Enzymatic Stress Responses of Coreius guichenoti to Microplastics with Different Particle Sizes
Abstract
:1. Introduction Test
2. Materials and Methods
2.1. Microplastics
2.2. Source of Experimental Organism and Experimental Design
2.3. Tissue Sample Collection
2.4. Enzymatic Activities Analysis
2.5. Data Analysis
3. Results
3.1. Enzymatic Stress Responses of Skin
3.2. Enzymatic Stress Responses of Gill
3.3. Enzymatic Stress Responses of Muscle
3.4. Enzymatic Stress Responses of Liver
3.5. Stress Adaptive Response of Intestine
3.6. Comprehensive Analysis among Different Organizations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fish Laboratory of Hubei Institute of Aquatic Biology. The Fishes of Yangtze River; Science Press: Beijing, China, 1976; ISBN 13031379. [Google Scholar]
- Ding, R.H. The Fishes of Sichuan; Sichuan Science and Technology Press: Chengdu, China, 1994; ISBN 9787536422957. [Google Scholar]
- Yang, Z.; Tang, H.; Tao, J.; Zhao, N. The effect of cascaded huge dams on the downstream movement of Coreius guichenoti (Sauvage & Dabry de Thiersant, 1874) in the upper Yangtze River. Environ. Biol. Fish. 2017, 100, 1507–1516. [Google Scholar] [CrossRef]
- Li, T.; Tang, L.; Wang, L.; An, L.; Wang, J.; Mo, K.L.; Chen, Q.W. Distribution characteristics and ecological types changes in fish communities under hydropower development from Xiluodu to Xiangjiaba reach. Acta Ecol. Sin. 2020, 40, 1473–1485. (In Chinese) [Google Scholar] [CrossRef]
- Li, T.; Mo, K.; Wang, J.; Chen, Q.; Zhang, J.; Zeng, C.; Zhang, H.; Yang, P. Mismatch between critical and accumulated temperature following river damming impacts fish spawning. Sci. Total Environ. 2021, 756, 144052. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, P.; Tang, H.; Gong, Y.; Dong, C.; Chen, X.; Zhao, N. The formation of habitat suitability curves for Coreius guichenoti (Sauvage & Dabry de Thiersant, 1874) of the lower Jinsha River. Ecol. Sci. 2017, 36, 129–137. (In Chinese) [Google Scholar]
- Luo, Y.P.; Wang, Q.Q. Effects of body mass and temperature on routine metabolic rate of juvenile largemouth bronze gudgeon Coreius guichenoti. J. Fish Biol. 2012, 80, 842–851. [Google Scholar] [CrossRef]
- Ministry of Agriculture and Rural Affairs of the People’s Republic of China. Announcement No. 948 of the Ministry of Agriculture and Rural Affairs of the People’s Republic of China. 2007. Available online: http://www.moa.gov.cn/gk/tzgg_1/gg/200801/t20080102_947565.htm (accessed on 6 May 2023).
- Liu, H.; Zhou, B. Current situation, problems and countermeasures of biological research on Coreius guichenoti. Plant Health Med. 2018, 31, 37–38. (In Chinese) [Google Scholar] [CrossRef]
