Health Risk Assessment of Heavy Metals in Indoor Household Dust in Urban and Rural Areas of Chiang Mai and Lamphun Provinces, Thailand
Abstract
:1. Introduction
2. Materials and Methods
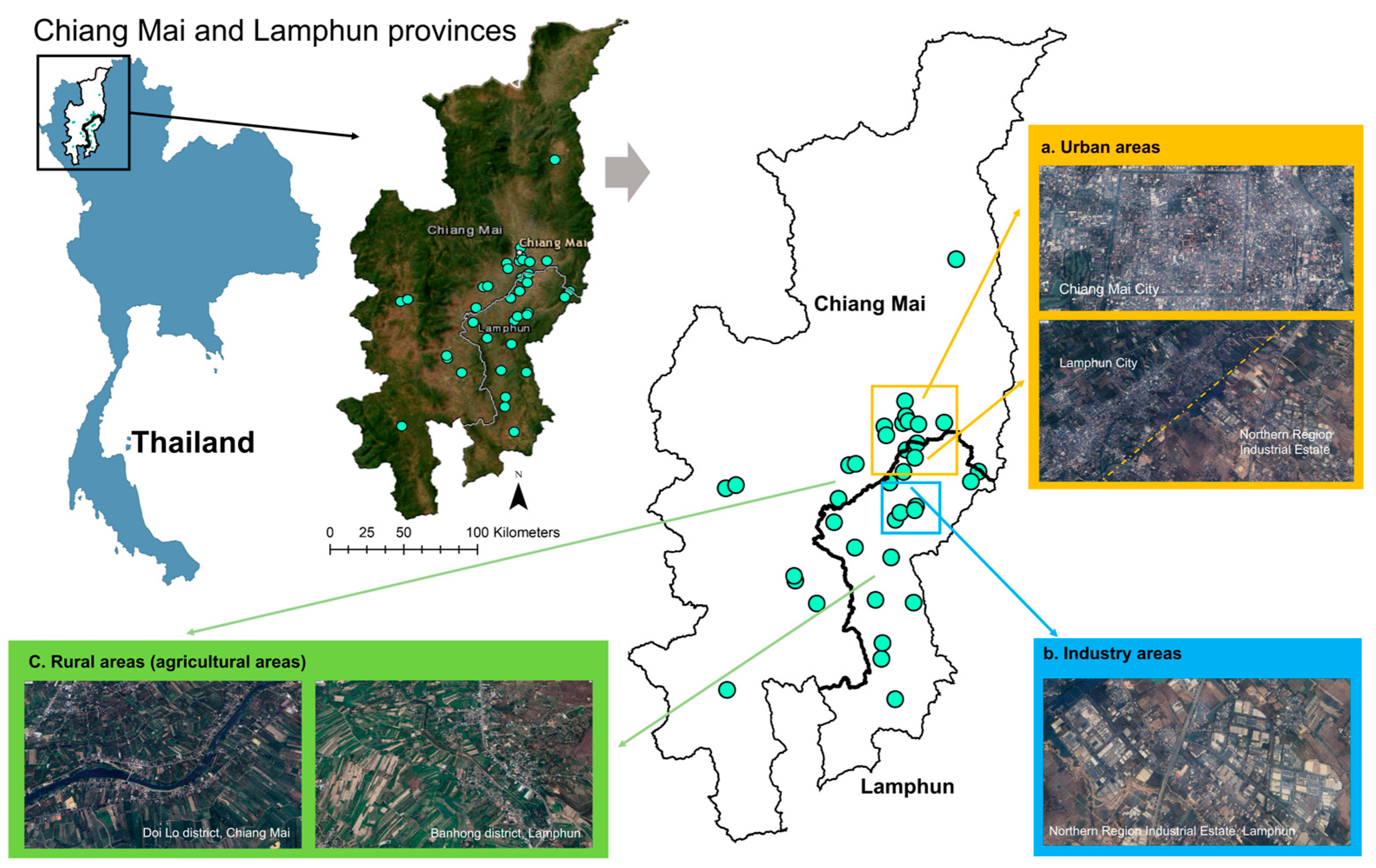
2.1. Study Area
2.2. Samples and Data Collection
2.3. Sample Analysis
2.4. Enrichment Factor (EF) Calculation
2.5. Health Risk Assessment
2.6. Source Apportionment Model
2.7. Statistical Analysis
3. Results and Discussion
3.1. Heavy Metal Concentrations in Indoor Household Dust
| Countries (n) | Heavy Metal Concentrations in Indoor Dust (mg kg−1) | Ref. | |||||||
|---|---|---|---|---|---|---|---|---|---|
| As | Cd | Cr | Cu | Mn | Ni | Pb | Zn | ||
| 35 Countries (n = 2265) a | 25.3 | 128 | 264 | 333 | 77.6 | 224 | 1470 | [17] | |
| 35 Countries (n = 2265) b | 13.3 | 0.76 | 86 | 176 | 257 | 39 | 94 | 1110 | [17] |
| Australia (n = 1310) a | 31.9 | 105 | 232 | 336 | 49.4 | 305 | 1680 | [17] | |
| China (n = 111) a | 17.3 | 134 | 242 | 245 | 70.6 | 161 | 1190 | [17] | |
| Ghana (n = 54) a | 6.2 | 43.4 | 74.9 | 185 | 48 | 163 | 252 | [17] | |
| UK (n = 148) a | 6.9 | 93.6 | 136 | 269 | 35.8 | 131 | 532 | [17] | |
| USA (n = 345) a | 20.5 | 207 | 549 | 385 | 165.4 | 93.6 | 1785 | [17] | |
| Turkey (n = 85) b | 4.41 | 0.35 | 23.8 | 65.7 | 65.9 | 32.3 | 27.5 | 263 | [53] |
| Australia (n = 224) a | 20.2 | 99.8 | 298 | 247 | 56.7 | 364 | 2437 | [47] | |
| Across China (n = 3392) b | 15.6 | 2.73 | 85.9 | 136.2 | 40.7 | 161.5 | 602.7 | [18] | |
| Canada (n = 125) a | 13 | 11 | 92 | 1900 | 250 | 60 | 4500 | 14,000 | [54] |
| UK (n = 32) c | 1.2 | 301 | 524 | 53.1 | 150 | 622 | [55] | ||
| Japan (n = 100) c | 1.02 | 67.8 | 304 | 226 | 59.6 | 57.9 | 920 | [56] | |
| Sukhothai, Thailand (n = 16) a | 9 | 3 | 226 | 1051 | [57] | ||||
| Ubon Ratchathani, Thailand (n = 56) a | 0.99 | 0.92 | 3.00 | [58] | |||||
| This study (n = 100) a | 12.5 | 2.2 | 38.5 | 107.3 | 577.6 | 34.9 | 62.1 | 408.7 | |
| This study (n = 100) b | 10.3 | 0.9 | 32.4 | 82.5 | 542.7 | 28.9 | 44.8 | 352.7 | |
| Areas | Median, IQR Concentration, (mg kg−1) | |||||||
|---|---|---|---|---|---|---|---|---|
| As | Cd | Cr | Cu | Mn | Ni | Pb | Zn | |
| Urbanized | ||||||||
| Rural (agriculture) (79) | 10.97, 5.89 | 0.89, 1.31 | 30.97, 18.00 | 79.18, 57.25 | 577.41, 284.14 | 27.37, 18.93 | 45.25, 29.18 | 332.33, 193.00 |
| Urban (21) | 8.80, 4.14 | 1.08, 1.53 | 41.89, 15.12 | 84.13, 57.58 | 461.83, 288.92 | 30.94, 17.36 | 44.16, 41.19 | 420.46, 234.74 |
| p-Value | 0.042 | 0.308 | 0.137 | 0.557 | 0.102 | 0.245 | 0.632 | 0.053 |
| Industrial area nearby | ||||||||
| Yes (21) | 10.97, 6.20 | 1.37, 9.49 | 34.61, 16.33 | 81.83, 34.49 | 519.73, 205.02 | 29.86, 24.55 | 49.29, 31.13 | 384.10, 199.74 |
| No (74) | 10.25, 6.50 | 0.83, 1.18 | 31.41, 19.91 | 84.16, 63.33 | 573.72, 281.19 | 27.92, 16.89 | 42.18, 30.28 | 344.83, 214.65 |
| p-Value | 0.784 | 0.028 | 0.654 | 0.438 | 0.510 | 0.319 | 0.247 | 0.654 |
3.2. Source Identification of Heavy Metal in Indoor Household Dust
3.3. Health Risk Assessment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klepeis, N.E.; Nelson, W.C.; Ott, W.R.; Robinson, J.P.; Tsang, A.M.; Switzer, P.; Behar, J.V.; Hern, S.C.; Engelmann, W.H. The National Human Activity Pattern Survey (NHAPS): A resource for assessing exposure to environmental pollutants. J. Expo. Sci. Environ. Epidemiol. 2001, 11, 231–252. [Google Scholar] [CrossRef] [PubMed]
- Nwanaji-Enwerem, J.C.; Allen, J.G.; Beamer, P.I. Another invisible enemy indoors: COVID-19, human health, the home, and United States indoor air policy. J. Expo. Sci. Environ. Epidemiol. 2020, 30, 773–775. [Google Scholar] [CrossRef] [PubMed]
- González-Martín, J.; Kraakman, N.J.R.; Pérez, C.; Lebrero, R.; Muñoz, R. A state–of–the-art review on indoor air pollution and strategies for indoor air pollution control. Chemosphere 2021, 262, 128376. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Household Air Pollution and Health. Available online: https://www.who.int/news-room/fact-sheets/detail/household-air-pollution-and-health (accessed on 28 August 2022).
