The Lethal and Sublethal Effects of Lambda-Cyhalothrin and Emamectin Benzoate on the Soybean Pest Riptortus pedestris (Fabricius)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Insects and Chemicals
2.2. Bioassays
2.3. Dual-Choice Behavior Assays
2.4. Analyses of the Sublethal Effects of Insecticides on the F0 Generation
2.5. Analyses of Sublethal Effects on the Traits of the F1 Generation
2.6. Life Table Data Analyses
2.7. qRT-PCR
2.8. Statistical Analysis
3. Results
3.1. LCT and EMB Have Toxic Effects on R. pedestris Nymphs
3.2. LCT and EMB Exhibit Repellant Activity
3.3. Sublethal Doses of LCT and EMB Impact the Developmental Duration and Longevity of R. pedestris in the F0 and F1 Generations
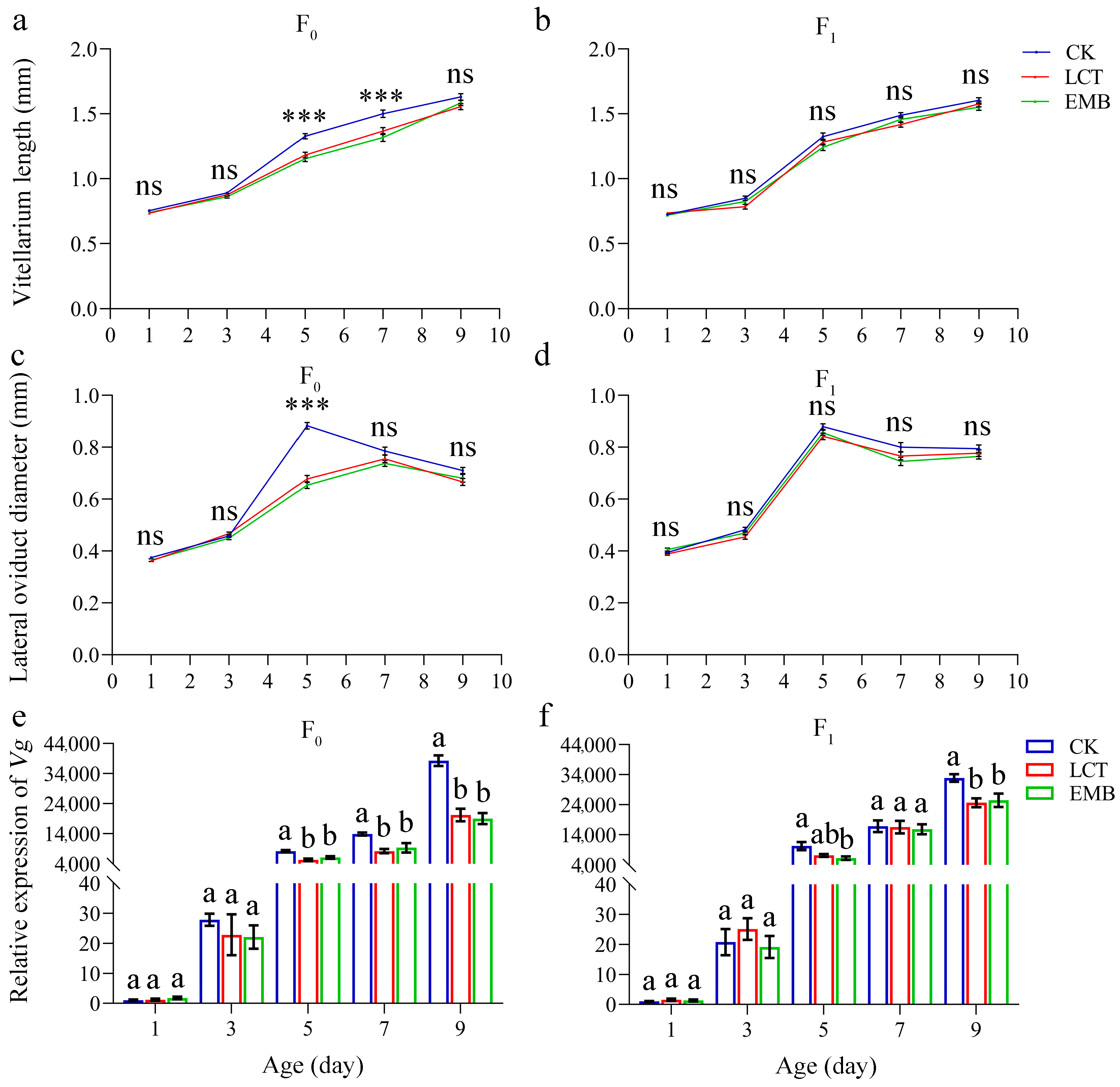
3.4. Sublethal Effects of LCT and EMB on R. pedestris Reproductive Parameters, Ovarian Development, and Vitellogenin Expression in the F0 and F1 Generations
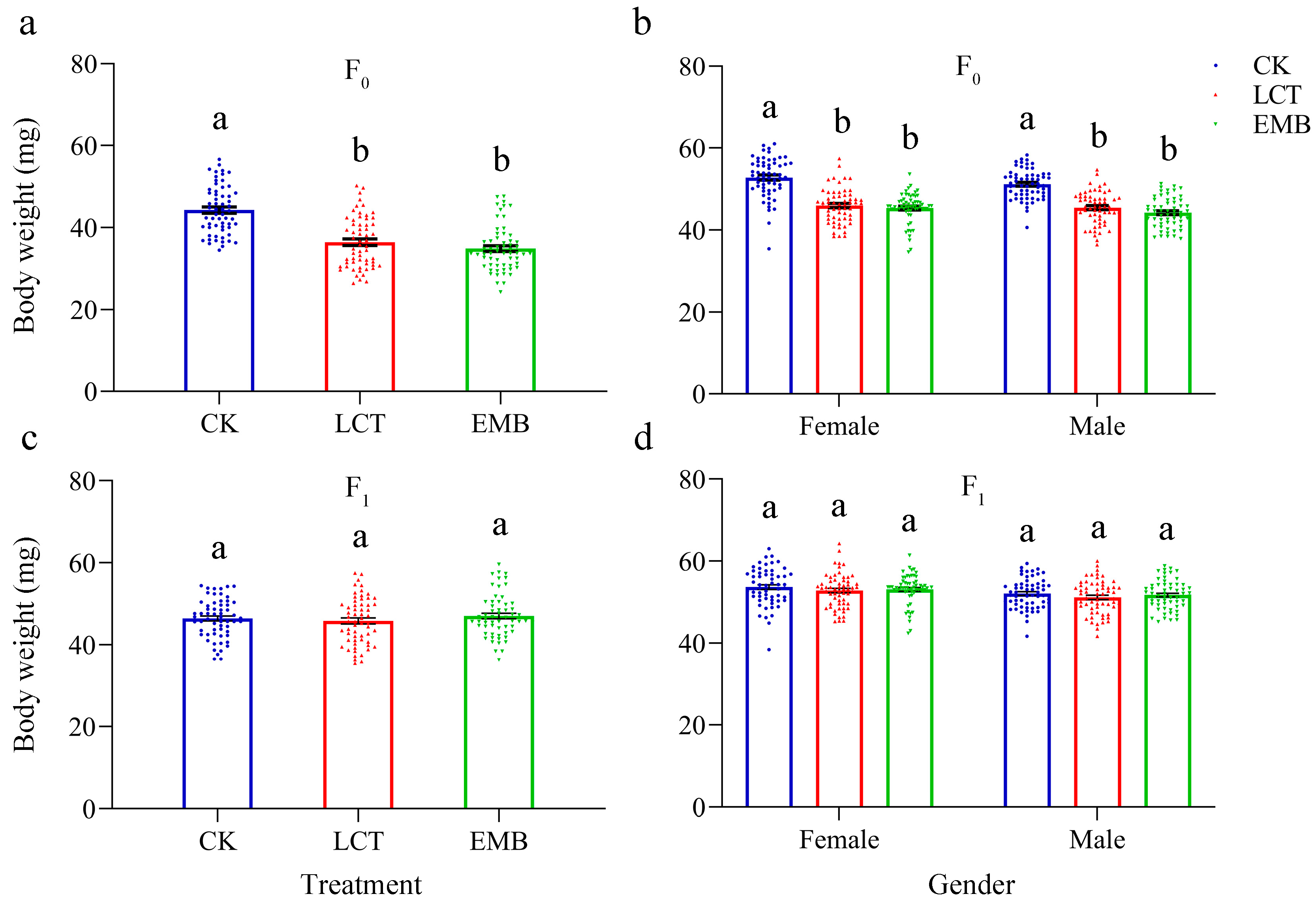
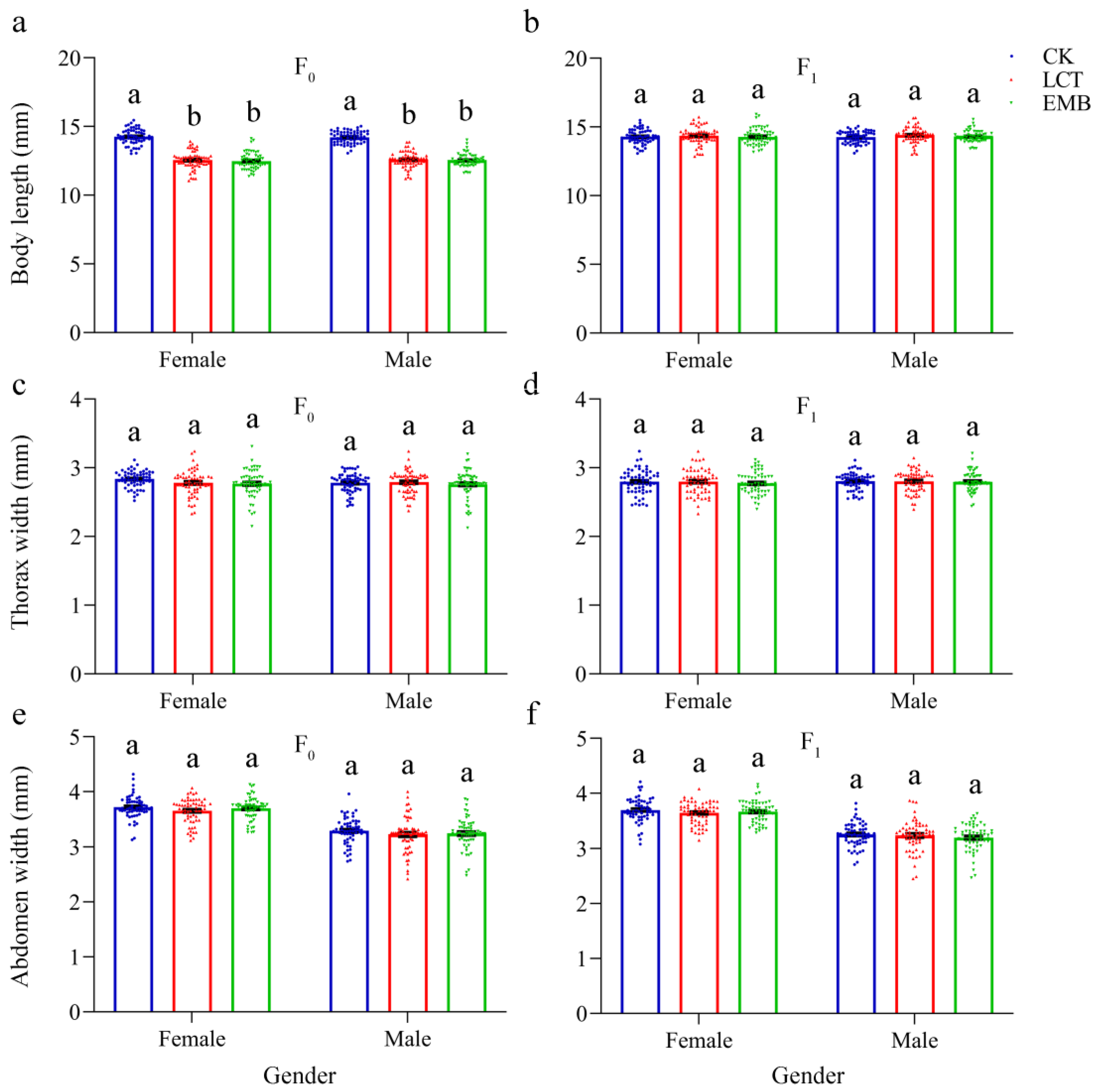
3.5. Sublethal Effects of LCT and EMB on the Body Weight and Morphology of R. pedestris in the F0 and F1 Generations
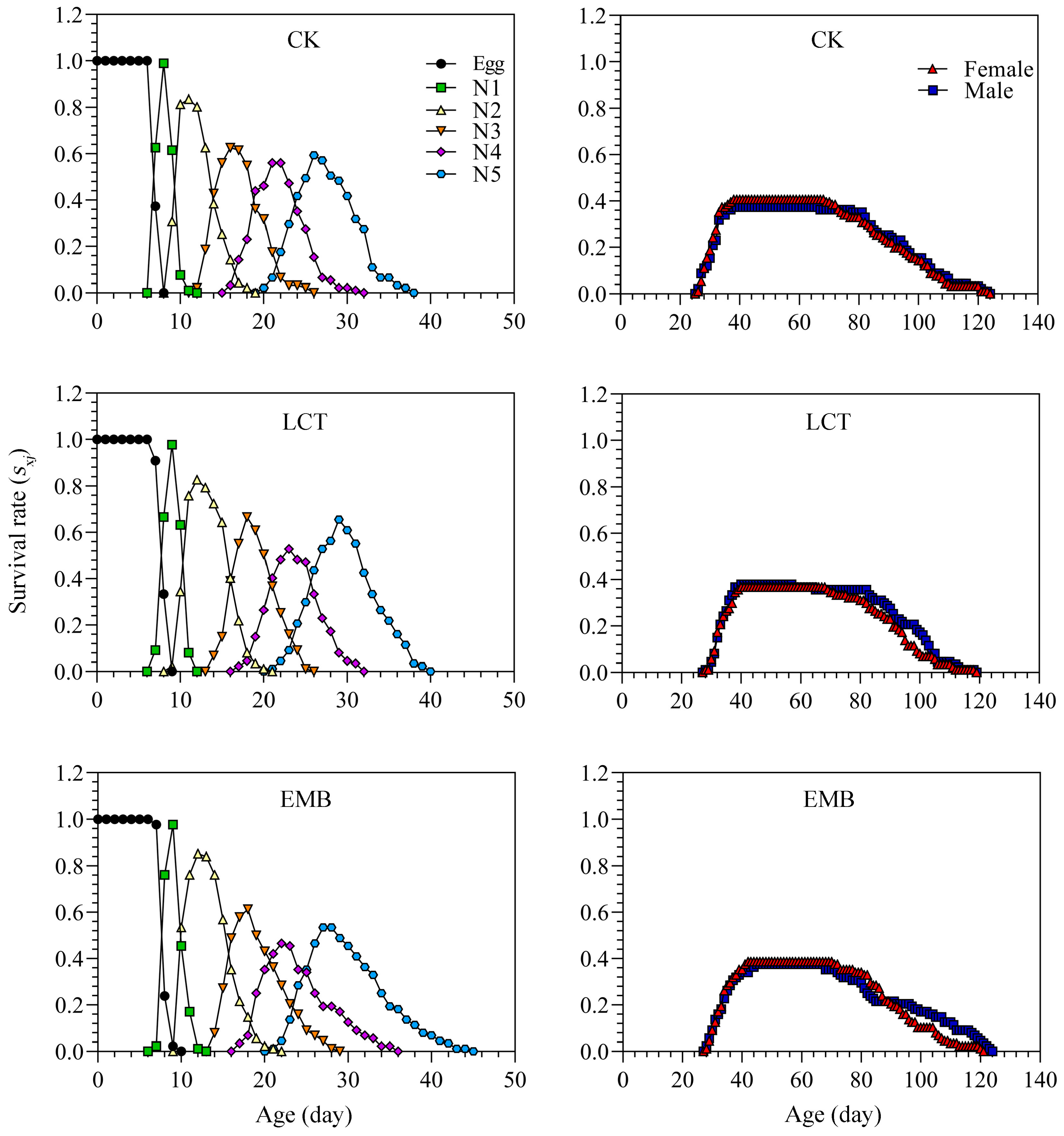
3.6. Sublethal Effects of LCT and EMB on R. pedestris Population Parameters in the F1 Generation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shreinemachers, P.; Tipraqsa, P. Agricultural pesticides and land use intensification in high, middle and low income countries. Food Policy 2012, 37, 616–626. [Google Scholar] [CrossRef]
- Bantz, A.; Camon, J.; Froger, J.A.; Goven, D.; Raymond, V. Exposure to sublethal doses of insecticide and their effects on insects at cellular and physiological levels. Curr. Opin. Insect. Sci. 2018, 30, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Shreinemachers, P.; Schad, I.; Tipraqsa, P.; Williams, P.M.; Neef, A.; Riwthong, S.; Sangchan, W.; Grovermann, C. Can public GAP standards reduce agricultural pesticide use? The case of fruit and vegetable farming in northern Thailand. Agric. Hum. Values 2012, 29, 519–529. [Google Scholar] [CrossRef]
- Hladik, M.L.; Main, A.R.; Goulson, D. Environmental risks and challenges associated with neonicotinoid insecticides. Environ. Sci. Technol. 2018, 52, 3329–3335. [Google Scholar] [CrossRef] [PubMed]