- National Forestry and Grassland Administration and Ministry of Agriculture and Rural Affairs of the People’s Republic of China. Announcement No. 3 of the National Forestry and Grassland Administration and Ministry of Agriculture and Rural Affairs of the People’s Republic of China in 2021. 2021. Available online: http://www.moa.gov.cn/govpublic/YYJ/202102/t20210205_6361292.htm (accessed on 6 May 2023).
- Sun, Z.; Zhu, T.; Yang, D.; Li, C.; Wu, X. Progress and prospect on the artificial domestication and reproduction of Coreius guichenoti. Freshw. Fish. 2020, 50, 107–112. (In Chinese) [Google Scholar] [CrossRef]
- Andrady, A.L.; Neal, M.A. Applications and societal benefits of plastics. Philos. Trans. R. Soc. B 2009, 364, 1977–1984. [Google Scholar] [CrossRef]
- Wang, R.; Pu, X.X.; Zhao, Y.L. Research progress on the toxic effects of microplastics. J. Baotou Med. Coll. 2023, 9, 84–90. (In Chinese) [Google Scholar] [CrossRef]
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Plastic waste inputs from land into the ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef] [PubMed]
- Frias, J.P.G.L.; Nash, R. Microplastics: Finding a consensus on the definition. Mar. Pollut. Bull. 2019, 138, 145–147. [Google Scholar] [CrossRef]
- Wu, P.; Huang, J.; Zheng, Y.; Yang, Y.; Zhang, Y.; He, F.; Chen, H.; Quan, G.; Yan, J.; Li, T.; et al. Environmental occurrences, fate, and impacts of microplastics. Ecotox. Environ. Saf. 2019, 184, 109612. [Google Scholar] [CrossRef]
- Hirt, N.; Body-Malapel, M. Immunotoxicity and intestinal effects of nano-and microplastics: A review of the literature. Part. Fibre Toxicol. 2020, 17, 57. [Google Scholar] [CrossRef]
- Karbalaei, S.; Hanachi, P.; Walker, T.R.; Cole, M. Occurrence, sources, human health impacts and mitigation of microplastic pollution. Environ. Sci. Pollut. Res. 2018, 25, 36046–36063. [Google Scholar] [CrossRef]
- Borrelle, S.B.; Ringma, J.; Law, K.L.; Monnahan, C.C.; Lebreton, L.; Mcgivern, A.; Murphy, E.; Jambeck, J.; Leonard, G.H.; Hilleary, M.A.; et al. Predicted growth in plastic waste exceeds efforts to mitigate plastic pollution. Science 2020, 369, 1515–1518. [Google Scholar] [CrossRef]
- Choi, D.; Bang, J.; Kim, T.; Oh, Y.; Hwang, Y.; Hong, J. In vitro chemical and physical toxicities of polystyrene microfragments in human-derived cells. J. Hazard. Mater. 2020, 400, 123308. [Google Scholar] [CrossRef]