- Cincinelli, A.; Martellini, T. Indoor Air Quality and Health. Int. J. Environ. Res. Public. Health 2017, 14, 1286. [Google Scholar] [CrossRef] [PubMed]
- Somsunun, K.; Prapamontol, T.; Pothirat, C.; Liwsrisakun, C.; Pongnikorn, D.; Fongmoon, D.; Chantara, S.; Wongpoomchai, R.; Naksen, W.; Autsavapromporn, N.; et al. Estimation of lung cancer deaths attributable to indoor radon exposure in upper northern Thailand. Sci. Rep. 2022, 12, 5169. [Google Scholar] [CrossRef] [PubMed]
- Barrio-Parra, F.; De Miguel, E.; Lázaro-Navas, S.; Gómez, A.; Izquierdo, M. Indoor Dust Metal Loadings: A Human Health Risk Assessment. Expo. Health 2018, 10, 41–50. [Google Scholar] [CrossRef]
- Al-Khashman, O.A. The investigation of metal concentrations in street dust samples in Aqaba city, Jordan. Environ. Geochem. Health 2007, 29, 197–207. [Google Scholar] [CrossRef]
- Lu, X.; Wang, L.; Li, L.Y.; Lei, K.; Huang, L.; Kang, D. Multivariate statistical analysis of heavy metals in street dust of Baoji, NW China. J. Hazard. Mater. 2010, 173, 744–749. [Google Scholar] [CrossRef]
- Hejami, A.A.; Davis, M.; Prete, D.; Lu, J.; Wang, S. Heavy metals in indoor settled dusts in Toronto, Canada. Sci. Total Environ. 2020, 703, 134895. [Google Scholar] [CrossRef]
- Charlesworth, S.; De Miguel, E.; Ordóñez, A. A review of the distribution of particulate trace elements in urban terrestrial environments and its application to considerations of risk. Environ. Geochem. Health 2011, 33, 103–123. [Google Scholar] [CrossRef]
- Turner, A. Oral bioaccessibility of trace metals in household dust: A review. Environ. Geochem. Health 2011, 33, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Zhang, X.; Li, L.Y.; Chen, H. Assessment of metals pollution and health risk in dust from nursery schools in Xi’an, China. Environ. Res. 2014, 128, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Chen, L.-J.; Li, H.-H.; Lin, J.-Q.; Yang, Z.-B.; Yang, Y.-X.; Xu, X.-X.; Xian, J.-R.; Shao, J.-R.; Zhu, X.-M. Characteristics and health risk assessment of heavy metals exposure via household dust from urban area in Chengdu, China. Sci. Total Environ. 2018, 619–620, 621–629. [Google Scholar] [CrossRef]
- Rasmussen, P.E.; Levesque, C.; Chénier, M.; Gardner, H.D. Contribution of metals in resuspended dust to indoor and personal inhalation exposures: Relationships between PM10 and settled dust. Build. Environ. 2018, 143, 513–522. [Google Scholar] [CrossRef]
- Deering, K.; Spiegel, E.; Quaisser, C.; Nowak, D.; Schierl, R.; Bose-O’Reilly, S.; Garí, M. Monitoring of arsenic, mercury and organic pesticides in particulate matter, ambient air and settled dust in natural history collections taking the example of the Museum für Naturkunde, Berlin. Environ. Monit. Assess. 2019, 191, 375. [Google Scholar] [CrossRef] [PubMed]
- Isley, C.F.; Fry, K.L.; Liu, X.; Filippelli, G.M.; Entwistle, J.A.; Martin, A.P.; Kah, M.; Meza-Figueroa, D.; Shukle, J.T.; Jabeen, K.; et al. International Analysis of Sources and Human Health Risk Associated with Trace Metal Contaminants in Residential Indoor Dust. Environ. Sci. Technol. 2022, 56, 1053–1068. [Google Scholar] [CrossRef]
- Wang, M.; Lv, Y.; Lv, X.; Wang, Q.; Li, Y.; Lu, P.; Yu, H.; Wei, P.; Cao, Z.; An, T. Distribution, sources and health risks of heavy metals in indoor dust across China. Chemosphere 2023, 313, 137595. [Google Scholar] [CrossRef]
- Shi, G.; Chen, Z.; Xu, S.; Zhang, J.; Wang, L.; Bi, C.; Teng, J. Potentially toxic metal contamination of urban soils and roadside dust in Shanghai, China. Environ. Pollut. 2008, 156, 251–260. [Google Scholar] [CrossRef]