- Ullah, F.; Gul, H.; Desneux, N.; Said, F.; Gao, X.W.; Song, D.L. Fitness costs in chlorfenapyr-resistant populations of the chive maggot, Bradysia odoriphaga. Ecotoxicol. 2020, 29, 407–416. [Google Scholar] [CrossRef] [PubMed]
- He, F.L.; Sun, S.A.; Tan, H.L.; Sun, X.; Shang, D.L.; Yao, C.T.; Qin, C.; Ji, S.M.; Li, X.D.; Zhang, J.W. Compatibility of chlorantraniliprole with the generalist predator Coccinella septempunctata L. (Coleoptera: Coccinellidae) based toxicity, life-cycle development and population parameters in laboratory microcosms. Chemosphere 2019, 225, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Ju, D.; Liu, Y.X.; Liu, X.; Dewer, Y.; Mota-Sanchez, D.; Yang, X.Q. Exposure to lambda-cyhalothrin and abamectin drives sublethal and transgenerational effects on the development and reproduction of Cydia pomonella. Ecotoxicol. Environ. Saf. 2023, 252, 114581. [Google Scholar] [CrossRef] [PubMed]
- Desneux, N.; Decourtye, A.; Delpuech, J.M. The sublethal effects of pesticides on beneficial arthropods. Annu. Rev. Entomol. 2007, 52, 81–106. [Google Scholar] [CrossRef]
- De Franca, S.M.; Breda, M.O.; Barbosa, D.R.S.; Araujo, A.M.N.; Guedes, C.A. The sublethal effects of insecticides in insects. In Biological Control of Pest and Vector Insects; Shields, V.D.C., Ed.; Intech Open: London, UK, 2017; pp. 23–39. [Google Scholar] [CrossRef]
- Kong, F.F.; Song, Y.Q.; Zhang, Q.; Wang, Z.Y.; Liu, Y.Q. Sublethal effects of chlorantraniliprole on Spodoptera litura (Lepidoptera: Noctuidae) moth: Implication for attract-and-kill strategy. Toxics 2021, 9, 20. [Google Scholar] [CrossRef]
- Ma, K.S.; Tang, Q.L.; Liang, P.Z.; Li, J.H.; Gao, X.W. A sublethal concentration of afidopyropen suppresses the population growth of the cotton aphid, Aphis gossypii Glover (Hemiptera: Aphididae). J. Integr. Agric. 2022, 21, 2055–2064. [Google Scholar] [CrossRef]
- Tamilselvan, R.; Kennedy, J.S.; Suganthi, A. Sublethal and transgenerational effects of spinetoram on the biological traits of Plutella xylostella (L.) (Lepidoptera: Plutellidae). Ecotoxicology 2021, 30, 667–677. [Google Scholar] [CrossRef] [PubMed]
