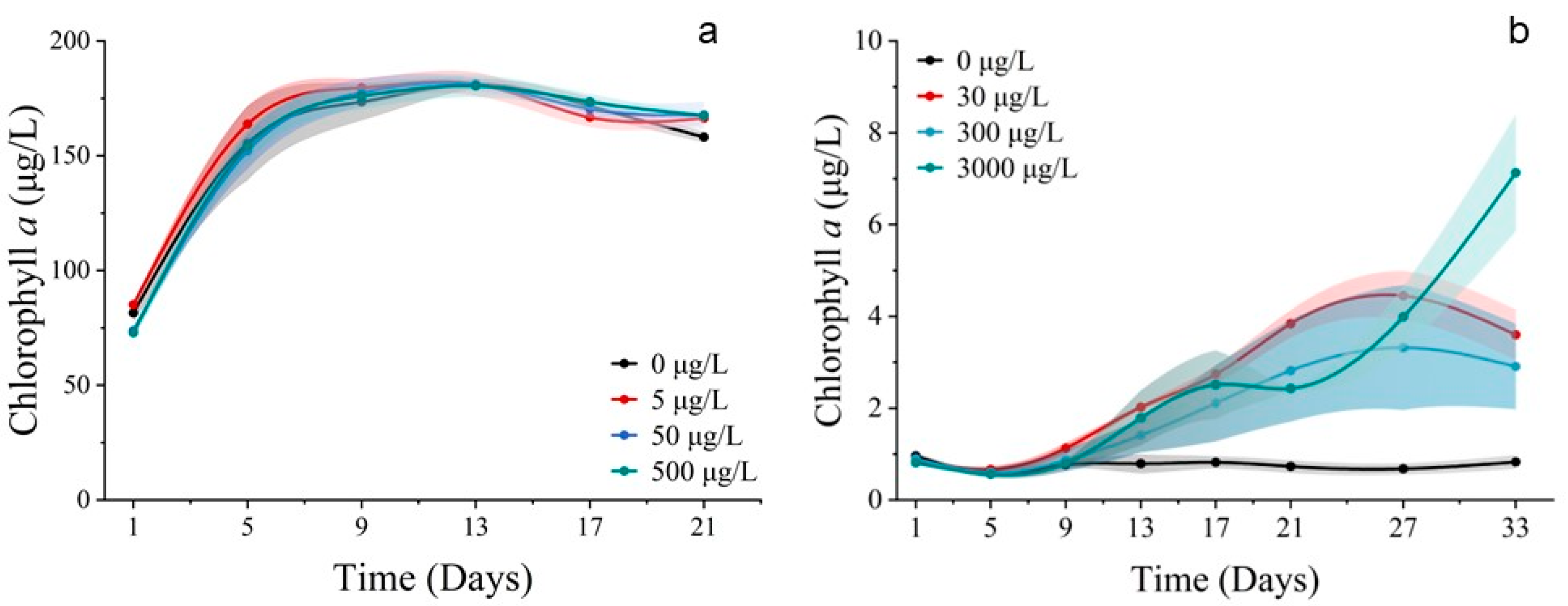
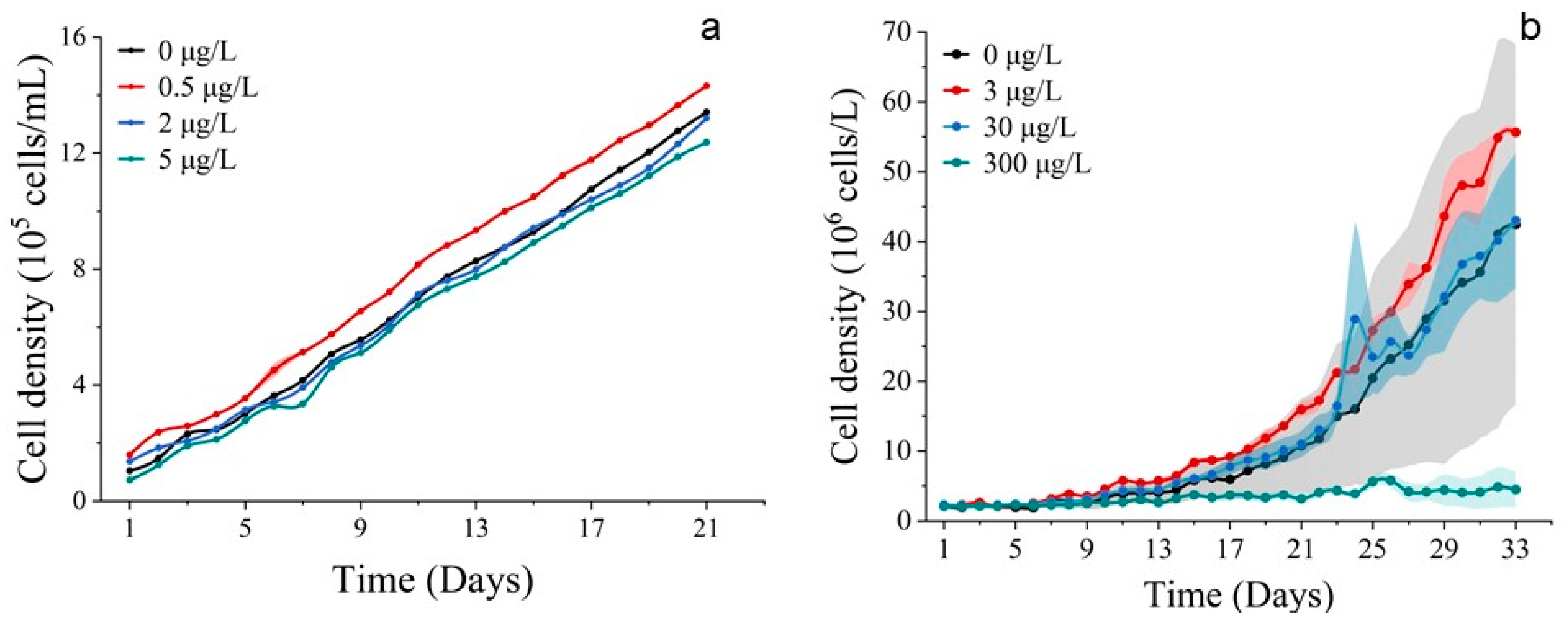
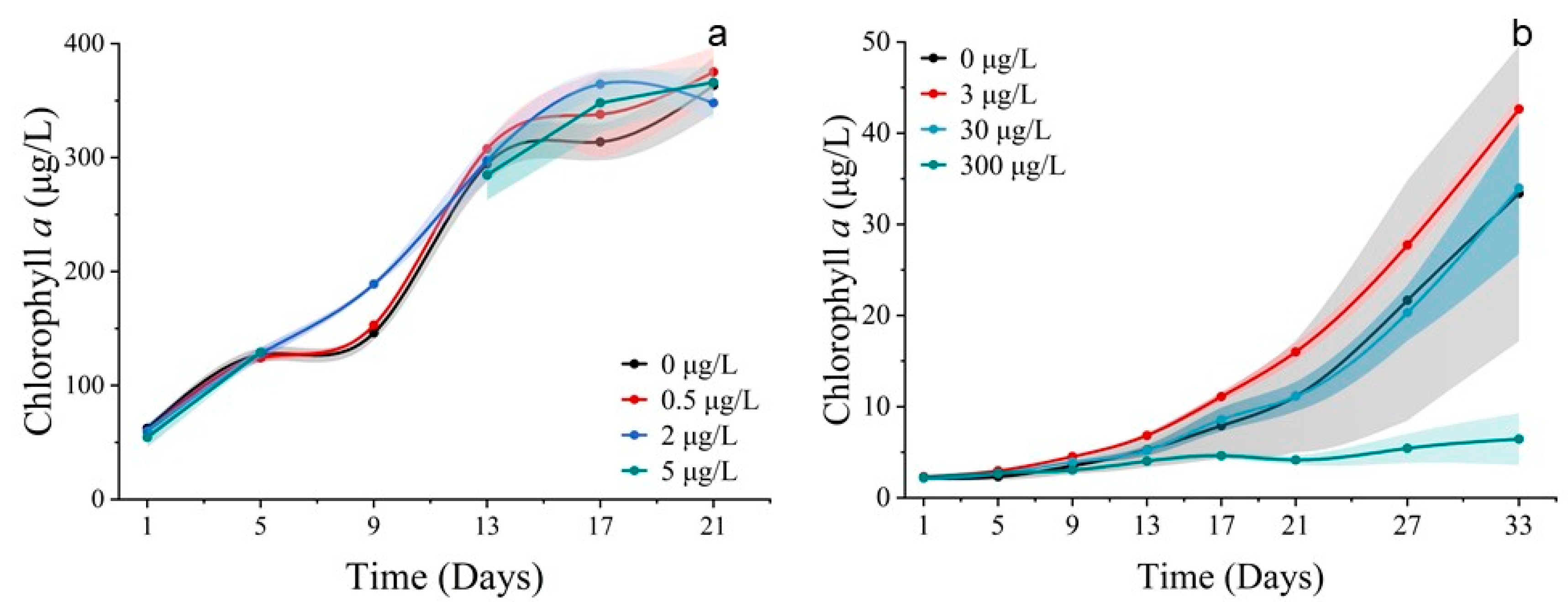
3.2.1. Cell Abundance and Chlorophyll a Concentration
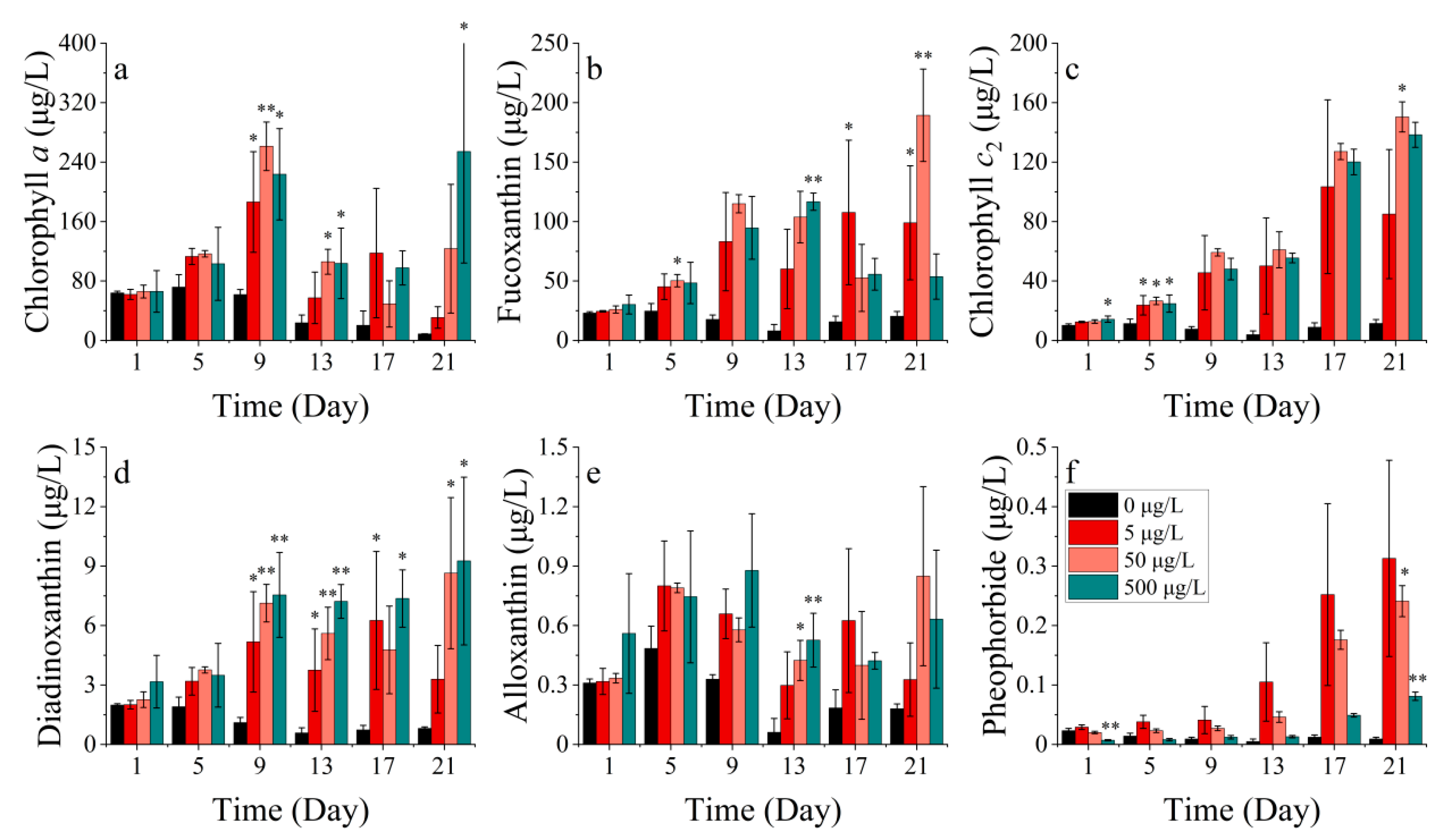
3.2.2. Pigments
- Pigments of S. costatum
- Pigments of P. donghaiense
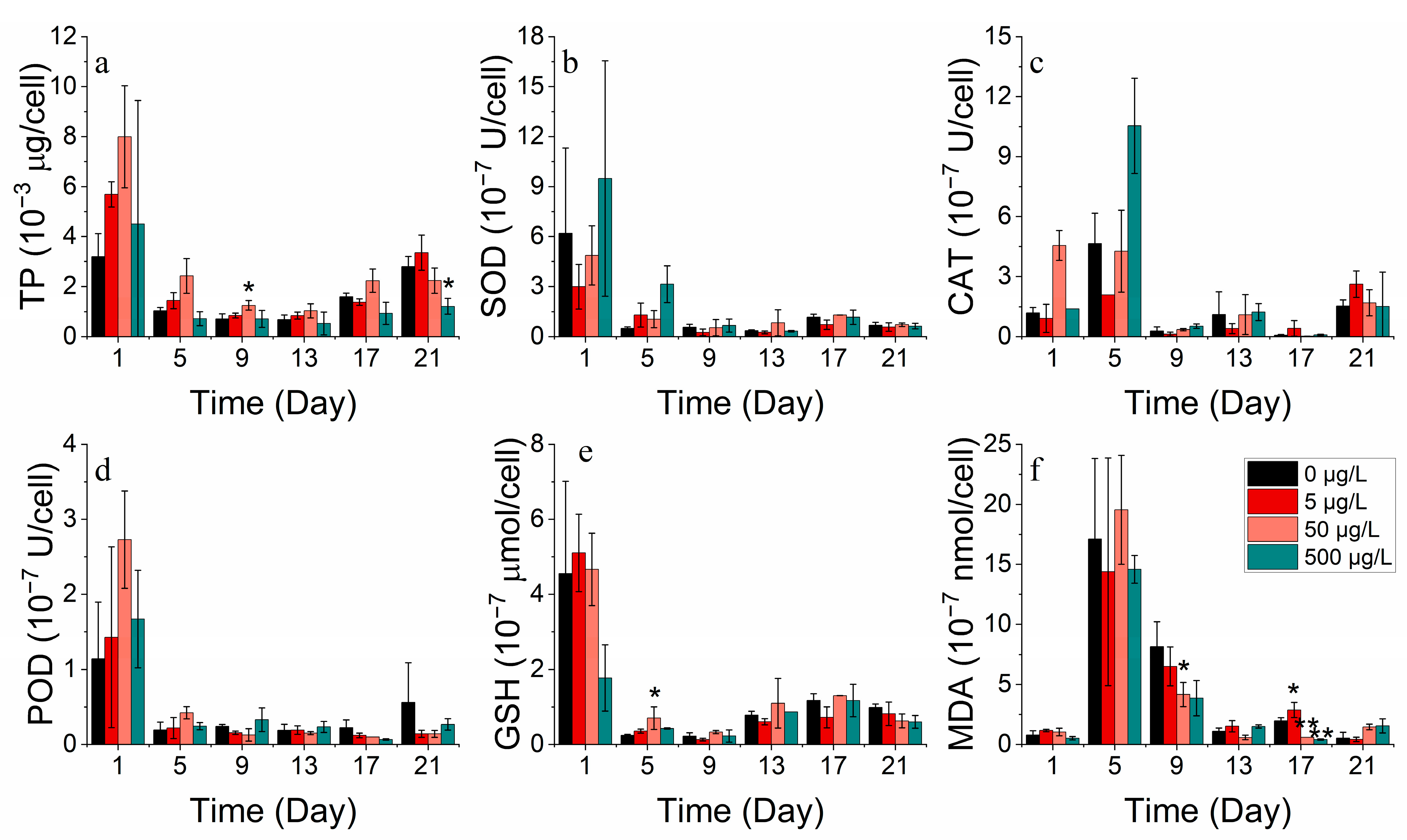
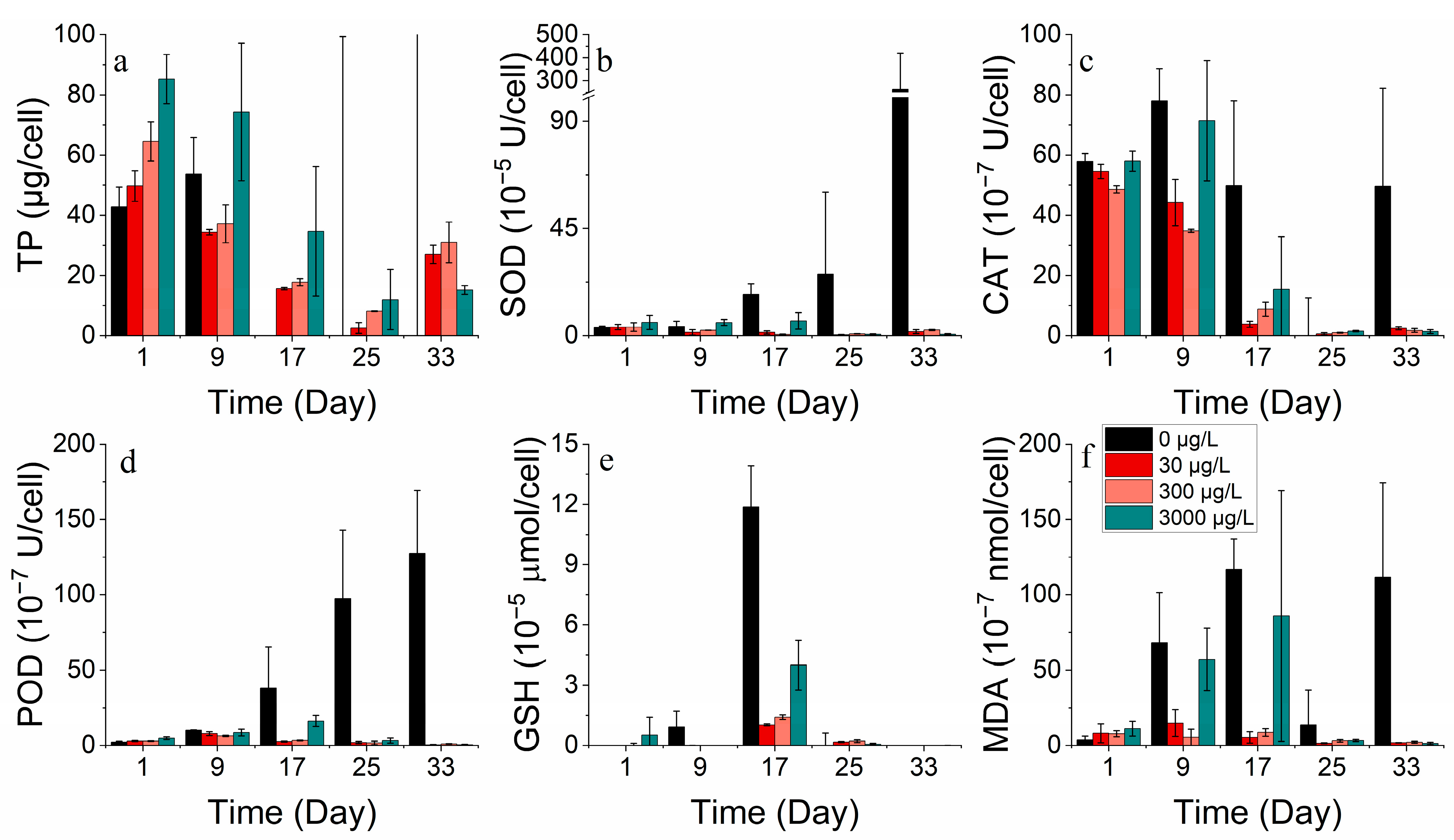
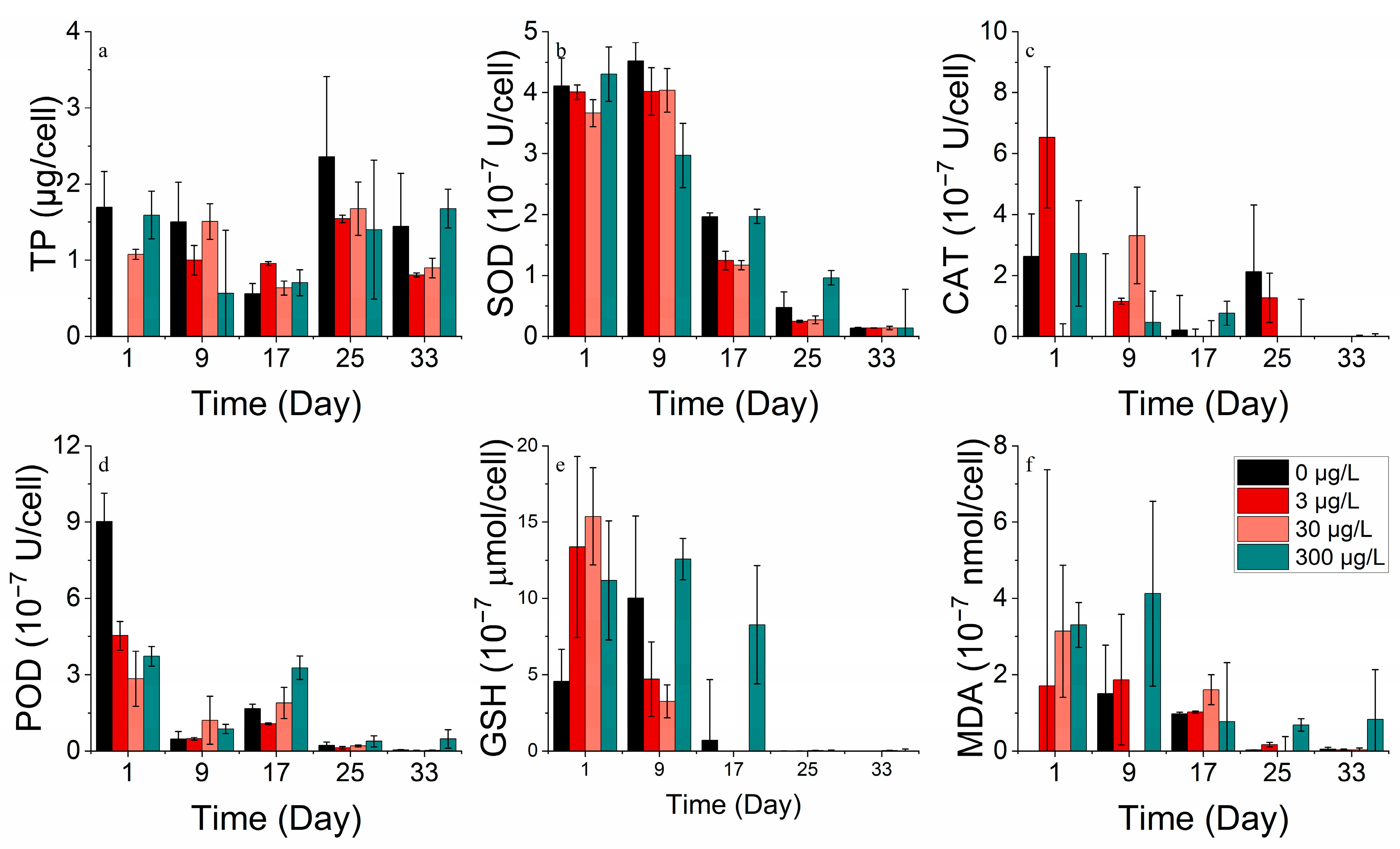
3.2.3. Protein and Antioxidant Enzyme Markers
3.2.4. Metal Absorption
3.3. Nickel Toxic Effects
3.3.1. Cell Abundance and Chlorophyll a Concentration
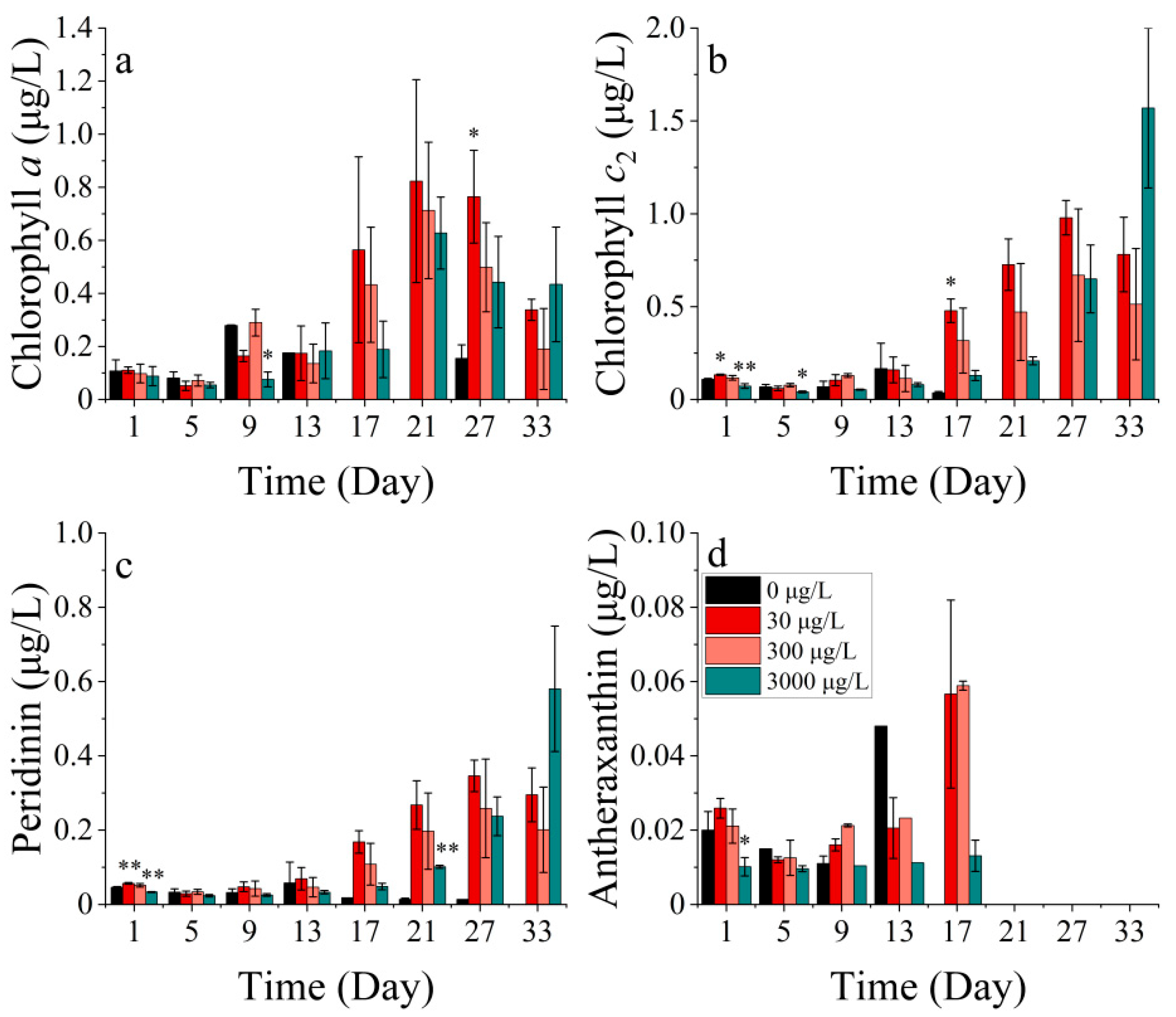
3.3.2. Pigments
3.3.3. Protein and Antioxidant Enzyme Markers
3.3.4. Metal Absorption
4. Discussion
4.1. Potential Role for Co/Ni in Coregulating Phytoplankton Growth
4.2. Interaction between Metals
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cheng, Y.; Dai, Y.; Zhang, Y.; Yang, C.; Liu, C. Status and Prospects of the Development of Deep-Sea Polymetallic Nodule-Collecting Technology. Sustainability 2023, 15, 4572. [Google Scholar] [CrossRef]
- Hund, K.; La Porta, D.; Fabregas, T.P.; Laing, T.; Drexhage, J. Minerals for Climate Action: The Mineral Intensity of the Clean Energy Transition; World Bank: Washington, DC, USA, 2023. [Google Scholar]
- Miller, K.A.; Thompson, K.F.; Johnston, P.; Santillo, D. An Overview of Seabed Mining Including the Current State of Development, Environmental Impacts, and Knowledge Gaps. Front. Mar. Sci. 2018, 4, 418. [Google Scholar] [CrossRef]
- Christiansen, B.; Denda, A.; Christiansen, S. Potential effects of deep seabed mining on pelagic and benthopelagic biota. Mar. Policy 2020, 114, 103442. [Google Scholar] [CrossRef]
- Cormier, R.; Londsdale, J. Risk assessment for deep sea mining: An overview of risk. Mar. Policy 2020, 114, 103485. [Google Scholar] [CrossRef]