- Gurung, A.; Bell, M.L. The state of scientific evidence on air pollution and human health in Nepal. Environ. Res. 2013, 124, 54–64. [Google Scholar] [CrossRef]
- Kong, S.; Lu, B.; Ji, Y.; Zhao, X.; Chen, L.; Li, Z.; Han, B.; Bai, Z. Levels, risk assessment and sources of PM10 fraction heavy metals in four types dust from a coal-based city. Microchem. J. 2011, 98, 280–290. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, Y.J.; Seo, Y.R. An Overview of Carcinogenic Heavy Metal: Molecular Toxicity Mechanism and Prevention. J. Cancer Prev. 2015, 20, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Carver, A.; Gallicchio, V. Heavy Metals and Cancer; IntechOpen: London, UK, 2018. [Google Scholar]
- Lin, Y.; Ma, J.; Zhang, Z.; Zhu, Y.; Hou, H.; Zhao, L.; Sun, Z.; Xue, W.; Shi, H. Linkage between human population and trace elements in soils of the Pearl River Delta: Implications for source identification and risk assessment. Sci. Total Environ. 2018, 610–611, 944–950. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zhao, J.; Chang, S.X.; Collins, C.; Xu, J.; Liu, X. Status assessment and probabilistic health risk modeling of metals accumulation in agriculture soils across China: A synthesis. Environ. Int. 2019, 128, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Kapaj, S.; Peterson, H.; Liber, K.; Bhattacharya, P. Human health effects from chronic arsenic poisoning--a review. J. Environ. Sci. Health A Tox Hazard. Subst. Environ. Eng. 2006, 41, 2399–2428. [Google Scholar] [CrossRef] [PubMed]
- Mohammed Abdul, K.S.; Jayasinghe, S.S.; Chandana, E.P.S.; Jayasumana, C.; De Silva, P.M.C.S. Arsenic and human health effects: A review. Environ. Toxicol. Pharmacol. 2015, 40, 828–846. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Sun, Y.; Yao, W.; Ba, Q.; Wang, H. Effects of Cadmium Exposure on the Immune System and Immunoregulation. Front. Immunol. 2021, 12, 695484. [Google Scholar] [CrossRef] [PubMed]
- Genchi, G.; Sinicropi, M.S.; Lauria, G.; Carocci, A.; Catalano, A. The Effects of Cadmium Toxicity. Int. J. Environ. Res. Public. Health 2020, 17, 3782. [Google Scholar] [CrossRef]
- Hossini, H.; Shafie, B.; Niri, A.D.; Nazari, M.; Esfahlan, A.J.; Ahmadpour, M.; Nazmara, Z.; Ahmadimanesh, M.; Makhdoumi, P.; Mirzaei, N.; et al. A comprehensive review on human health effects of chromium: Insights on induced toxicity. Environ. Sci. Pollut. Res. 2022, 29, 70686–70705. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention, The National Institute for Occupational Safety and Health (NIOSH). Mangnese. Available online: https://www.cdc.gov/niosh/topics/manganese/default.html (accessed on 8 October 2023).
- Genchi, G.; Carocci, A.; Lauria, G.; Sinicropi, M.S.; Catalano, A. Nickel: Human Health and Environmental Toxicology. Int. J. Environ. Res. Public. Health 2020, 17, 679. [Google Scholar] [CrossRef]
- World Health Organization. Lead Poisoning. Available online: https://www.who.int/news-room/fact-sheets/detail/lead-poisoning-and-health (accessed on 8 October 2023).
- International Agency for Research on Cancer (IARC). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC: Lyon, France, 2012. [Google Scholar]
- IARC. Arsenic, metals, fibres, and dusts. IARC Monogr. Eval. Carcinog. Risks Hum. 2012, 100, 11–465. [Google Scholar]
- Pietrzak, S.; Wójcik, J.; Baszuk, P.; Marciniak, W.; Wojtyś, M.; Dębniak, T.; Cybulski, C.; Gronwald, J.; Alchimowicz, J.; Masojć, B.; et al. Influence of the Levels of Arsenic, Cadmium, Mercury and Lead on Overall Survival in Lung Cancer. Biomolecules 2021, 11, 1160. [Google Scholar] [CrossRef] [PubMed]
- Geoinformatics Center. Impact Assessment of Urbanization on Environmental Quality in Chiang Mai-Lumphun Valley, Northern Thailand. Available online: https://geoinfo.ait.ac.th/impact-assessment-of-urbanization-on-environmental-quality-in-chiang-mai-lumphun-valley-northern-thailand/ (accessed on 10 October 2023).