- Fouad, E.A.; EI-Sherif, S.A.N.; Mokbel, E.M.S. Flupyradifurone induces transgenerational hormesis effects in the cowpea aphid, Aphis craccivora. Ecotoxicology 2022, 31, 909–918. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.; Lee, D.H. Characterization of overwintering behaviors and sites of bean bug, Riptortus pedestris (Hemiptera: Alydidae), under laboratory and field conditions. Environ. Entomol. 2018, 47, 1280–1286. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.J.; Choi, K.S.; Koh, S. Population parameters and growth of Riptortus pedestris (Fabricius) (Hemiptera: Alydidae) under elevated CO2 concentrations. Entomol. Res. 2020, 51, 12–23. [Google Scholar] [CrossRef]
- Wei, Z.Y.; Guo, W.B.; Jiang, S.S.; Yan, D.K.; Shi, Y.; Wu, B.; Xin, X.Q.; Chen, L.; Cai, Y.P.; Zhang, H.H.; et al. Transcriptional profiling reveals a critical role of GmFT2a in soybean staygreen syndrome caused by the pest Riptortus pedestris. New Phytol. 2022, 237, 1876–1890. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Chen, J.H.; Shi, S.S. Research progress on soybean stink bug (Riptortus pedestris). Chin. J. Oil Crop Sci. 2019, 41, 804–815. [Google Scholar] [CrossRef]
- Zhen, C.A.; Tan, Y.; Miao, L.; Wu, J.; Gao, X.W. Overexpression of cytochrome P450s in a lambda-cyhalothrin resistant population of Apolygus lucorum (Meyer-Dür). PLoS ONE 2018, 13, e0198671. [Google Scholar] [CrossRef]
- Li, X.R.; Li, Y.; Wang, W.; He, N.; Tan, X.L.; Yang, X.Q. LC50 of lambda-cyhalothrin stimulates reproduction on the moth Mythimna separata (Walker). Pestic. Biochem. Phys. 2019, 153, 47–54. [Google Scholar] [CrossRef]
- Gao, X.W.; Liang, P.; Gu, S.H.; Zhang, L.; Zhu, B. Insect Toxicology, 1st ed.; China Agricultural University Press: Beijing, China, 2022. [Google Scholar]
- Xu, Z.; Bai, J.Y.; Li, L.; Liang, L.W.; Ma, X.Q.; Ma, L. Sublethal concentration of emamectin benzoate inhibits the growth of gypsy moth by inducing digestive dysfunction and nutrient metabolism discorder. Pest Manag. Sci. 2021, 77, 4073–4083. [Google Scholar] [CrossRef]
- Ishaaya, I.; Barazani, A.; Kontsedalov, S.; Horowitz, A.R. Insecticides with novel modes of action: Mechanism, selectivity and cross-resistance. Entomol. Res. 2007, 37, 148–152. [Google Scholar] [CrossRef]