- Huang, W.; Yin, H.; Yang, Y.; Jin, L.; Lu, G.; Dang, Z. Influence of the co-exposure of microplastics and tetrabromobisphenol A on human gut: Simulation in vitro with human cell Caco-2 and gut microbiota. Sci. Total Environ. 2021, 778, 146264. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.W.; Wu, Y.C.; Liao, V.H.C. Early developmental nanoplastics exposure disturbs circadian rhythms associated with stress resistance decline and modulated by DAF-16 and PRDX-2 in C. elegans. J. Hazard. Mater. 2022, 423, 127091. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Deng, C.; Dong, L.; Liu, L.; Li, H.; Wu, J.; Ye, C. Microplastic pollution in the Yangtze River Basin: Heterogeneity of abundances and characteristics in different environments. Environ. Pollut. 2021, 287, 117580. [Google Scholar] [CrossRef] [PubMed]
- Li, T.C.; Huang, X.L.; Wu, C.X.; Guo, W.S. Current situation, prevention and control measures of microplastic pollution in water bodies of the Yangtze River Basin. J. Changjiang River Sci. Res. Inst. 2021, 38, 143–150. (In Chinese) [Google Scholar]
- He, D.; Chen, X.; Zhao, W.; Zhu, Z.; Qi, X.; Zhou, L.; Chen, W.; Wan, C.; Li, D.; Zou, X.; et al. Microplastics contamination in the surface water of the Yangtze River from upstream to estuary based on different sampling methods. Environ. Res. 2021, 196, 110908. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Mintenig, S.M.; Cheng, C.; Wu, J.; Koelmans, A.A. Extent and risks of microplastic pollution in the Yangtze River. State of the science. Sci. Total Environ. 2023, 910, 168538. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Chatterjee, S. Microplastic pollution, a threat to marine ecosystem and human health: A short review. Environ. Sci. Pollut. Res. 2017, 24, 21530–21547. [Google Scholar] [CrossRef]
- Yin, K.; Wang, Y.; Zhao, H.; Wang, D.; Guo, M.; Mu, M.; Liu, Y.; Nie, X.; Li, B.; Li, J.; et al. A comparative review of microplastics and nanoplastics: Toxicity hazards on digestive, reproductive and nervous system. Sci. Total Environ. 2021, 774, 145758. [Google Scholar] [CrossRef]
- Xiao, K.; Song, L.; Li, Y.; Li, C.; Zhang, S. Dietary intake of microplastics impairs digestive performance, induces hepatic dysfunction, and shortens lifespan in the annual fish Nothobranchius guentheri. Biogerontology 2023, 24, 207–223. [Google Scholar] [CrossRef]
- Jovanović, B. Ingestion of microplastics by fish and its potential consequences from a physical perspective. Integr. Environ. Assess. Manag. 2017, 13, 510–515. [Google Scholar] [CrossRef]
- Sequeira, I.F.; Prata, J.C.; da Costa, J.P.; Duarte, A.C.; Rocha-Santos, T. Worldwide contamination of fish with microplastics: A brief global overview. Mar. Pollut. Bull. 2020, 160, 111681. [Google Scholar] [CrossRef]