- Kermel-Torrès, D. Atlas of Thailand: Spatial Structures and Development. Available online: https://books.openedition.org/irdeditions/32537 (accessed on 10 October 2023).
- Falciani, R.; Novaro, E.; Marchesini, M.; Gucciardi, M. Multi-element analysis of soil and sediment by ICP-MS after a microwave assisted digestion method. J. Anal. At. Spectrom. 2000, 15, 561–565. [Google Scholar] [CrossRef]
- Zhao, X.; Li, Z.; Wang, D.; Tao, Y.; Qiao, F.; Lei, L.; Huang, J.; Ting, Z. Characteristics, source apportionment and health risk assessment of heavy metals exposure via household dust from six cities in China. Sci. Total Environ. 2021, 762, 143126. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Lin, L.; Zhang, Y. Contamination and human health risks of metals in indoor dust from university libraries: A case study from Qingdao, China. Hum. Ecol. Risk Assess. Int. J. 2021, 27, 152–161. [Google Scholar] [CrossRef]
- Liu, E.; Yan, T.; Birch, G.; Zhu, Y. Pollution and health risk of potentially toxic metals in urban road dust in Nanjing, a mega-city of China. Sci. Total Environ. 2014, 476–477, 522–531. [Google Scholar] [CrossRef]
- Rubio, B.; Nombela, M.A.; Vilas, F. Geochemistry of Major and Trace Elements in Sediments of the Ria de Vigo (NW Spain): An Assessment of Metal Pollution. Mar. Pollut. Bull. 2000, 40, 968–980. [Google Scholar] [CrossRef]
- Taylor, S.R. Abundance of chemical elements in the continental crust: A new table. Geochim. Et. Cosmochim. Acta 1964, 28, 1273–1285. [Google Scholar] [CrossRef]
- Cao, S.; Chen, X.; Zhang, L.; Xing, X.; Wen, D.; Wang, B.; Qin, N.; Wei, F.; Duan, X. Quantificational exposure, sources, and health risks posed by heavy metals in indoor and outdoor household dust in a typical smelting area in China. Indoor Air 2020, 30, 872–884. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. Exposure Factors Handbook 2011 Edition (Final Report). Available online: https://cfpub.epa.gov/ncea/risk/recordisplay.cfm?deid=236252 (accessed on 4 September 2023).
- Doyi, I.N.Y.; Isley, C.F.; Soltani, N.S.; Taylor, M.P. Human exposure and risk associated with trace element concentrations in indoor dust from Australian homes. Environ. Int. 2019, 133, 105125. [Google Scholar] [CrossRef]
- Zhou, L.; Liu, G.; Shen, M.; Hu, R.; Liu, Y. Source identification of heavy metals and stable carbon isotope in indoor dust from different functional areas in Hefei, China. Sci. Total Environ. 2020, 710, 135599. [Google Scholar] [CrossRef]
- Hunt, A.; Johnson, D.L. Suspension and resuspension of dry soil indoors following track-in on footwear. Environ. Geochem. Health 2012, 34, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Naccarato, A.; Tassone, A.; Cavaliere, F.; Elliani, R.; Pirrone, N.; Sprovieri, F.; Tagarelli, A.; Giglio, A. Agrochemical treatments as a source of heavy metals and rare earth elements in agricultural soils and bioaccumulation in ground beetles. Sci. Total Environ. 2020, 749, 141438. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.P.; Turnbull, R.E.; Rissmann, C.W.; Rieger, P. Heavy metal and metalloid concentrations in soils under pasture of southern New Zealand. Geoderma Reg. 2017, 11, 18–27. [Google Scholar] [CrossRef]
- He, C.-T.; Zheng, X.-B.; Yan, X.; Zheng, J.; Wang, M.-H.; Tan, X.; Qiao, L.; Chen, S.-J.; Yang, Z.-Y.; Mai, B.-X. Organic contaminants and heavy metals in indoor dust from e-waste recycling, rural, and urban areas in South China: Spatial characteristics and implications for human exposure. Ecotoxicol. Environ. Saf. 2017, 140, 109–115. [Google Scholar] [CrossRef]
- Gul, H.K.; Gullu, G.; Babaei, P.; Nikravan, A.; Kurt-Karakus, P.B.; Salihoglu, G. Assessment of house dust trace elements and human exposure in Ankara, Turkey. Environ. Sci. Pollut. Res. 2023, 30, 7718–7735. [Google Scholar] [CrossRef] [PubMed]
- Dingle, J.H.; Kohl, L.; Khan, N.; Meng, M.; Shi, Y.A.; Pedroza-Brambila, M.; Chow, C.-W.; Chan, A.W.H. Sources and composition of metals in indoor house dust in a mid-size Canadian city. Environ. Pollut. 2021, 289, 117867. [Google Scholar] [CrossRef]
- Turner, A.; Simmonds, L. Elemental concentrations and metal bioaccessibility in UK household dust. Sci. Total Environ. 2006, 371, 74–81. [Google Scholar] [CrossRef]
- Yoshinaga, J.; Yamasaki, K.; Yonemura, A.; Ishibashi, Y.; Kaido, T.; Mizuno, K.; Takagi, M.; Tanaka, A. Lead and other elements in house dust of Japanese residences—Source of lead and health risks due to metal exposure. Environ. Pollut. 2014, 189, 223–228. [Google Scholar] [CrossRef]
- Srithawirat, T.; Chimjan, O. Contamination assessment of heavy metals in dust deposited on residential building walls in agricultural areas of Sukhothai. J. Environ. Manag. 2017, 13, 20–33. [Google Scholar] [CrossRef]
- Kuntawee, C.; Tantrakarnapa, K.; Limpanont, Y.; Lawpoolsri, S.; Phetrak, A.; Mingkhwan, R.; Worakhunpiset, S. Exposure to Heavy Metals in Electronic Waste Recycling in Thailand. Int. J. Environ. Res. Public. Health 2020, 17, 2996. [Google Scholar] [CrossRef]
- Barbieri, M. The Importance of Enrichment Factor (EF) and Geoaccumulation Index (Igeo) to Evaluate the Soil Contamination. J. Geol. Geophys. 2016, 5, 1–4. [Google Scholar] [CrossRef]