- Moustafa, M.A.M.; Kákai, Á.; Awad, M.; Fónagy, A. Sublethal effects of spinosad and emamectin benzoate on larval development and reproductive activities of the cabbage moth, Mamestra brassicae L. (Lepidoptera: Noctuidae). Crop Prot. 2016, 90, 197–204. [Google Scholar] [CrossRef]
- Mokbel, E.S.; Huesien, A. Sublethal effects of emamectin benzoate on life table parameters of the cotton leafworm, Spodoptera littoralis (Boisd.). Bull. Natl. Res. Cent. 2020, 44, 155. [Google Scholar] [CrossRef]
- Yao, Q.; Xu, S.; Dong, Y.Z.; Que, Y.L.; Quan, L.F.; Chen, B.X. Characterization of vitellogenin and vitellogenin receptor of Conopomorpha sinensis Bradley and their responses to sublethal concentrations of insecticide. Front. Physiol. 2018, 9, 1250. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Yang, X.B.; Yang, H.; Long, G.Y.; Jin, D.C. Effects of sublethal concentrations of insecticides on the fecundity of Sogatella furcifera (Hemiptera: Delphacidae) via the regulation of vitellogenin and its receptor. J. Insect Sci. 2020, 20, 1–9. [Google Scholar] [CrossRef]
- Goodman, D. Optimal life histories, optimal notation, and the value of reproductive value. Am. Nat. 1982, 119, 803–823. [Google Scholar] [CrossRef]
- Chi, H.; Liu, H. Two new methods for the study of insect population ecology. Bull. Inst. Zool. Acad. Sin. Acad. Sin. 1985, 24, 225–240. [Google Scholar]
- Chi, H.; Su, H.Y. Age-stage, two-sex life tables of Aphidius gifuensis (Ashmead) (Hymenoptera: Braconidae) and its host Myzus persicae (Sulzer) (Homoptera: Aphididae) with mathematical proof of the relationship between female fecundity and the net reproductive rate. Environ. Entomol. 2006, 35, 10–21. [Google Scholar] [CrossRef]
- Tuan, S.J.; Lee, C.C.; Chi, H. Population and damage projection of Spodoptera litura (F.) on peanuts (Arachis hypogaea L.) under different conditions using the age-stage, two-sex life table. Pest Manag. Sci. 2014, 70, 805–813, Erratum in: Pest Manag. Sci. 2014, 70, 1936. [Google Scholar] [CrossRef]
- Chi, H. TWOSEX-MS Chart: A Computer Program for the Age-Stage, Two-Sex Life Table Analysis. 2023. Available online: http://140.120.197.173/Ecology/prod02.htm (accessed on 21 March 2023).