- Sharifinia, M.; Bahmanbeigloo, Z.A.; Keshavarzifard, M.; Khanjani, M.H.; Lyons, B.P. Microplastic pollution as a grand challenge in marine research: A closer look at their adverse impacts on the immune and reproductive systems. Ecotox. Environ. Saf. 2020, 204, 111109. [Google Scholar] [CrossRef]
- Qiang, L.; Cheng, J. Exposure to polystyrene microplastics impairs gonads of zebrafish (Danio rerio). Chemosphere 2021, 263, 128161. [Google Scholar] [CrossRef]
- Huang, J.N.; Wen, B.; Xu, L.; Ma, H.C.; Li, X.X.; Gao, J.Z.; Chen, Z.Z. Micro/nano-plastics cause neurobehavioral toxicity in discus fish (Symphysodon aequifasciatus): Insight from brain-gut-microbiota axis. J. Hazard. Mater. 2022, 421, 126830. [Google Scholar] [CrossRef]
- Gao, S.B.; Tang, H.Y.; Hong, F. Preliminary test on domestication of larval and juvenile Coreius guichenoti. J. Hydr. 2012, 33, 150–152. (In Chinese) [Google Scholar] [CrossRef]
- Shu, Q.; Huang, J.; Xu, X.; Hu, R. Effects of different diets on the growth and survival rate in the fries of Coreius guichenoti. J. Aquac. 2020, 41, 32–36. (In Chinese) [Google Scholar] [CrossRef]
- Liu, X.; Huang, Y.; Yuan, X.; Xu, M.; Tu, Z. Swimming ability and behavior of artificially propagated largemouth bronze gudgeon (Coreius guichenoti). J. Hydr. 2021, 42, 94–100. (In Chinese) [Google Scholar] [CrossRef]
- Chen, P.; Zhao, Y.; Yang, Y.J.; Yang, J.; Qu, H.T. The effect of high feeding rate on antioxidant, immune function, and lipid metabolism in the liver of young Coreius guichenoti. Chin. J. Ani. Nutr. 2023, 35, 5870–5885. (In Chinese) [Google Scholar]
- Li, X.; Wu, X.; Yang, D.; Zhu, Y. Development of microsatellite markers for Coreius guichenoti based on transcriptome sequencing. J. Hydroecol. 2021, 42, 97–103. (In Chinese) [Google Scholar] [CrossRef]
- He, Y.F.; Zhu, Y.J.; Gong, J.L.; Zhu, T.B.; Wu, X.B.; Li, X.M.; Meng, Z.H.; Yang, D.G. Genetic diversity and population history dynamics analysis of Coreius guichenoti in the middle and lower reaches of the Jinsha river. Acta Hydr. Sinica 2022, 46, 37–47. (In Chinese) [Google Scholar]
- Liu, X.Y. Molecular Cloning and Expression Analysis of the HPG Axis Genes in the Coreius guichenoti; Central China Normal University: Wuhan, China, 2012. [Google Scholar]
- Li, X.M.; Wu, X.B.; Gong, J.L.; Zhu, Y.J.; Yang, D.G. Comparative analysis of liver transcriptome of parent and offspring Coreius guichenoti. Acta Hydrobiol. Sin. 2020, 44, 774–780. (In Chinese) [Google Scholar] [CrossRef]