- Drazen, J.C.; Smith, C.R.; Gjerde, K.M.; Haddock, S.H.D.; Carter, G.S.; Choy, C.A.; Clark, M.R.; Dutrieux, P.; Goetze, E.; Hauton, C.; et al. Midwater ecosystems must be considered when evaluating environmental risks of deep-sea mining. Proc. Natl. Acad. Sci. USA 2020, 117, 17455–17460. [Google Scholar] [CrossRef] [PubMed]
- Perelman, J.N.; Firing, E.; van der Grient, J.M.A.; Jones, B.A.; Drazen, J.C. Mesopelagic Scattering Layer Behaviors Across the Clarion-Clipperton Zone: Implications for Deep-Sea Mining. Front. Mar. Sci. 2021, 8, 632764. [Google Scholar] [CrossRef]
- Hauton, C.; Brown, A.; Thatje, S.; Mestre, N.C.; Bebianno, M.J.; Martins, I.; Bettencourt, R.; Canals, M.; Sanchez-Vidal, A.; Shillito, B.; et al. Identifying Toxic Impacts of Metals Potentially Released during Deep-Sea Mining—A Synthesis of the Challenges to Quantifying Risk. Front. Mar. Sci. 2017, 4, 368. [Google Scholar] [CrossRef]
- Ou, R.; Cai, L.; Qiu, J.; Huang, H.; Ou, D.; Li, W.; Lin, F.; He, X.; Wang, L.; Wu, R. Simulation Experiment of Environmental Impact of Deep-Sea Mining: Response of Phytoplankton Community to Polymetallic Nodules and Sediment Enrichment in Surface Water. Toxics 2022, 10, 610. [Google Scholar] [CrossRef] [PubMed]
- Zeeshan, M.; Murugadas, A.; Ghaskadbi, S.; Ramaswamy, B.R.; Akbarsha, M.A. Ecotoxicological assessment of cobalt using Hydra model: ROS, oxidative stress, DNA damage, cell cycle arrest, and apoptosis as mechanisms of toxicity. Environ. Pollut. 2017, 224, 54–69. [Google Scholar] [CrossRef]
- Bruland, K.W.; Donat, J.R.; Hutchins, D.A. Interactive influences of bioactive trace metals on biological production in oceanic waters. Limnol. Oceanogr. 1991, 36, 1555–1577. [Google Scholar] [CrossRef]
- Ho, T.Y.; Quigg, A.; Finkel, Z.V.; Milligan, A.J.; Wyman, K.; Falkowski, P.G.; Morel, F.M. The elemental composition of some marine phytoplankton. J. Phycol. 2003, 39, 1145–1159. [Google Scholar] [CrossRef]