- Chi, H. Life–table analysis incorporating both sexes and variable development rates among individuals. Environ. Entomol. 1988, 17, 26–34. [Google Scholar] [CrossRef]
- Lee, J.B.; Park, K.E.; Lee, S.A.; Jang, S.H.; Eo, H.J.; Jang, H.A.; Kim, C.H.; Ohbayashi, T.; Matsuura, Y.; Kikuchi, Y.; et al. Gut symbiotic bacteria stimulate insect growth and egg production by modulating hexamerin and vitellogenin gene expression. Dev. Comp. Immunol. 2017, 69, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Endo, S.; Tsurumachi, M. Insecticide susceptibility of brown plant hopper and white-backed plant hopper collected from Southeast Asia. J. Pestic. Sci. 2001, 26, 82–86. [Google Scholar] [CrossRef]
- Ragsdale, D.W.; Landis, D.A.; Brodeur, J.; Heimpel, G.E.; Desneux, N. Ecology and management of the soybean aphid in North America. Annu. Rev. Entomol. 2011, 56, 375–399. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Ali, E.; Li, W.H.; He, B.Y.; Gong, P.P.; Xu, P.F.; Li, J.H.; Wan, H. Sublethal effects of sulfoxaflor on the development and reproduction of the brown planthopper, Nilaparvata lugens (Stål). Crop Prot. 2019, 118, 6–14. [Google Scholar] [CrossRef]
- Arifunnahar, M.; Khatun, M.M.; Hossain, M.A.; Alim, M.A. Toxicity evaluation of different chemical pesticides against Riptortus pedestris (Hemiptera: Alydidae) under laboratory condition in Bangladesh. J. Bangl. Agric. Univ. 2021, 19, 192–197. [Google Scholar] [CrossRef]
- Guedes, R.N.C.; Smagghe, G.; Stark, J.D.; Desneus, N. Pesticide-induced stress in arthropod pests for optimized integrated pest management programs. Annu. Rev. Entomol. 2016, 61, 43–62. [Google Scholar] [CrossRef]
- Romero, A.; Potter, M.F.; Haynes, K.F. Behavioral responses of the bed bug to insecticide residues. J. Med. Entomol. 2009, 46, 51–57. [Google Scholar] [CrossRef]
- Maharjan, R.; Jung, C. Insecticide-mediated behavioral avoidance by bean bug, Riptortus pedestris (Heteroptera: Alydidae). Entomol. Res. 2015, 45, 184–192. [Google Scholar] [CrossRef]