- Xia, Y.G.; Zhang, L.; Chen, S.B.; Perera, H.A.C.C.; Li, Z.J.; Zhang, T.L.; Ye, S.W.; Yuan, J.; Liu, J.S. The analysis of heavy metals in the muscles and the livers of Coreius heterodon and Coreivs guichenoti after the impoundment in the three gorges reservoir. Acta Hydrobiol. Sin. 2015, 39, 861–868. [Google Scholar] [CrossRef]
- Dong, C.; Chen, X.; Wan, C.; Tang, H.; Yang, Z. Nutrient composition analysis of Coreius guichenoti at different ages and gonadal development stages. J. Hydroecol. 2021, 43, 108–115. (In Chinese) [Google Scholar] [CrossRef]
- Ran, J.; Zhang, Q.; Wei, M. Population ecology of Micracanthorkynchina motamurai in Coreius guichenoti in the upper reach of Yangtze River. Chin. J. Ecol. 2005, 24, 203–706. (In Chinese) [Google Scholar] [CrossRef]
- Fu, Y.W.; Zhu, C.K.; Zhang, Q.Z. Molecular characterization and expression analysis of complement components C3 and C9 in largemouth bronze gudgeon (Coreius guichenoti) in response to Ichthyophthirius multifiliis infection. Aquaculture 2019, 506, 270–279. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, D. Histopathological observation of Coreius guichenoti infected by Ichthyophthirius multifiliis Fouquet, 1876. J. Southwest China Nor. Univ. (Nat. Sci. Ed.) 2005, 6, 1112–1115. (In Chinese) [Google Scholar] [CrossRef]
- Zhao, J.H. Studies on the Physiological Mechanism and Coping Strategies of Stress of Coreius guichenoti; Huazhong Agricultural University: Wuhan, China, 2014. [Google Scholar]
- Zhao, J.; Zhu, Y.; He, Y.; Chen, J.; Feng, X.; Li, X.; Xiong, B.; Yang, D. Effects of Temperature Reduction and MS-222 on Water Quality and Blood Biochemistry in Simulated Transport Experiment of Largemouth Bronze Gudgeon, Coreius guichenoti. J. World Aquacult. Soc. 2014, 45, 719. [Google Scholar] [CrossRef]
- Zhu, T.; Li, F.; Wu, X.; Zhu, Y.; Guo, W.; Yang, D. Effects of Electronarcosis on the Behavior and Blood Biochemical Indices of Juvenile Coreius guichenoti. Sichuan J. Zool. 2015, 34, 885–888. (In Chinese) [Google Scholar] [CrossRef]
- Dong, C.; Pan, L.; He, D.; Xie, J.; Tang, H.; Yang, Z.; Wang, X.; Yang, S. The Efficacy of MS-222 as Anesthetic Agent in Largemouth Bronze Gudgeon Coreius guichenoti. N. Am. J. Aquacult. 2017, 79, 123–127. [Google Scholar] [CrossRef]
- Wang, W. Investigation of heavy metal content in fish at Chongqing section of the Yangtze River before water storage in the Three Gorges Reservoir. Water Resour. Prot. 2008, 5, 34–37. (In Chinese) [Google Scholar]
- Hale, R.C.; Seeley, M.E.; La Guardia, M.J.; Mai, L.; Zeng, E.Y. A global perspective on microplastics. J. Geophys. Res.-Oceans 2020, 125, e2018JC014719. [Google Scholar] [CrossRef]