- Twining, B.S.; Baines, S.B.; Bozard, J.B.; Vogt, S.; Walker, E.A.; Nelson, D.M. Metal quotas of plankton in the equatorial Pacific Ocean. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2011, 58, 325–341. [Google Scholar] [CrossRef]
- Hong, H.-S.; Wang, M.-H.; Huang, X.-G.; Wang, D.-Z. Effects of macronutrient additions on nickel uptake and distribution in the dinoflagellate Prorocentrum donghaiense Lu. Environ. Pollut. 2009, 157, 1933–1938. [Google Scholar] [CrossRef] [PubMed]
- Niyogi, S.; Wood, C.M. Biotic Ligand Model, a Flexible Tool for Developing Site-Specific Water Quality Guidelines for Metals. Environ. Sci. Technol. 2004, 38, 6177–6192. [Google Scholar] [CrossRef] [PubMed]
- Twining, B.S.; Baines, S.B. The trace metal composition of marine phytoplankton. Ann. Rev. Mar. Sci. 2013, 5, 191–215. [Google Scholar] [CrossRef] [PubMed]
- Morel, F.M.M.; Lam, P.J.; Saito, M.A. Trace Metal Substitution in Marine Phytoplankton. Annu. Rev. Earth Planet. Sci. 2020, 48, 491–517. [Google Scholar] [CrossRef]
- Zeng, Y.; Wang, L.; Jiang, L.; Cai, X.; Li, Y. Joint Toxicity of Lead, Chromium, Cobalt and Nickel to Photobacterium phosphoreum at No Observed Effect Concentration. Bull. Environ. Contam. Toxicol. 2015, 95, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Ciğerci, İ.H.; Ali, M.M.; Kaygısız, Ş.Y.; Liman, R. Genotoxicity assessment of cobalt chloride in Eisenia hortensis earthworms coelomocytes by comet assay and micronucleus test. Chemosphere 2016, 144, 754–757. [Google Scholar] [CrossRef]
- Singh, N.; Bhagat, J.; Ingole, B.S. Genotoxicity of two heavy metal compounds: Lead nitrate and cobalt chloride in Polychaete Perinereis cultrifera. Environ. Monit. Assess. 2017, 189, 308. [Google Scholar] [CrossRef]