- Zhang, Q.Q.; Li, W.Q.; Lu, Z.B.; Li, L.L.; Yu, Y.; Li, C.; Men, X.Y. Sublethal effects of beta-cypermethrin on the bird cherry-oat aphid Rhopalosiphum padi (Hemiptera: Aphididae). J. Asia-Pac. Entomol. 2019, 22, 693–698. [Google Scholar] [CrossRef]
- Khan, M.M.; Ali, M.W.; Hafeez, M.; Fan, Z.Y.; Ali, S.; Qiu, B.L. Lethal and sublethal effects of emamectin benzoate on life-table and physiological parameters of citrus red mite, Panonychus citri. Exp. Appl. Acarol. 2021, 85, 173–190. [Google Scholar] [CrossRef]
- Khan, M.M.; Nawaz, M.; Hua, H.X.; Cai, W.L.; Zhao, J. Lethal and sublethal effects of emamectin benzoate on the rove beetle Paederus fuscipes, a non-target predator of rice brown planthopper, Nilaparvata lugens. Ecotoxicol. Environ. Saf. 2018, 165, 19–24. [Google Scholar] [CrossRef]
- Müller, T.; Prosche, A.; Müller, C. Sublethal insecticide exposure affects reproduction, chemical phenotype as well as offspring development and antennae symmetry of a leaf beetle. Environ. Pollut. 2017, 230, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Lv, N.N.; Ma, K.S.; Li, R.; Liang, P.Z.; Liang, P.; Gao, X.W. Sublethal and lethal effects of the imidacloprid on the metabolic characteristics based on high-throughput non-targeted metabolomics in Aphis gossypii Glover. Ecotoxicol. Environ. Saf. 2012, 211, 111969. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.A.A. Pyriproxyfen induces lethal and sublethal effects on biological traits and demographic growth parameters in Musca domestica. Ecotoxicology 2021, 30, 610–621. [Google Scholar] [CrossRef] [PubMed]
- Houri-Ze’evi, L.; Korem, Y.; Sheftel, H.; Faigenbloom, L.; Toker, I.A.; Dagan, Y.; Award, L.; Degani, L.; Alon, U.; Rechavi, O. A tunable mechanism determines the duration of the transgenerational small RNA inheritance in C. elegans. Cell 2016, 165, 88–99. [Google Scholar] [CrossRef]
- Heard, E.; Martienssen, R.A. Transgenerational epigenetic inheritance: Myths and mechanism. Cell 2014, 157, 95–109. [Google Scholar] [CrossRef]
- Stark, J.D.; Banks, J.E. Population-level effects of pesticides and other toxicants on arthropods. Annu. Rev. Entomol. 2003, 48, 505–519. [Google Scholar] [CrossRef]