- da Rosa Couto, R.; Faversani, J.; Ceretta, C.A.; Ferreira, P.A.A.; Marchezan, C.; Basso Facco, D.; Garlet, L.P.; Silva, J.S.; Comin, J.J.; Bizzi, C.A.; et al. Health risk assessment and soil and plant heavy metal and bromine contents in field plots after ten years of organic and mineral fertilization. Ecotoxicol. Environ. Saf. 2018, 153, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Liu, G.; Shen, M.; Liu, Y. Potential ecological and health risks of heavy metals for indoor and corresponding outdoor dust in Hefei, Central China. Chemosphere 2022, 302, 134864. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.L.; Yin, C.-Y.; Yap, S.Y. Particle size and metals concentrations of dust from a paint manufacturing plant. J. Hazard. Mater. 2010, 174, 839–842. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Pi, L.; Hu, W.; Chen, M.; Luo, Y.; Li, Z.; Su, S.; Gan, Z.; Ding, S. Concentrations and health risk assessment of metal(loid)s in indoor dust from two typical cities of China. Environ. Sci. Pollut. Res. 2016, 23, 9082–9092. [Google Scholar] [CrossRef] [PubMed]
- Davis, H.T.; Marjorie Aelion, C.; McDermott, S.; Lawson, A.B. Identifying natural and anthropogenic sources of metals in urban and rural soils using GIS-based data, PCA, and spatial interpolation. Environ. Pollut. 2009, 157, 2378–2385. [Google Scholar] [CrossRef] [PubMed]
- Marx, S.K.; Rashid, S.; Stromsoe, N. Global-scale patterns in anthropogenic Pb contamination reconstructed from natural archives. Environ. Pollut. 2016, 213, 283–298. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.P.; Isley, C.F.; Fry, K.L.; Liu, X.; Gillings, M.M.; Rouillon, M.; Soltani, N.S.; Gore, D.B.; Filippelli, G.M. A citizen science approach to identifying trace metal contamination risks in urban gardens. Environ. Int. 2021, 155, 106582. [Google Scholar] [CrossRef]
- O’Connor, D.; Hou, D.; Ye, J.; Zhang, Y.; Ok, Y.S.; Song, Y.; Coulon, F.; Peng, T.; Tian, L. Lead-based paint remains a major public health concern: A critical review of global production, trade, use, exposure, health risk, and implications. Environ. Int. 2018, 121, 85–101. [Google Scholar] [CrossRef]
- Shi, T.; Wang, Y. Heavy metals in indoor dust: Spatial distribution, influencing factors, and potential health risks. Sci. Total Environ. 2021, 755, 142367. [Google Scholar] [CrossRef]
- Rasmussen, P.E.; Levesque, C.; Chénier, M.; Gardner, H.D.; Jones-Otazo, H.; Petrovic, S. Canadian House Dust Study: Population-based concentrations, loads and loading rates of arsenic, cadmium, chromium, copper, nickel, lead, and zinc inside urban homes. Sci. Total Environ. 2013, 443, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Böhlandt, A.; Schierl, R.; Diemer, J.; Koch, C.; Bolte, G.; Kiranoglu, M.; Fromme, H.; Nowak, D. High concentrations of cadmium, cerium and lanthanum in indoor air due to environmental tobacco smoke. Sci. Total Environ. 2012, 414, 738–741. [Google Scholar] [CrossRef] [PubMed]
- Kurt-Karakus, P.B. Determination of heavy metals in indoor dust from Istanbul, Turkey: Estimation of the health risk. Environ. Int. 2012, 50, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Luippold, R.S.; Mundt, K.A.; Austin, R.P.; Liebig, E.; Panko, J.; Crump, C.; Crump, K.; Proctor, D. Lung cancer mortality among chromate production workers. Occup. Environ. Med. 2003, 60, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Park, R.M.; Bena, J.F.; Stayner, L.T.; Smith, R.J.; Gibb, H.J.; Lees, P.S. Hexavalent chromium and lung cancer in the chromate industry: A quantitative risk assessment. Risk Anal. 2004, 24, 1099–1108. [Google Scholar] [CrossRef] [PubMed]
- Mahiout, S.; Kiilunen, M.; Vermeire, T.; Viegas, S.; Woutersen, M.; Santonen, T. Occupational exposure to Cr(VI) in Finland in 1980–2016 and related lung cancer risk assessment. Regul. Toxicol. Pharmacol. 2022, 136, 105276. [Google Scholar] [CrossRef] [PubMed]
- Catrambone, M.; Canepari, S.; Perrino, C. Determination of Cr(III), Cr(VI) and total chromium in atmospheric aerosol samples. E3S Web Conf. 2013, 1, 07005. [Google Scholar] [CrossRef]
- Ukhurebor, K.E.; Aigbe, U.O.; Onyancha, R.B.; Nwankwo, W.; Osibote, O.A.; Paumo, H.K.; Ama, O.M.; Adetunji, C.O.; Siloko, I.U. Effect of hexavalent chromium on the environment and removal techniques: A review. J. Environ. Manag. 2021, 280, 111809. [Google Scholar] [CrossRef]
- Fry, K.L.; Gillings, M.M.; Isley, C.F.; Gunkel-Grillon, P.; Taylor, M.P. Trace element contamination of soil and dust by a New Caledonian ferronickel smelter: Dispersal, enrichment, and human health risk. Environ. Pollut. 2021, 288, 117593. [Google Scholar] [CrossRef]
- Aungkulanon, S.; Tangcharoensathien, V.; Shibuya, K.; Bundhamcharoen, K.; Chongsuvivatwong, V. Post universal health coverage trend and geographical inequalities of mortality in Thailand. Int. J. Equity Health 2016, 15, 190. [Google Scholar] [CrossRef]
- Imsamran, W.; Chaiwerawattana, A.; Wiangnon, S.; Pongnikorn, D.; Suwanrungrung, K.; Sangrajrang, S.; Buasom, R. Cancer in Thailand Vol. VIII, 2010–2012; National Cancer Institute: Bangkok, Thailand, 2015. [Google Scholar]
- Pongnikorn, D.; Daoprasert, K.; Waisri, N.; Laversanne, M.; Bray, F. Cancer incidence in northern Thailand: Results from six population-based cancer registries 1993-2012. Int. J. Cancer 2018, 142, 1767–1775. [Google Scholar] [CrossRef] [PubMed]
- Olujimi, O.; Steiner, O.; Goessler, W. Pollution indexing and health risk assessments of trace elements in indoor dusts from classrooms, living rooms and offices in Ogun State, Nigeria. J. Afr. Earth Sci. 2015, 101, 396–404. [Google Scholar] [CrossRef]
- Hou, S. Pollution characteristics, sources, and health risk assessment of human exposure to Cu, Zn, Cd and Pb pollution in urban street dust across China between 2009 and 2018. Environ. Int. 2019, 128, 430–437. [Google Scholar] [CrossRef] [PubMed]
- United States Environmental Protection Agency. US EPA Regional Screening Levels (RSL) Calculator. Available online: https://epa-prgs.ornl.gov/cgi-bin/chemicals/csl_search (accessed on 4 September 2023).