- Barrio-Parra, F.; Elío, J.; De Miguel, E.; García-González, J.E.; Izquierdo, M.; Álvarez, R. Environmental risk assessment of cobalt and manganese from industrial sources in an estuarine system. Environ. Geochem. Health 2018, 40, 737–748. [Google Scholar] [CrossRef]
- Yamatani, K.; Saito, K.; Ikezawa, Y.; Ohnuma, H.; Sugiyama, K.; Manaka, H.; Takahashi, K.; Sasaki, H. Relative Contribution of Ca2+-Dependent Mechanism in Glucagon-Induced Glucose Output from the Liver. Arch. Biochem. Biophys. 1998, 355, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Howe, P.L.; Reichelt-Brushett, A.J.; Clark, M.W. Investigating lethal and sublethal effects of the trace metals cadmium, cobalt, lead, nickel and zinc on the anemone Aiptasia pulchella, a cnidarian representative for ecotoxicology in tropical marine environments. Mar. Freshw. Res. 2014, 65, 551–561. [Google Scholar] [CrossRef]
- Sunda, W.G. Trace metal interactions with marine phytoplankton. Biol. Oceanogr. 1989, 6, 411–442. [Google Scholar] [CrossRef]
- Sunda, W.G. Feedback interactions between trace metal nutrients and phytoplankton in the ocean. Front. Microbiol. 2012, 3, 204. [Google Scholar] [CrossRef] [PubMed]
- Guillard, R.R.L.; Ryther, J.H. Studies of marine planktonic diatoms. I. Cyclotella nana Hustedt and Detonula confervacea Cleve. Can. J. Microbiol. 1962, 8, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Parsons, T.; Maita, Y.; Lalli, C.M. Amanual of chemical and biological methods for seawater analysis. In Biological Oceanographic Processes; Parsons, T., Ed.; Pergamon Press: New York, NY, USA, 1984; p. 173. [Google Scholar]
- Barlow, R.; Mantoura, R.; Gough, M.; Fileman, T. Pigment signatures of the phytoplankton composition in the northeastern Atlantic during the 1990 spring bloom. Deep Sea Res. Part II Top. Stud. Oceanogr. 1993, 40, 459–477. [Google Scholar] [CrossRef]