- Ghramh, H.A.; Sadip, N.; Naqqash, M.N.; Abid, A.D.; Shahzad, S.; Saeed, S.; Iqbal, N.; Khan, K.A. Transgenerational effects of lambda-cyhalothrin on Musca domestica L. (Diptera: Muscidae). Sci. Rep. 2022, 12, 19228. [Google Scholar] [CrossRef]



| Insecticide | Slope ± SE a | LC30 mg L−1 (95% CL) b | LC50 mg L−1 (95% CL) b | LC70 mg L−1 (95% CL) b | LC90 mg L−1 (95% CL) b | χ2 | p |
|---|---|---|---|---|---|---|---|
| lambda-cyhalothrin | 1.227 ± 0.136 | 1.002 (0.649–1.369) | 2.680 (2.058–3.404) | 7.166 (5.523–9.998) | 29.655 (19.061–57.087) | 3.335 | 0.503 |
| emamectin benzoate | 1.309 ± 0.138 | 3.053 (2.107–4.026) | 7.681 (6.057–9.620) | 19.323 (15.051–26.588) | 73.207 (48.307–133.801) | 5.199 | 0.267 |
| Stages or Parameters | Control (CK) (n) | Lambda-Cyhalothrin (LCT) (n) | Emamectin Benzoate (EMB) (n) |
|---|---|---|---|
| Third instar (d) | 4.96 ± 0.10 b (189) | 6.63 ± 0.10 a (160) | 6.85 ± 0.09 a (151) |
| Fourth instar (d) | 5.03 ± 0.09 c (183) | 7.30 ± 0.10 a (154) | 5.68 ± 0.11 b (145) |
| Fifth instar (d) | 6.67 ± 0.08 b (183) | 7.83 ± 0.09 a (154) | 7.57 ± 0.10 a (145) |
| Female longevity (d) | 65.43 ± 1.65 a (60) | 43.88 ± 1.37 b (60) | 41.22 ± 1.38 b (60) |
| Male longevity (d) | 73.25 ± 1.73 a (60) | 52.32 ± 1.56 b (60) | 49.73 ± 1.63 b (60) |
| APOP a (d) | 6.37 ± 0.16 c (60) | 8.47 ± 0.21 b (60) | 8.67 ± 0.19 a (60) |
| Oviposition days (d) | 50.38 ± 1.66 a (60) | 30.20 ± 1.30 b (60) | 24.00 ± 1.31 c (60) |
| Fecundity (eggs/female) | 262.48 ± 8.57 a (60) | 163.92 ± 7.67 b (60) | 146.12 ± 7.46 b (60) |
| Stages or Parameters | Control (CK) (n) | Lambda-Cyhalothrin (LCT) (n) | Emamectin Benzoate (EMB) (n) |
|---|---|---|---|
| Egg period (d) | 7.37 ± 0.05 b (91) | 8.24 ± 0.07 a (87) | 8.23 ± 0.06 a (88) |
| First instar (d) | 2.36 ± 0.06 a (81) | 2.40 ± 0.06 a (75) | 2.36 ± 0.07 a (78) |
| Second instar (d) | 4.99 ± 0.16 b (74) | 5.96 ± 0.15 a (68) | 5.97 ± 0.17 a (74) |
| Third instar (d) | 4.87 ± 0.12 a (71) | 4.90 ± 0.18 a (68) | 5.03 ± 0.19 a (69) |
| Fourth instar (d) | 4.94 ± 0.12 a (71) | 4.86 ± 0.11 a (66) | 5.01 ± 0.13 a (69) |
| Fifth instar (d) | 6.32 ± 0.13 b (71) | 7.37 ± 0.15 a (65) | 7.15 ± 0.14 a (67) |
| Preadult (d) | 30.86 ± 0.36 b (71) | 33.68 ± 0.36 a (65) | 33.85 ± 0.50 a (67) |