- Wang, S.; Wang, L.; Huan, Y.; Wang, R.; Liang, T. Concentrations, spatial distribution, sources and environmental health risks of potentially toxic elements in urban road dust across China. Sci. Total Environ. 2022, 805, 150266. [Google Scholar] [CrossRef]
- US EPA. Supplemental Guidance for Developing Soil Screening Levels for Superfund Sites. Available online: https://www.epa.gov/superfund/superfund-soil-screening-guidance (accessed on 4 September 2023).
- Ferreira-Baptista, L.; De Miguel, E. Geochemistry and risk assessment of street dust in Luanda, Angola: A tropical urban environment. Atmos. Environ. 2005, 39, 4501–4512. [Google Scholar] [CrossRef]
- Wignall, J.A.; Muratov, E.; Sedykh, A.; Guyton, K.Z.; Tropsha, A.; Rusyn, I.; Chiu, W.A. Conditional Toxicity Value (CTV) Predictor: An In Silico Approach for Generating Quantitative Risk Estimates for Chemicals. Environ. Health Perspect. 2018, 126, 057008. [Google Scholar] [CrossRef]
- Office of Environmental Health Hazard Assessment (OEHHA). Technical Support Doc.ument for Cancer Potency Factors 2009. Available online: https://oehha.ca.gov/air/crnr/technical-support-document-cancer-potency-factors-2009 (accessed on 5 September 2023).


| Heavy Metals | Concentration (mg kg−1) | |||||
|---|---|---|---|---|---|---|
| Mean | SD | Median | IQR | Min. | Max. | |
| As | 12.5 | 8.8 | 10.3 | 6.1 | 4.2 | 62.1 |
| Cd | 2.2 | 3.9 | 0.9 | 1.5 | 0.2 | 20.2 |
| Cr | 38.5 | 20.1 | 32.4 | 18.3 | 17.2 | 148.0 |
| Cu | 107.3 | 78.2 | 82.5 | 56.3 | 25.1 | 401.5 |
| Mn | 577.6 | 222.5 | 542.7 | 270.3 | 204.8 | 1318.1 |
| Ni | 34.9 | 23.6 | 28.9 | 17.9 | 11.2 | 146.2 |
| Pb | 62.1 | 57.8 | 44.8 | 27.7 | 18.0 | 426.4 |
| Zn | 408.7 | 246.9 | 352.7 | 210.1 | 123.8 | 1527.2 |
| Heavy Metals | n | Enrichment Factor | |||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Median | IQR | Min. | Max. | ||
| As | 100 | 11.13 | 5.66 | 10.13 | 4.93 | 3.82 | 47.88 |
| Cd | 96 | 18.18 | 27.69 | 9.43 | 13.41 | 1.47 | 141.17 |
| Cr | 100 | 0.70 | 0.48 | 0.61 | 0.39 | 0.21 | 4.15 |
| Cu | 97 | 3.76 | 3.24 | 2.87 | 2.14 | 0.72 | 19.77 |
| Ni | 99 | 0.84 | 0.61 | 0.65 | 0.50 | 0.18 | 3.60 |
| Pb | 100 | 7.99 | 5.14 | 6.48 | 5.03 | 2.41 | 27.99 |
| Zn | 99 | 10.84 | 7.68 | 9.21 | 8.77 | 1.85 | 51.82 |
| Heavy Metal | Noncarcinogenic Risk | |||||||
|---|---|---|---|---|---|---|---|---|
| Adult | Child | |||||||
| HQing | HQinh | HQdermal | HI | HQing | HQinh | HQdermal | HI | |
| As | 6.20 × 10−2 | 1.49 × 10−4 | 1.81 × 10−2 | 8.02 × 10−2 | 5.21 × 10−1 | 2.91 × 10−4 | 1.81 × 10−2 | 5.39 × 10−1 |
| Urban | 5.79 × 10−2 | 1.39 × 10−4 | 1.69 × 10−2 | 7.49 × 10−2 | 4.86 × 10−1 | 2.72 × 10−4 | 1.69 × 10−2 | 5.03 × 10−1 |
| Rural | 6.31 × 10−2 | 1.51 × 10−4 | 1.84 × 10−2 | 8.17 × 10−2 | 5.30 × 10−1 | 2.96 × 10−4 | 1.84 × 10−2 | 5.49 × 10−1 |
| Cd | 1.09 × 10−2 | 2.29 × 10−4 | 1.74 × 10−3 | 1.28 × 10−2 | 9.14 × 10−2 | 4.48 × 10−4 | 8.68 × 10−3 | 1.01 × 10−1 |
| Urban | 3.23 × 10−3 | 6.79 × 10−5 | 5.15 × 10−4 | 3.81 × 10−3 | 2.71 × 10−2 | 1.33 × 10−4 | 2.57 × 10−3 | 2.98 × 10−2 |
| Rural | 1.29 × 10−2 | 2.72 × 10−4 | 2.06 × 10−3 | 1.53 × 10−2 | 1.09 × 10−1 | 5.32 × 10−4 | 1.03 × 10−2 | 1.19 × 10−1 |