- Milne, A.; Landing, W.; Bizimis, M.; Morton, P. Determination of Mn, Fe, Co, Ni, Cu, Zn, Cd and Pb in seawater using high resolution magnetic sector inductively coupled mass spectrometry (HR-ICP-MS). Anal. Chim. Acta 2010, 665, 200–207. [Google Scholar] [CrossRef]
- Correggia, M.; Iorio, L.D.; Bastianoni, A.B.; Yücel, M.; Cordone, A.; Giovannelli, D. Standard Operating Procedure for the analysis of trace elements in hydrothermal fluids by Inductively Coupled Plasma Mass Spectrometry (ICP-MS). Open Res. Eur. 2023, 3, 90. [Google Scholar] [CrossRef]
- Organization for Economic Cooperation and Development (OECD). Guideline for Testing Chemicals. No.201: Alga Growth Inhibition Test; OECD: Paris, France, 1984. [Google Scholar]
- Dunnett, C.W. A Multiple Comparison Procedure for Comparing Several Treatments with a Control. J. Am. Stat. Assoc. 1955, 50, 1096–1121. [Google Scholar] [CrossRef]
- Manimaran, K.; Karthikeyan, P.; Ashokkumar, S.; Ashok Prabu, V.; Sampathkumar, P. Effect of copper on growth and enzyme activities of marine diatom, Odontella mobiliensis. Bull. Environ. Contam. Toxicol. 2012, 88, 30–37. [Google Scholar] [CrossRef]
- Karthikeyan, P.; Marigoudar, S.R.; Nagarjuna, A.; Sharma, K.V. Toxicity assessment of cobalt and selenium on marine diatoms and copepods. Environ. Chem. Ecotoxicol. 2019, 1, 36–42. [Google Scholar] [CrossRef]
- Meyer, J.S.; Lyons-Darden, T.; Garman, E.R.; Middleton, E.T.; Schlekat, C.E. Toxicity of Nanoparticulate Nickel to Aquatic Organisms: Review and Recommendations for Improvement of Toxicity Tests. Environ. Toxicol. Chem. 2020, 39, 1861–1883. [Google Scholar] [CrossRef]
- Gissi, F.; Stauber, J.L.; Binet, M.T.; Golding, L.A.; Adams, M.S.; Schlekat, C.E.; Garman, E.R.; Jolley, D.F. A review of nickel toxicity to marine and estuarine tropical biota with particular reference to the South East Asian and Melanesian region. Environ. Pollut. 2016, 218, 1308–1323. [Google Scholar] [CrossRef] [PubMed]