- Hong, J.; Chen, X.; Liu, S.; Fu, Z.; Han, M.; Wang, Y.; Gu, Z.; Ma, Z. Impact of fish density on water quality and physiological response of golden pompano (Trachinotus ovatus) flingerlings during transportation. Aquaculture 2019, 507, 260–265. [Google Scholar] [CrossRef]
- Banaee, M.; Beitsayah, A.; Prokić, M.D.; Petrović, T.G.; Zeidi, A.; Faggio, C. Effects of cadmium chloride and biofertilizer (Bacilar) on biochemical parameters of freshwater fish, Alburnus mossulensis. Comp. Biochem. Phys. C 2023, 268, 109614. [Google Scholar] [CrossRef]
- Hossen, S.; Hanif, M.A.; Kho, K.H. Glutathione reductase, a biomarker of pollutant and stress in Pacific abalone. Mar. Pollut. Bull. 2023, 192, 115139. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Luo, H.; Wang, D.; Zheng, Y.; Zhu, W.; Zhang, W.; Chen, Z.; Chen, X.; Shao, J. Partial Substitution of Fish Meal with Soy Protein Concentrate on Growth, Liver Health, Intestinal Morphology, and Microbiota in Juvenile Large Yellow Croaker (Larimichthys crocea). Aquacult. Nutr. 2023, 2023, 3706709. [Google Scholar] [CrossRef] [PubMed]
- Zare, M.; Kazempour, M.; Hosseini, H.; Choupani, S.M.H.; Akhavan, S.R.; Rombenso, A.; Esmaeili, N. Fish Meal Replacement and Early Mild Stress Improve Stress Responsiveness and Survival of Fish after Acute Stress. Animals 2023, 13, 1314. [Google Scholar] [CrossRef] [PubMed]
- De Magalhães, C.R.; Farinha, A.P.; Carrilho, R.; Schrama, D.; Cerqueira, M.; Rodrigues, P.M. A new window into fish welfare: A proteomic discovery study of stress biomarkers in the skin mucus of gilthead seabream (Sparus aurata). J. Proteom. 2023, 281, 104904. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.N.; Wen, B.; Li, X.X.; Xu, L.; Gao, J.Z.; Chen, Z.Z. Astaxanthin mitigates oxidative stress caused by microplastics at the expense of reduced skin pigmentation in discus fish. Sci. Total Environ. 2023, 874, 162494. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Shi, J.R.; Song, K.; Zhao, T.T.; Liu, S.; Yue, X.J. Research progress of secretion mechanisms, components and function of fishes mucus. J. Anhui Agric. Sci. 2014, 42, 7445–7448+7458. (In Chinese) [Google Scholar] [CrossRef]
- Sanahuja, I.; Guerreiro, P.M.; Girons, A.; Fernandez-Alacid, L.; Ibarz, A. Evaluating the repetitive mucus extraction effects on mucus biomarkers, mucous cells, and the skin-barrier status in a marine fish model. Front. Mar. Sci. 2023, 9, 1095246. [Google Scholar] [CrossRef]