| Cr | 1.94 × 10−2 | 6.99 × 10−5 | 3.88 × 10−3 | 2.34 × 10−2 | 1.63 × 10−1 | 1.37 × 10−4 | 1.94 × 10−2 | 1.83 × 10−1 |
| Urban | 2.09 × 10−2 | 7.50 × 10−5 | 4.16 × 10−3 | 2.51 × 10−2 | 1.75 × 10−1 | 1.47 × 10−4 | 2.08 × 10−2 | 1.96 × 10−1 |
| Rural | 1.90 × 10−2 | 6.85 × 10−5 | 3.80 × 10−3 | 2.29 × 10−2 | 1.60 × 10−1 | 1.34 × 10−4 | 1.90 × 10−2 | 1.79 × 10−1 |
| Cu | 4.99 × 10−3 | 3.47 × 10−5 | 6.64 × 10−5 | 5.09 × 10−3 | 4.19 × 10−2 | 6.79 × 10−5 | 3.32 × 10−4 | 4.23 × 10−2 |
| Urban | 3.88 × 10−3 | 2.70 × 10−5 | 5.16 × 10−5 | 3.96 × 10−3 | 3.26 × 10−2 | 5.28 × 10−5 | 2.58 × 10−4 | 3.29 × 10−2 |
| Rural | 5.29 × 10−3 | 3.68 × 10−5 | 7.04 × 10−5 | 5.40 × 10−3 | 4.44 × 10−2 | 7.20 × 10−5 | 3.51 × 10−4 | 4.49 × 10−2 |
| Mn | 3.64 × 10−2 | 2.10 × 10−3 | 1.90 × 10−3 | 4.04 × 10−2 | 3.06 × 10−1 | 4.10 × 10−3 | 9.47 × 10−3 | 3.20 × 10−1 |
| Urban | 3.19 × 10−2 | 1.83 × 10−3 | 1.66 × 10−3 | 3.53 × 10−2 | 2.68 × 10−1 | 3.59 × 10−3 | 8.28 × 10−3 | 2.79 × 10−1 |
| Rural | 3.77 × 10−2 | 2.17 × 10−3 | 1.96 × 10−3 | 4.18 × 10−2 | 3.16 × 10−1 | 4.24 × 10−3 | 9.79 × 10−3 | 3.30 × 10−1 |
| Ni | 2.69 × 10−3 | 3.13 × 10−7 | 3.97 × 10−5 | 2.73 × 10−3 | 2.26 × 10−2 | 6.12 × 10−7 | 1.98 × 10−4 | 2.28 × 10−2 |
| Urban | 2.94 × 10−3 | 3.42 × 10−7 | 4.35 × 10−5 | 2.98 × 10−3 | 2.47 × 10−2 | 6.70 × 10−7 | 2.17 × 10−4 | 2.49 × 10−2 |
| Rural | 2.62 × 10−3 | 3.05 × 10−7 | 3.87 × 10−5 | 2.66 × 10−3 | 2.20 × 10−2 | 5.97 × 10−7 | 1.93 × 10−4 | 2.22 × 10−2 |
| Pb | 2.68 × 10−2 | 3.19 × 10−6 | 7.12 × 10−4 | 2.75 × 10−2 | 2.25 × 10−1 | 6.25 × 10−6 | 3.56 × 10−3 | 2.28 × 10−1 |
| Urban | 2.81 × 10−2 | 3.34 × 10−6 | 7.46 × 10−4 | 2.88 × 10−2 | 2.36 × 10−1 | 6.55 × 10−6 | 3.73 × 10−3 | 2.39 × 10−1 |
| Rural | 2.64 × 10−2 | 3.15 × 10−6 | 7.03 × 10−4 | 2.71 × 10−2 | 2.22 × 10−1 | 6.17 × 10−6 | 3.51 × 10−3 | 2.25 × 10−1 |
| Zn | 2.11 × 10−3 | 2.53 × 10−7 | 4.22 × 10−5 | 2.16 × 10−3 | 1.78 × 10−2 | 4.96 × 10−7 | 2.11 × 10−4 | 1.80 × 10−2 |
| Urban | 2.66 × 10−3 | 3.18 × 10−7 | 5.30 × 10−5 | 2.71 × 10−3 | 2.23 × 10−2 | 6.24 × 10−7 | 2.65 × 10−4 | 2.26 × 10−2 |
| Rural | 1.97 × 10−3 | 2.36 × 10−7 | 3.93 × 10−5 | 2.01 × 10−3 | 1.65 × 10−2 | 4.62 × 10−7 | 1.96 × 10−4 | 1.67 × 10−2 |
| Total | 1.65 × 10−1 | 2.58 × 10−3 | 2.65 × 10−2 | 1.94 × 10−1 | 1.39 | 5.06 × 10−3 | 5.99 × 10−2 | 1.45 |
| Urban | 1.51 × 10−1 | 2.15 × 10−3 | 2.41 × 10−2 | 1.78 × 10−1 | 1.27 | 4.20 × 10−3 | 5.30 × 10−2 | 1.33 |
| Rural | 1.69 × 10−1 | 2.70 × 10−3 | 2.71 × 10−2 | 1.99 × 10−1 | 1.42 | 5.28 × 10−3 | 6.17 × 10−2 | 1.49 |
| Heavy Metals | Carcinogenic Risk (CR) | |||||||
|---|---|---|---|---|---|---|---|---|
| Adult | Child | |||||||
| CRing | CRinh | CRdermal | LCR | CRing | CRinh | CRdermal | LCR | |
| As | 5.02 × 10−5 | 1.27 × 10−8 | 2.73 × 10−6 | 5.30 × 10−5 | 3.18 × 10−4 | 3.18 × 10−9 | 2.73 × 10−6 | 3.21 × 10−4 |
| Urban | 4.69 × 10−5 | 1.19 × 10−8 | 2.55 × 10−6 | 4.94 × 10−5 | 2.97 × 10−4 | 2.96 × 10−9 | 2.55 × 10−6 | 2.99 × 10−4 |
| Rural | 5.11 × 10−5 | 1.29 × 10−8 | 2.78 × 10−6 | 5.39 × 10−5 | 3.24 × 10−4 | 3.23 × 10−9 | 2.78 × 10−6 | 3.26 × 10−4 |