| Female longevity (d) | 62.65 ± 2.30 a (37) | 58.78 ± 2.04 a (32) | 59.71 ± 2.14 a (34) |
| Male longevity (d) | 66.32 ± 2.22 a (34) | 62.55 ± 2.27 a (33) | 62.97 ± 3.15 a (33) |
| Total longevity (d) | 95.27 ± 1.69 a (71) | 94.37 ± 1.55 a (65) | 95.16 ± 1.93 a (67) |
| APOP a | 6.57 ± 0.16 a (37) | 7.19 ± 0.38 a (32) | 6.85 ± 0.23 a (34) |
| TPOP b | 37.41 ± 0.49 b (37) | 40.84 ± 0.70 a (32) | 40.56 ± 0.71 a (34) |
| Oviposition days (d) | 49.08 ± 2.04 a (37) | 42.09 ± 1.41 b (32) | 43.32 ± 1.77 b (34) |
| Fecundity (eggs/female) | 253.43 ± 11.38 a (37) | 191.56 ± 8.50 b (32) | 176.97 ± 8.94 b (34) |
| Parameters | Control (CK) | Lambda-Cyhalothrin | Emamectin Benzoate |
|---|---|---|---|
| Net reproductive rate (R0) | 103.044 ± 13.847 a | 70.460 ± 10.368 b | 68.375 ± 9.819 b |
| Intrinsic rate of increase (d−1) (r) | 0.086 ±0.029 a | 0.075 ± 0.030 b | 0.075 ± 0.031 b |
| Finite rate of increase (d−1) (λ) | 1.090 ± 0.032 a | 1.077 ± 0.032 b | 1.078 ± 0.033 b |
| Mean generation time (d) (T) | 54.023 ± 0.765 a | 57.035 ± 0.897 a | 56.355 ± 1.162 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, J.; An, J.; Chang, H.; Li, Y.; Dang, Z.; Wu, C.; Gao, Z. The Lethal and Sublethal Effects of Lambda-Cyhalothrin and Emamectin Benzoate on the Soybean Pest Riptortus pedestris (Fabricius). Toxics 2023, 11, 971. https://doi.org/10.3390/toxics11120971
Guo J, An J, Chang H, Li Y, Dang Z, Wu C, Gao Z. The Lethal and Sublethal Effects of Lambda-Cyhalothrin and Emamectin Benzoate on the Soybean Pest Riptortus pedestris (Fabricius). Toxics. 2023; 11(12):971. https://doi.org/10.3390/toxics11120971
Chicago/Turabian StyleGuo, Jianglong, Jingjie An, Hong Chang, Yaofa Li, Zhihong Dang, Chi Wu, and Zhanlin Gao. 2023. "The Lethal and Sublethal Effects of Lambda-Cyhalothrin and Emamectin Benzoate on the Soybean Pest Riptortus pedestris (Fabricius)" Toxics 11, no. 12: 971. https://doi.org/10.3390/toxics11120971
APA StyleGuo, J., An, J., Chang, H., Li, Y., Dang, Z., Wu, C., & Gao, Z. (2023). The Lethal and Sublethal Effects of Lambda-Cyhalothrin and Emamectin Benzoate on the Soybean Pest Riptortus pedestris (Fabricius). Toxics, 11(12), 971. https://doi.org/10.3390/toxics11120971