- Kim, J.H.; Yu, Y.B.; Choi, J.H. Toxic effects on bioaccumulation, hematological parameters, oxidative stress, immune responses and neurotoxicity in fish exposed to microplastics: A review. J. Hazard. Mater. 2021, 413, 125423. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Liu, B.; Li, H.; Tang, Y.; Ge, X.; Liu, B.; Xu, P. Protective effects of emodin on oxidized fish oil-induced metabolic disorder and oxidative stress through Notch-Nrf2 crosstalk in the liver of teleost Megalobrama amblycephala. Antioxidants 2022, 11, 1179. [Google Scholar] [CrossRef]
- Espinosa, C.; Esteban, M.Á.; Cuesta, A. Dietary administration of PVC and PE microplastics produces histological damage, oxidative stress and immunoregulation in European sea bass (Dicentrarchus labrax L.). Fish Shellfish. Immune 2019, 95, 574–583. [Google Scholar] [CrossRef]
- Roch, S.; Friedrich, C.; Brinker, A. Uptake routes of microplastics in fishes: Practical and theoretical approaches to test existing theories. Sci. Rep. 2020, 10, 3896. [Google Scholar] [CrossRef]
- Li, B.; Liang, W.; Liu, Q.X.; Fu, S.; Ma, C.; Chen, Q.; Su, L.; Craig, N.J.; Shi, H. Fish ingest microplastics unintentionally. Environ. Sci. Technol. 2021, 55, 10471–10479. [Google Scholar] [CrossRef] [PubMed]
- Roch, S.; Ros, A.F.H.; Friedrich, C.; Brinker, A. Microplastic evacuation in fish is particle size-dependent. Freshw. Biol. 2021, 66, 926–935. [Google Scholar] [CrossRef]
- Naidoo, T.; Thompson, R.C.; Rajkaran, A. Quantification and characterisation of microplastics ingested by selected juvenile fish species associated with mangroves in KwaZulu-Natal, South Africa. Environ. Pollut. 2020, 257, 113635. [Google Scholar] [CrossRef] [PubMed]
- Kaloyianni, M.; Bobori, D.C.; Xanthopoulou, D.; Malioufa, G.; Sampsonidis, I.; Kalogiannis, S.; Feidantsis, K.; Kastrinaki, G.; Dimitriadi, A.; Koumoundouros, G.; et al. Toxicity and functional tissue responses of two freshwater fish after exposure to polystyrene microplastics. Toxics 2021, 9, 289. [Google Scholar] [CrossRef] [PubMed]
- Wen, B.; Zhang, N.; Jin, S.R.; Chen, Z.Z.; Gao, J.Z.; Liu, Y.; Liu, H.P.; Xu, Z. Microplastics have a more profound impact than elevated temperatures on the predatory performance, digestion and energy metabolism of an Amazonian cichlid. Aquat. Toxicol. 2018, 195, 67–76. [Google Scholar] [CrossRef]
- Trestrail, C.; Walpitagama, M.; Miranda, A.; Nugegoda, D.; Shimeta, J. Microplastics alter digestive enzyme activities in the marine bivalve, Mytilus galloprovincialis. Sci. Total Environ. 2021, 779, 146418. [Google Scholar] [CrossRef]
- Frank, Y.A.; Interesova, E.A.; Solovyev, M.M.; Xu, J.; Vorobiev, D.S. Effect of Microplastics on the Activity of Digestive and Oxidative-Stress-Related Enzymes in Peled Whitefish (Coregonus peled Gmelin) Larvae. Int. J. Mol. Sci. 2023, 24, 10998. [Google Scholar] [CrossRef]