- Whitfield, M. Interactions between phytoplankton and trace metals in the ocean. Adv. Mar. Biol. 2001, 41, 1–128. [Google Scholar] [CrossRef]
- Guo, J.A.; Strzepek, R.; Willis, A.; Ferderer, A.; Bach, L.T. Investigating the effect of nickel concentration on phytoplankton growth to assess potential side-effects of ocean alkalinity enhancement. Biogeoscience 2022, 19, 3683–3697. [Google Scholar] [CrossRef]
- Browning, T.J.; Rapp, I.; Schlosser, C.; Gledhill, M.; Achterberg, E.P.; Bracher, A.; Moigne, F.A.C.L. Influence of Iron, Cobalt, and Vitamin B12 Supply on Phytoplankton Growth in the Tropical East Pacific During the 2015 El Niño. Geophys. Res. Lett. 2018, 45, 6150–6159. [Google Scholar] [CrossRef]
- Zigman, M.; Dubinsky, Z.; Iluz, D. The Xanthophyll Cycle in Aquatic Phototrophs and Its Role in the Mitigation of Photoinhibition and Photodynamic Damage; InTech: London, UK, 2012; p. 191. [Google Scholar]
- Chmiel, R.J.; Kell, R.M.; Rao, D.; Moran, D.M.; DiTullio, G.R.; Saito, M.A. Low cobalt inventories in the Amundsen and Ross seas driven by high demand for labile cobalt uptake among native phytoplankton communities. Biogeoscience 2023, 20, 3997–4027. [Google Scholar] [CrossRef]
- Osman, M.E.H.; El-Naggar, A.H.; El-Sheekh, M.M.; El-Mazally, E.E. Differential effects of Co2+ and Ni2+ on protein metabolism in Scenedesmus obliquus and Nitzschia perminuta. Environ. Toxicol. Pharmacol. 2004, 16, 169–178. [Google Scholar] [CrossRef]
- Mccain, J.S.P.; Bertrand, E.M. Phytoplankton antioxidant systems and their contributions to cellular elemental stoichiometry. Limnol. Oceanogr. Lett. 2021, 7, 96–111. [Google Scholar] [CrossRef]
- Rueter, J.G.; Petersen, R.R. Micronutrient effects on cyanobacterial growth and physiology. N. Z. J. Mar. Freshw. Res. 1987, 21, 435–445. [Google Scholar] [CrossRef]