| Cd | 7.44 × 10−6 | 1.22 × 10−5 | 5.39 × 10−7 | 2.02 × 10−5 | 4.71 × 10−5 | 3.05 × 10−6 | 5.39 × 10−7 | 5.07 × 10−5 |
| Urban | 2.21 × 10−6 | 3.61 × 10−6 | 1.60 × 10−7 | 5.98 × 10−6 | 1.40 × 10−5 | 9.04 × 10−7 | 1.60 × 10−7 | 1.50 × 10−5 |
| Rural | 8.84 × 10−6 | 1.45 × 10−5 | 6.41 × 10−7 | 2.39 × 10−5 | 5.60 × 10−5 | 3.62 × 10−6 | 6.41 × 10−7 | 6.02 × 10−5 |
| Cr | 5.25 × 10−5 | 3.89 × 10−4 | 9.50 × 10−8 | 4.41 × 10−4 | 3.32 × 10−4 | 9.72 × 10−5 | 9.50 × 10−8 | 4.29 × 10−4 |
| Urban | 5.63 × 10−5 | 4.18 × 10−4 | 1.02 × 10−7 | 4.74 × 10−4 | 3.57 × 10−4 | 1.04 × 10−4 | 1.02 × 10−7 | 4.61 × 10−4 |
| Rural | 5.14 × 10−5 | 3.81 × 10−4 | 9.32 × 10−8 | 4.33 × 10−4 | 3.26 × 10−4 | 9.53 × 10−5 | 9.32 × 10−8 | 4.21 × 10−4 |
| Ni | 1.49 × 10−5 | 2.22 × 10−9 | 1.59 × 10−7 | 1.51 × 10−5 | 9.43 × 10−5 | 5.55 × 10−10 | 1.59 × 10−7 | 9.45 × 10−5 |
| Urban | 1.63 × 10−5 | 2.43 × 10−9 | 1.75 × 10−7 | 1.65 × 10−5 | 1.03 × 10−4 | 6.07 × 10−10 | 1.75 × 10−7 | 1.03 × 10−4 |
| Rural | 1.45 × 10−5 | 2.16 × 10−9 | 1.55 × 10−7 | 1.47 × 10−5 | 9.20 × 10−5 | 5.41 × 10−10 | 1.55 × 10−7 | 9.21 × 10−5 |
| Pb | 1.43 × 10−6 | 1.79 × 10−10 | 6.42 × 10−8 | 1.50 × 10−6 | 9.08 × 10−6 | 4.46 × 10−11 | 6.42 × 10−8 | 9.14 × 10−6 |
| Urban | 1.50 × 10−6 | 1.87 × 10−10 | 6.73 × 10−8 | 1.57 × 10−6 | 9.52 × 10−6 | 4.68 × 10−11 | 6.73 × 10−8 | 9.58 × 10−6 |
| Rural | 1.42 × 10−6 | 1.76 × 10−10 | 6.33 × 10−8 | 1.48 × 10−6 | 8.96 × 10−6 | 4.41 × 10−11 | 6.33 × 10−8 | 9.03 × 10−6 |
| Total | 1.26 × 10−4 | 4.01 × 10−4 | 3.59 × 10−6 | 5.31 × 10−4 | 8.01 × 10−4 | 1.00 × 10−4 | 3.59 × 10−6 | 9.05 × 10−4 |
| Urban | 1.23 × 10−4 | 4.21 × 10−4 | 3.05 × 10−6 | 5.47 × 10−4 | 7.80 × 10−4 | 1.05 × 10−4 | 3.05 × 10−6 | 8.89 × 10−4 |
| Rural | 1.27 × 10−4 | 3.96 × 10−4 | 3.73 × 10−6 | 5.27 × 10−4 | 8.06 × 10−4 | 9.89 × 10−5 | 3.73 × 10−6 | 9.09 × 10−4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Somsunun, K.; Prapamontol, T.; Kuanpan, T.; Santijitpakdee, T.; Kohsuwan, K.; Jeytawan, N.; Thongjan, N. Health Risk Assessment of Heavy Metals in Indoor Household Dust in Urban and Rural Areas of Chiang Mai and Lamphun Provinces, Thailand. Toxics 2023, 11, 1018. https://doi.org/10.3390/toxics11121018
Somsunun K, Prapamontol T, Kuanpan T, Santijitpakdee T, Kohsuwan K, Jeytawan N, Thongjan N. Health Risk Assessment of Heavy Metals in Indoor Household Dust in Urban and Rural Areas of Chiang Mai and Lamphun Provinces, Thailand. Toxics. 2023; 11(12):1018. https://doi.org/10.3390/toxics11121018
Chicago/Turabian StyleSomsunun, Kawinwut, Tippawan Prapamontol, Todsabhorn Kuanpan, Teetawat Santijitpakdee, Kanyapak Kohsuwan, Natwasan Jeytawan, and Nathaporn Thongjan. 2023. "Health Risk Assessment of Heavy Metals in Indoor Household Dust in Urban and Rural Areas of Chiang Mai and Lamphun Provinces, Thailand" Toxics 11, no. 12: 1018. https://doi.org/10.3390/toxics11121018
APA StyleSomsunun, K., Prapamontol, T., Kuanpan, T., Santijitpakdee, T., Kohsuwan, K., Jeytawan, N., & Thongjan, N. (2023). Health Risk Assessment of Heavy Metals in Indoor Household Dust in Urban and Rural Areas of Chiang Mai and Lamphun Provinces, Thailand. Toxics, 11(12), 1018. https://doi.org/10.3390/toxics11121018