- Zhang, C.; Pan, Z.; Wang, S.; Xu, G.; Zou, J. Size and concentration effects of microplastics on digestion and immunity of hybrid snakehead in developmental stages. Aquacult. Rep. 2022, 22, 100974. [Google Scholar] [CrossRef]
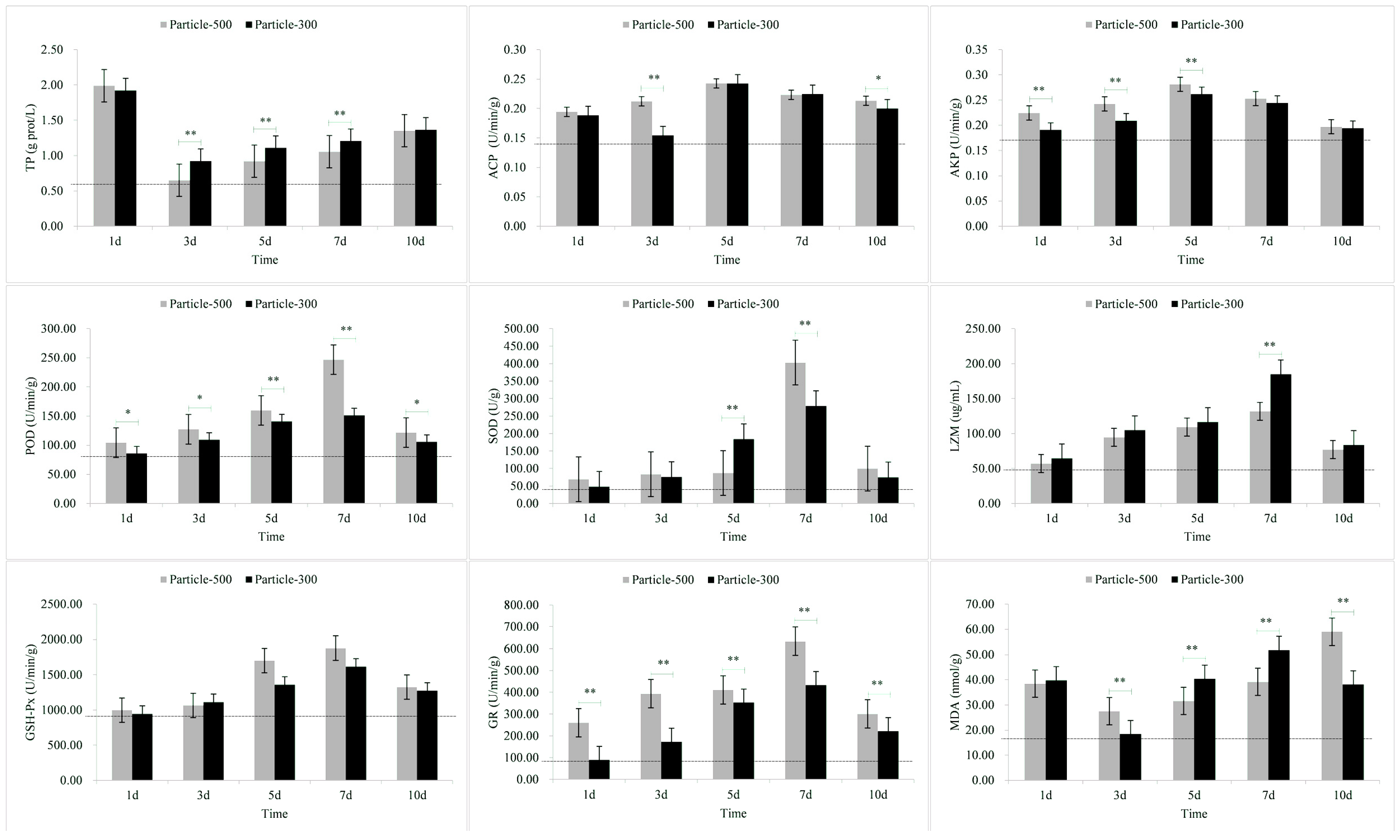

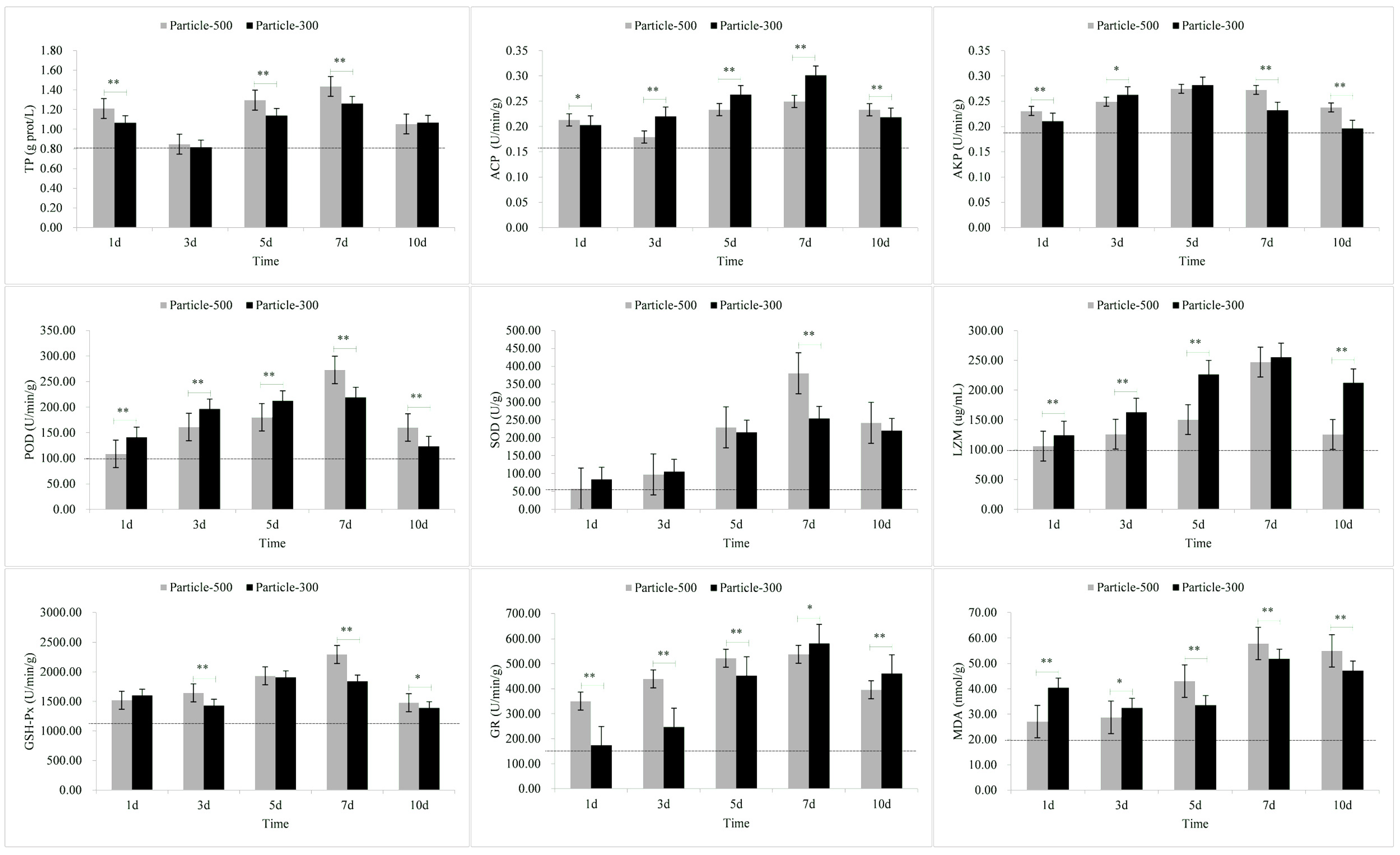
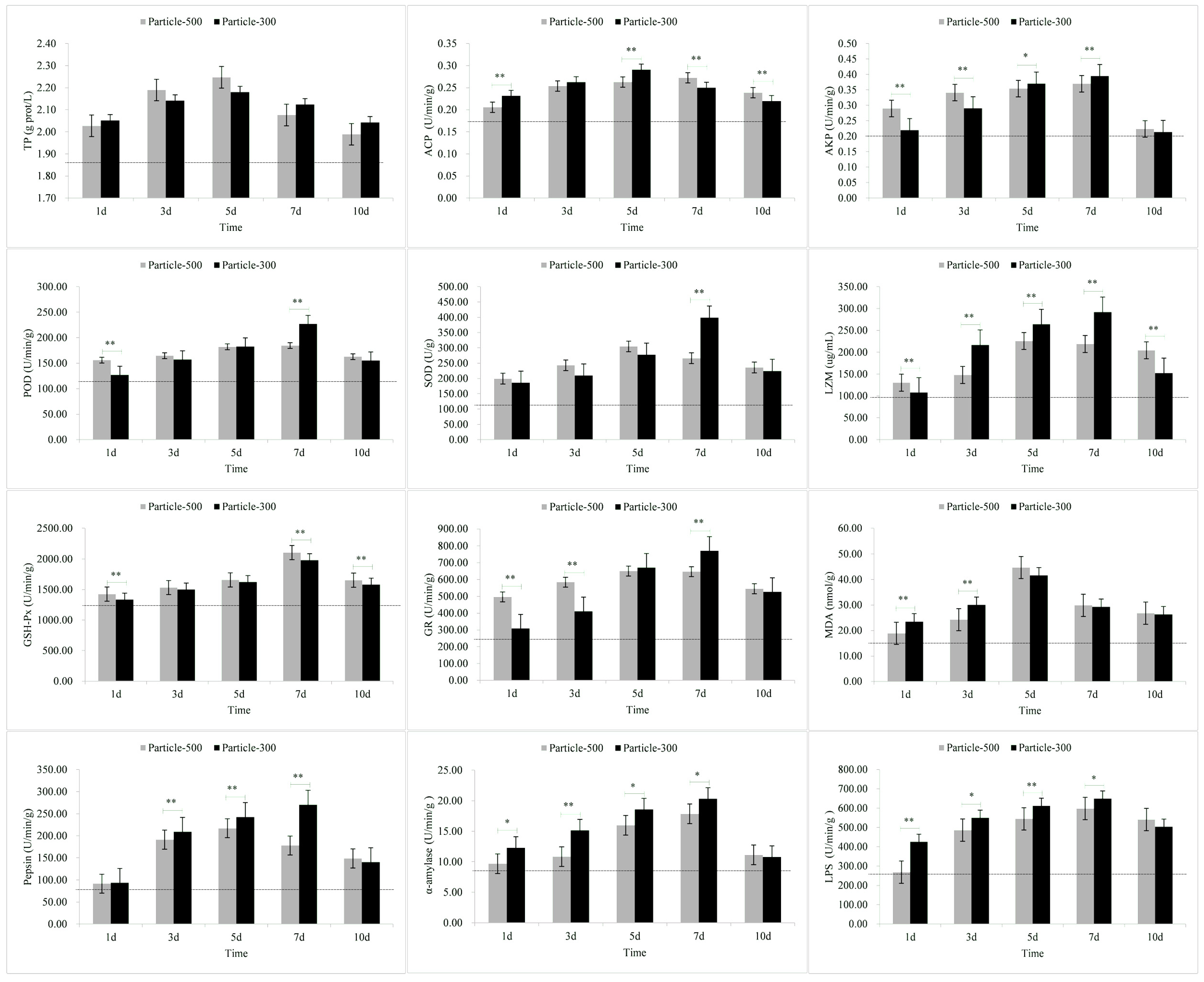
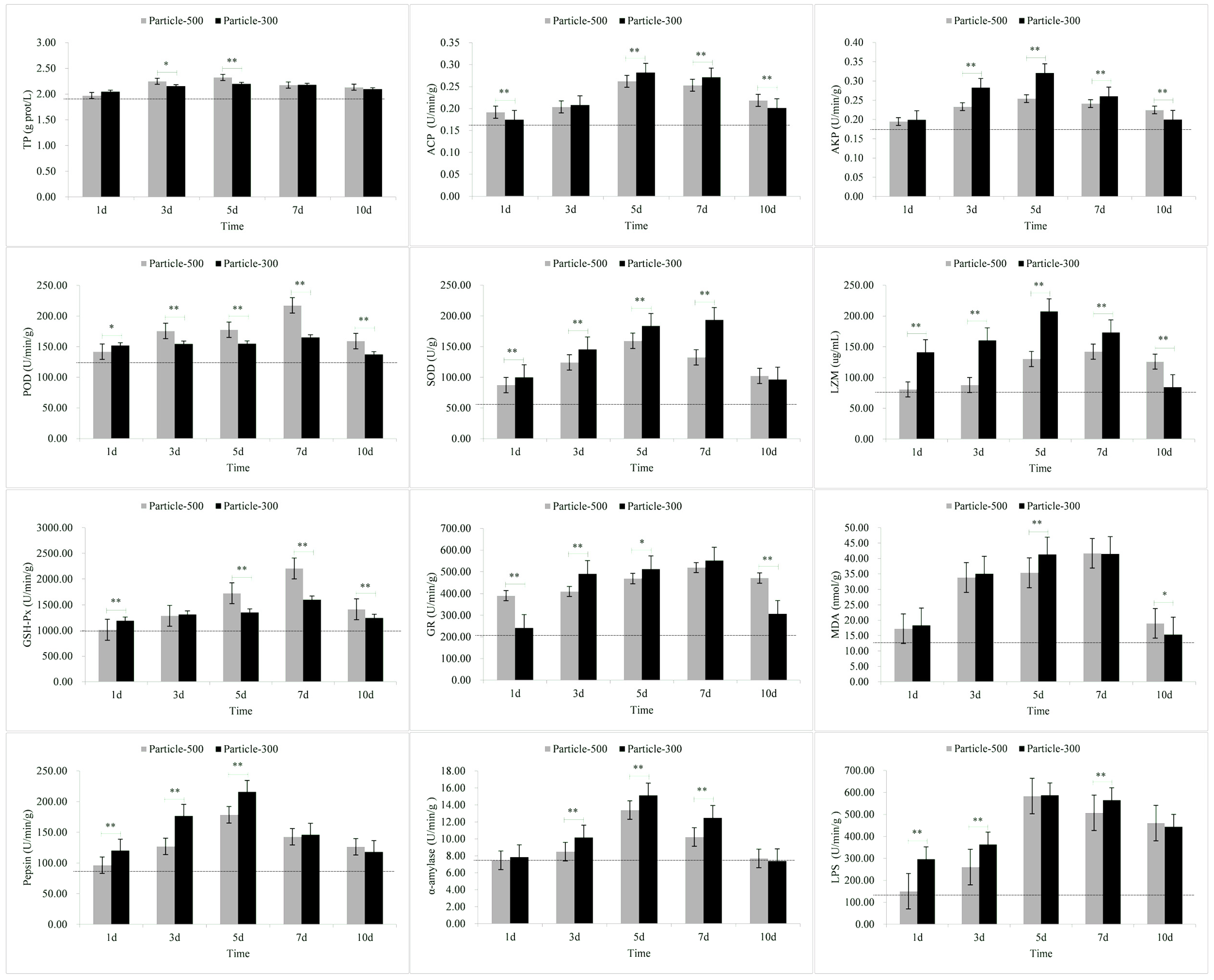
| Tissue | Stress Response Indicators | M5 | M3 |
|---|---|---|---|
| Skin | TP | − | + |
| ACP | + | − | |
| AKP | +’ | − | |
| POD | + | − | |
| SOD | +’ | − | |
| LZM | − | + | |
| GSH-Px | − | − | |
| GR | + | − | |
| MDA | − | +’ | |
| Gill | TP | − | − |
| ACP | +’ | − | |
| AKP | +’ | − | |
| POD | − | +’ | |
| SOD | +’ | − | |
| LZM | − | +’ | |
| GSH-Px | +’ | − | |
| GR | + | − | |
| MDA | +’ | − | |
| Muscle | TP | + | − |
| ACP | − | +’ | |
| AKP | +’ | − | |
| POD | +’ | − | |
| SOD | + | − | |
| LZM | − | + | |
| GSH-Px | − | + | |
| GR | − | + | |
| MDA | +’ | − | |
| Liver | TP | − | − |
| ACP | +’ | ||
| AKP | − | +’ | |
| POD | − | +’ | |
| SOD | − | + | |
| LZM | − | +’ | |
| GSH-Px | − | + | |
| GR | − | + | |
| MDA | − | + | |
| pepsin | − | + | |
| α-amylase | − | + | |
| LPS | − | + | |
| Iintestine | TP | + | − |
| ACP | − | +’ | |
| AKP | − | +’ | |
| POD | +’ | − | |
| SOD | − | + | |
| LZM | − | + | |
| GSH-Px | +’ | − | |
| GR | − | + | |
| MDA | − | + | |
| pepsin | − | + | |
| α-amylase | − | + | |
| LPS | − | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, W.; Qiu, J.; Lin, Y.; Li, X.; Li, W.; Ma, K.; Duan, Y.; Fu, Y. Enzymatic Stress Responses of Coreius guichenoti to Microplastics with Different Particle Sizes. Toxics 2023, 11, 1022. https://doi.org/10.3390/toxics11121022
Wu W, Qiu J, Lin Y, Li X, Li W, Ma K, Duan Y, Fu Y. Enzymatic Stress Responses of Coreius guichenoti to Microplastics with Different Particle Sizes. Toxics. 2023; 11(12):1022. https://doi.org/10.3390/toxics11121022
Chicago/Turabian StyleWu, Wenqiong, Junqiang Qiu, Yue Lin, Xike Li, Wenjuan Li, Keyi Ma, Yuanliang Duan, and Yuanshuai Fu. 2023. "Enzymatic Stress Responses of Coreius guichenoti to Microplastics with Different Particle Sizes" Toxics 11, no. 12: 1022. https://doi.org/10.3390/toxics11121022
APA StyleWu, W., Qiu, J., Lin, Y., Li, X., Li, W., Ma, K., Duan, Y., & Fu, Y. (2023). Enzymatic Stress Responses of Coreius guichenoti to Microplastics with Different Particle Sizes. Toxics, 11(12), 1022. https://doi.org/10.3390/toxics11121022