- Zhang, Y.; Gladyshev, V.N. General trends in trace element utilization revealed by comparative genomic analyses of Co, Cu, Mo, Ni, and Se. J. Biol. Chem. 2010, 285, 3393–3405. [Google Scholar] [CrossRef] [PubMed]
- Tebo, B.M.; Nealson, K.H.; Emerson, S.; Jacobs, L. Microbial mediation of Mn (II) and Co (II) precipitation at the O2/H2S interfaces in two anoxic fjords. Limnol. Oceanogr. 1984, 29, 1247–1258. [Google Scholar] [CrossRef]
- Lee, B.G.; Fisher, N.S. Microbially mediated cobalt oxidation in seawater revealed by radiotracer experiments. Limnol. Oceanogr. 1993, 38, 1593–1602. [Google Scholar] [CrossRef]
- Moffett, J.W.; Ho, J. Oxidation of cobalt and manganese in seawater via a common microbially catalyzed pathway. Geochim. Et Cosmochim. Acta 1996, 60, 3415–3424. [Google Scholar] [CrossRef]
- Balistrieri, L.S.; Murray, J.W. The surface chemistry of sediments from the Panama Basin: The influence of Mn oxides on metal adsorption. Geochim. Et Cosmochim. Acta 1986, 50, 2235–2243. [Google Scholar] [CrossRef]
- Tani, Y.; Ohashi, M.; Miyata, N.; Seyama, H.; Iwahori, K.; Soma, M. Sorption of Co (II), Ni (II), and Zn (II) on biogenic manganese oxides produced by a Mn-oxidizing fungus, strain KR21-2. J. Environ. Sci. Health Part A 2004, 39, 2641–2660. [Google Scholar] [CrossRef] [PubMed]
- Sunda, W.G.; Huntsman, S.A. Cobalt and zinc interreplacement in marine phytoplankton: Biological and geochemical implications. Limnol. Oceanogr. 1995, 40, 1404–1417. [Google Scholar] [CrossRef]
- Reis, L.L.d.; Alho, L.d.O.G.; Abreu, C.B.d.; Gebara, R.C.; Mansano, A.d.S.; Melão, M.d.G.G. Effects of cadmium and cobalt mixtures on growth and photosynthesis of Raphidocelis subcapitata (Chlorophyceae). Aquat. Toxicol. 2022, 244, 106077. [Google Scholar] [CrossRef]
- Tada, C.; Nishimura, O.; Itayama, T.; Inamori, Y.; Matsumura, M.; Sudo, R. The Influence of Materials Released from Lake Sediment on The Growth of Three Kinds of Algae. Jpn. J. Water Treat. Biol. 2001, 37, 161–172. [Google Scholar] [CrossRef][Green Version]








| Species | Metals | Parameters | NOEC | LOEC |
|---|---|---|---|---|
| S. costatum | cobalt | cell abundance | 14.140 | 26.077 |
| chlorophyll a | 16.251 | 22.874 | ||
| total protein | 45.374 | 66.428 | ||
| nickel | cell abundance | 3.771 | 5.505 | |
| chlorophyll a | 1.720 | 4.667 | ||
| total protein | 3.235 | 5.116 | ||
| P. donghaiense | cobalt | cell abundance | 53.271 | 89.852 |
| chlorophyll a | 37.247 | 66.140 | ||
| total protein | 42.854 | 81.237 | ||
| nickel | cell abundance | 18.428 | 42.313 | |
| chlorophyll a | 10.425 | 17.410 | ||
| total protein | 22.343 | 40.174 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ou, R.; Huang, H.; He, X.; Lin, S.; Ou, D.; Li, W.; Qiu, J.; Wang, L. Ecotoxicology of Polymetallic Nodule Seabed Mining: The Effects of Cobalt and Nickel on Phytoplankton Growth and Pigment Concentration. Toxics 2023, 11, 1005. https://doi.org/10.3390/toxics11121005
Ou R, Huang H, He X, Lin S, Ou D, Li W, Qiu J, Wang L. Ecotoxicology of Polymetallic Nodule Seabed Mining: The Effects of Cobalt and Nickel on Phytoplankton Growth and Pigment Concentration. Toxics. 2023; 11(12):1005. https://doi.org/10.3390/toxics11121005
Chicago/Turabian StyleOu, Rimei, Hao Huang, Xuebao He, Shuangshuang Lin, Danyun Ou, Weiwen Li, Jinli Qiu, and Lei Wang. 2023. "Ecotoxicology of Polymetallic Nodule Seabed Mining: The Effects of Cobalt and Nickel on Phytoplankton Growth and Pigment Concentration" Toxics 11, no. 12: 1005. https://doi.org/10.3390/toxics11121005
APA StyleOu, R., Huang, H., He, X., Lin, S., Ou, D., Li, W., Qiu, J., & Wang, L. (2023). Ecotoxicology of Polymetallic Nodule Seabed Mining: The Effects of Cobalt and Nickel on Phytoplankton Growth and Pigment Concentration. Toxics, 11(12), 1005. https://doi.org/10.3390/toxics11121005





